Abstract
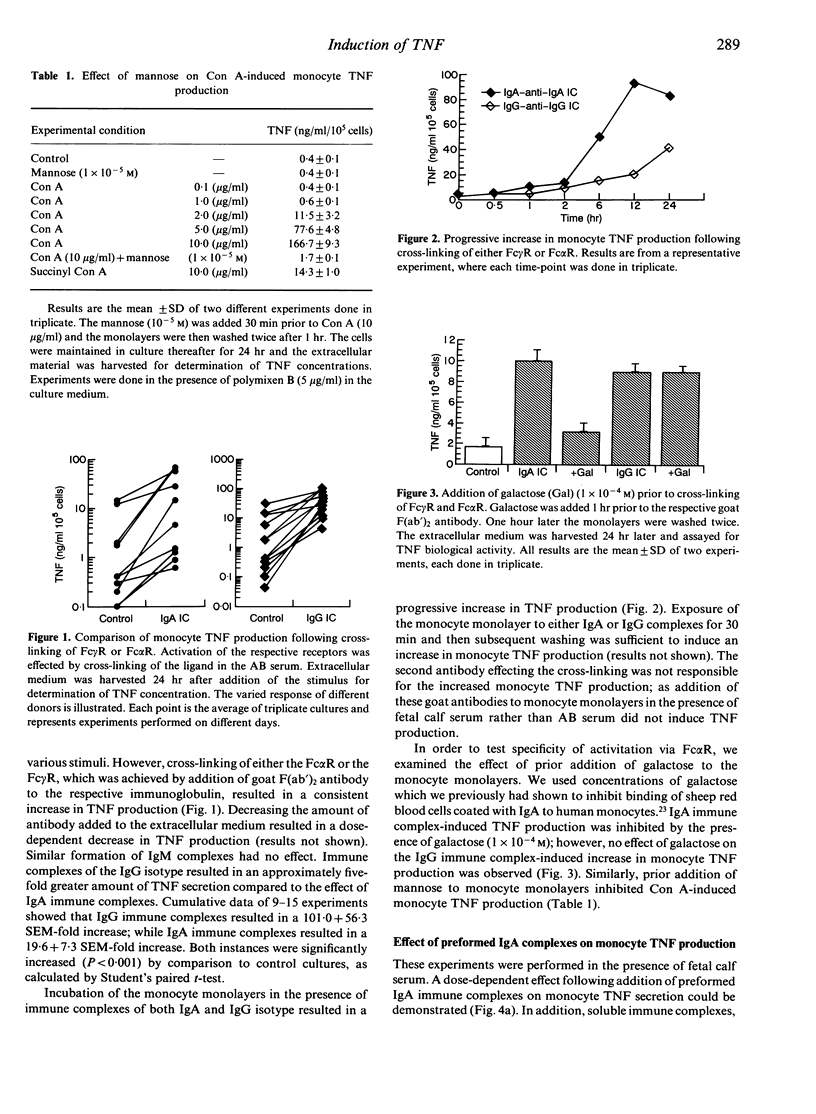
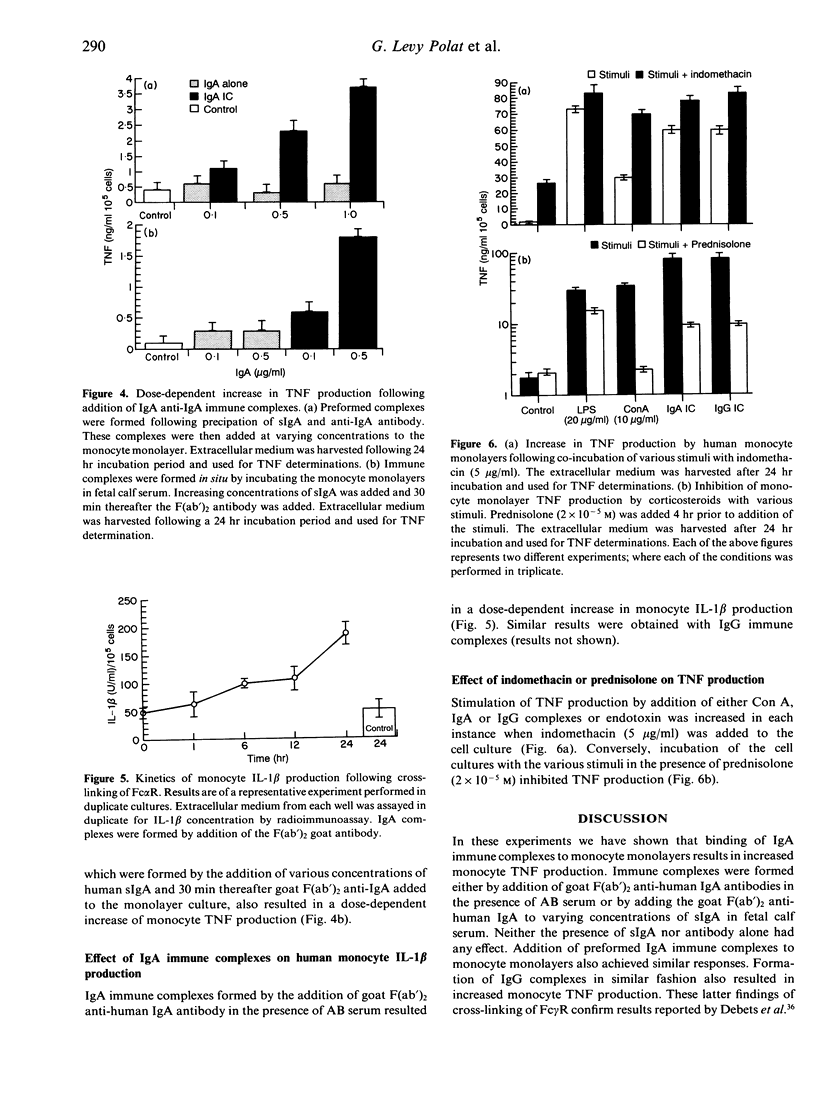
We have studied and compared the effects of IgA and IgG immune complexes and concanavalin A (Con A) on human monocyte tumour necrosis factor (TNF) production. The presence of IgA-containing immune complexes in monocyte monolayers resulted in a dose-dependent increase of TNF production. Similar results were obtained with IgG-containing immune complexes and Con A. The presence of monomeric IgA or IgG did not increase TNF secretion. Both IgA and IgG immune complexes also increased monocyte interleukin-1 beta (IL-1 beta) production. Galactose inhibited the effect of IgA but not IgG immune complexes, while mannose inhibited the effect of Con A. Prednisolone abrogated TNF production, while indomethacin enhanced TNF production in all instances where cross-linking of plasma membrane receptors was achieved. These results indicate that activation of Fc alpha receptors (Fc alpha R), Fc gamma R or mannose receptors of the human monocyte plasma membrane by cross-linking results in increased TNF and IL-1 beta secretion. These findings may be of particular relevance in the pathogenesis of IgA immune complex-mediated disease.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arima S., Nakayama M., Naito M., Sato T., Takahashi K. Significance of mononuclear phagocytes in IgA nephropathy. Kidney Int. 1991 Apr;39(4):684–692. doi: 10.1038/ki.1991.82. [DOI] [PubMed] [Google Scholar]
- Bancroft G. J., Sheehan K. C., Schreiber R. D., Unanue E. R. Tumor necrosis factor is involved in the T cell-independent pathway of macrophage activation in scid mice. J Immunol. 1989 Jul 1;143(1):127–130. [PubMed] [Google Scholar]
- Beutler B., Cerami A. Cachectin: more than a tumor necrosis factor. N Engl J Med. 1987 Feb 12;316(7):379–385. doi: 10.1056/NEJM198702123160705. [DOI] [PubMed] [Google Scholar]
- Beutler B., Mahoney J., Le Trang N., Pekala P., Cerami A. Purification of cachectin, a lipoprotein lipase-suppressing hormone secreted by endotoxin-induced RAW 264.7 cells. J Exp Med. 1985 May 1;161(5):984–995. doi: 10.1084/jem.161.5.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce N. W., Holdsworth S. R. Quantitation of the intrarenal uptake of immunoglobulin aggregates by macrophages in diffuse proliferative glomerulonephritis. Clin Exp Immunol. 1986 Jun;64(3):638–645. [PMC free article] [PubMed] [Google Scholar]
- Chantry D., Winearls C. G., Maini R. N., Feldmann M. Mechanism of immune complex-mediated damage: induction of interleukin 1 by immune complexes and synergy with interferon-gamma and tumor necrosis factor-alpha. Eur J Immunol. 1989 Jan;19(1):189–192. doi: 10.1002/eji.1830190130. [DOI] [PubMed] [Google Scholar]
