Abstract
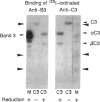
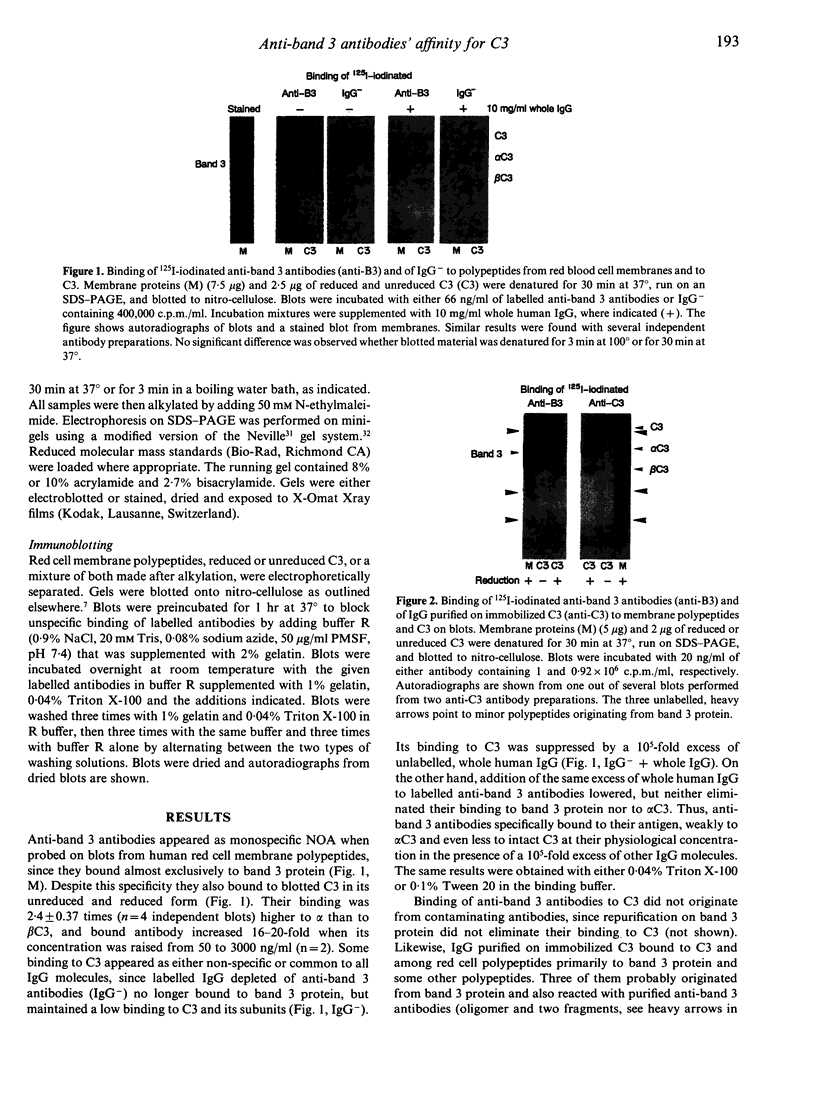
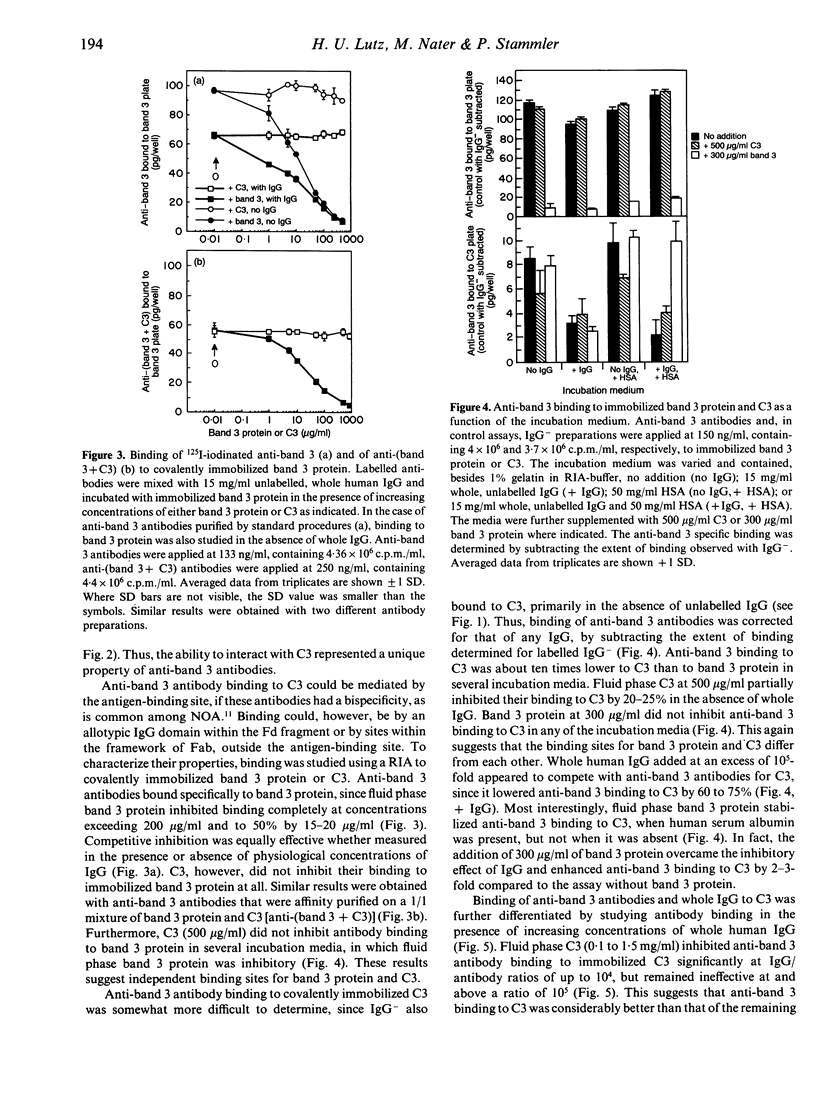
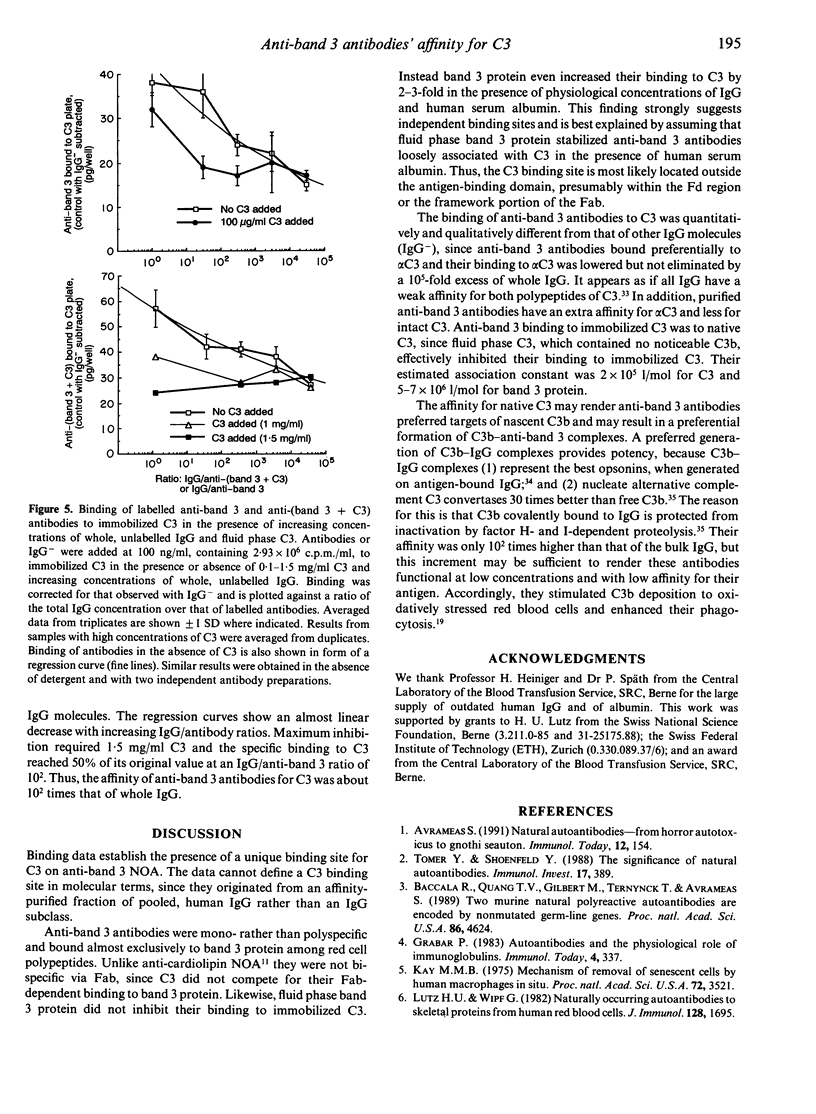
Naturally occurring anti-band 3 antibodies appear to mediate opsonization of oxidatively stressed and in vivo aged red cells. Their low concentration in plasma (< 100 ng/ml) and weak affinity (estimated association constant, 5-7 x 10(6) l/mol) contrasted with their biological efficiency. In compensating for their inadequate properties they have an affinity for C3 at a site independent of the antigen binding domain, with an estimated association constant of 2-3 x 10(5) l/mol. Though weak, their binding to C3 was about 100 times higher than that of whole IgG, which is known to have an affinity for C3. The affinity for C3 may render these antibodies preferred targets of the short-lived nascent C3b and result in a preferential C3b-anti-band 3 complex formation. C3b-IgG complexes represent the best opsonins and can nucleate alternative complement pathway C3 convertases by which opsonization is further enhanced.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avrameas S. Natural autoantibodies: from 'horror autotoxicus' to 'gnothi seauton'. Immunol Today. 1991 May;12(5):154–159. doi: 10.1016/S0167-5699(05)80045-3. [DOI] [PubMed] [Google Scholar]
- Baccala R., Quang T. V., Gilbert M., Ternynck T., Avrameas S. Two murine