- Chevailler A., Monteiro R. C., Kubagawa H., Cooper M. D. Immunofluorescence analysis of IgA binding by human mononuclear cells in blood and lymphoid tissue. J Immunol. 1989 Apr 1;142(7):2244–2249. [PubMed] [Google Scholar]
- Couser W. G. Mechanisms of glomerular injury in immune-complex disease. Kidney Int. 1985 Sep;28(3):569–583. doi: 10.1038/ki.1985.167. [DOI] [PubMed] [Google Scholar]
- Czerkinsky C., Koopman W. J., Jackson S., Collins J. E., Crago S. S., Schrohenloher R. E., Julian B. A., Galla J. H., Mestecky J. Circulating immune complexes and immunoglobulin A rheumatoid factor in patients with mesangial immunoglobulin A nephropathies. J Clin Invest. 1986 Jun;77(6):1931–1938. doi: 10.1172/JCI112522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debets J. M., Van der Linden C. J., Dieteren I. E., Leeuwenberg J. F., Buurman W. A. Fc-receptor cross-linking induces rapid secretion of tumor necrosis factor (cachectin) by human peripheral blood monocytes. J Immunol. 1988 Aug 15;141(4):1197–1201. [PubMed] [Google Scholar]
- Drapier J. C., Wietzerbin J., Hibbs J. B., Jr Interferon-gamma and tumor necrosis factor induce the L-arginine-dependent cytotoxic effector mechanism in murine macrophages. Eur J Immunol. 1988 Oct;18(10):1587–1592. doi: 10.1002/eji.1830181018. [DOI] [PubMed] [Google Scholar]
- Emancipator S. N., Gallo G. R., Lamm M. E. IgA nephropathy: perspectives on pathogenesis and classification. Clin Nephrol. 1985 Oct;24(4):161–179. [PubMed] [Google Scholar]
- Emancipator S. N., Lamm M. E. IgA nephropathy: pathogenesis of the most common form of glomerulonephritis. Lab Invest. 1989 Feb;60(2):168–183. [PubMed] [Google Scholar]
- Ezekowitz R. A., Sastry K., Bailly P., Warner A. Molecular characterization of the human macrophage mannose receptor: demonstration of multiple carbohydrate recognition-like domains and phagocytosis of yeasts in Cos-1 cells. J Exp Med. 1990 Dec 1;172(6):1785–1794. doi: 10.1084/jem.172.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanger M. W., Pugh J., Bernier G. M. The specificity of receptors for IgA on human peripheral polymorphonuclear cells and monocytes. Cell Immunol. 1981 May 15;60(2):324–334. doi: 10.1016/0008-8749(81)90274-4. [DOI] [PubMed] [Google Scholar]
- Fanger M. W., Shen L., Pugh J., Bernier G. M. Subpopulations of human peripheral granulocyes and monocytes express receptors for IgA. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3640–3644. doi: 10.1073/pnas.77.6.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorter A., Hiemstra P. S., Leijh P. C., van der Sluys M. E., van den Barselaar M. T., van Es L. A., Daha M. R. IgA- and secretory IgA-opsonized S. aureus induce a respiratory burst and phagocytosis by polymorphonuclear leucocytes. Immunology. 1987 Jul;61(3):303–309. [PMC free article] [PubMed] [Google Scholar]
- Gorter A., Hiemstra P. S., van der Voort E. A., van Es L. A., Daha M. R. Binding of human IgA1 to rat peritoneal macrophages. Immunology. 1988 Jun;64(2):207–212. [PMC free article] [PubMed] [Google Scholar]
- Havell E. A. Evidence that tumor necrosis factor has an important role in antibacterial resistance. J Immunol. 1989 Nov 1;143(9):2894–2899. [PubMed] [Google Scholar]
- Holdsworth S. R., Neale T. J., Wilson C. B. Abrogation of macrophage-dependent injury in experimental glomerulonephritis in the rabbit. Use of an antimacrophage serum. J Clin Invest. 1981 Sep;68(3):686–698. doi: 10.1172/JCI110304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob C. O., Aiso S., Michie S. A., McDevitt H. O., Acha-Orbea H. Prevention of diabetes in nonobese diabetic mice by tumor necrosis factor (TNF): similarities between TNF-alpha and interleukin 1. Proc Natl Acad Sci U S A. 1990 Feb;87(3):968–972. doi: 10.1073/pnas.87.3.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob C. O., McDevitt H. O. Tumour necrosis factor-alpha in murine autoimmune 'lupus' nephritis. Nature. 1988 Jan 28;331(6154):356–358. doi: 10.1038/331356a0. [DOI] [PubMed] [Google Scholar]
- Krane S. M., Simon L. S. Rheumatoid arthritis: clinical features and pathogenetic mechanisms. Med Clin North Am. 1986 Mar;70(2):263–284. doi: 10.1016/s0025-7125(16)30953-1. [DOI] [PubMed] [Google Scholar]
- Leist T. P., Frei K., Kam-Hansen S., Zinkernagel R. M., Fontana A. Tumor necrosis factor alpha in cerebrospinal fluid during bacterial, but not viral, meningitis. Evaluation in murine model infections and in patients. J Exp Med. 1988 May 1;167(5):1743–1748. doi: 10.1084/jem.167.5.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maliszewski C. R., March C. J., Schoenborn M. A., Gimpel S., Shen L. Expression cloning of a human Fc receptor for IgA. J Exp Med. 1990 Dec 1;172(6):1665–1672. doi: 10.1084/jem.172.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestecky J., McGhee J. R. Immunoglobulin A (IgA): molecular and cellular interactions involved in IgA biosynthesis and immune response. Adv Immunol. 1987;40:153–245. doi: 10.1016/s0065-2776(08)60240-0. [DOI] [PubMed] [Google Scholar]
- Monteiro R. C., Cooper M. D., Kubagawa H. Molecular heterogeneity of Fc alpha receptors detected by receptor-specific monoclonal antibodies. J Immunol. 1992 Mar 15;148(6):1764–1770. [PubMed] [Google Scholar]
- Monteiro R. C., Kubagawa H., Cooper M. D. Cellular distribution, regulation, and biochemical nature of an Fc alpha receptor in humans. J Exp Med. 1990 Mar 1;171(3):597–613. doi: 10.1084/jem.171.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padeh S., Jaffe C. L., Passwell J. H. Activation of human monocytes via their sIgA receptors. Immunology. 1991 Feb;72(2):188–193. [PMC free article] [PubMed] [Google Scholar]
- Passwell J. H., Dayer J. M., Gass K., Edelson P. J. Regulation by Fc fragments of the secretion of collagenase, PGE2, and lysozyme by mouse peritoneal macrophages. J Immunol. 1980 Aug;125(2):910–913. [PubMed] [Google Scholar]
- Passwell J. H., Dayer J. M., Merler E. Increased prostaglandin production by human monocytes after membrane receptor activation. J Immunol. 1979 Jul;123(1):115–120. [PubMed] [Google Scholar]
- Passwell J. H., Schreiner G. F., Wetsel R. A., Colten H. R. Complement gene expression in hepatic and extrahepatic tissues of NZB and NZB x W (F1) mouse strains. Immunology. 1990 Oct;71(2):290–294. [PMC free article] [PubMed] [Google Scholar]
- Passwell J., Schreiner G. F., Nonaka M., Beuscher H. U., Colten H. R. Local extrahepatic expression of complement genes C3, factor B, C2, and C4 is increased in murine lupus nephritis. J Clin Invest. 1988 Nov;82(5):1676–1684. doi: 10.1172/JCI113780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter D. H., Colten H. R. Molecular immunobiology of complement biosynthesis: a model of single-cell control of effector-inhibitor balance. Annu Rev Immunol. 1986;4:231–251. doi: 10.1146/annurev.iy.04.040186.001311. [DOI] [PubMed] [Google Scholar]
- Perlmutter D. H., Dinarello C. A., Punsal P. I., Colten H. R. Cachectin/tumor necrosis factor regulates hepatic acute-phase gene expression. J Clin Invest. 1986 Nov;78(5):1349–1354. doi: 10.1172/JCI112721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragsdale C. G., Arend W. P. Neutral protease secretion by human monocytes. Effect of surface-bound immune complexes. J Exp Med. 1979 Apr 1;149(4):954–968. doi: 10.1084/jem.149.4.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson G., Volovitz B., Passwell J. H. Identification of a secretory IgA receptor on breast-milk macrophages: evidence for specific activation via these receptors. Pediatr Res. 1991 May;29(5):429–434. doi: 10.1203/00006450-199105010-00004. [DOI] [PubMed] [Google Scholar]
- Spengler R. N., Spengler M. L., Strieter R. M., Remick D. G., Larrick J. W., Kunkel S. L. Modulation of tumor necrosis factor-alpha gene expression. Desensitization of prostaglandin E2-induced suppression. J Immunol. 1989 Jun 15;142(12):4346–4350. [PubMed] [Google Scholar]
- Szefler S. J., Norton C. E., Ball B., Gross J. M., Aida Y., Pabst M. J. IFN-gamma and LPS overcome glucocorticoid inhibition of priming for superoxide release in human monocytes. Evidence that secretion of IL-1 and tumor necrosis factor-alpha is not essential for monocyte priming. J Immunol. 1989 Jun 1;142(11):3985–3992. [PubMed] [Google Scholar]
- Wiggins R. C., Glatfelter A., Brukman J. Procoagulant activity in glomeruli and urine of rabbits with nephrotoxic nephritis. Lab Invest. 1985 Aug;53(2):156–165. [PubMed] [Google Scholar]


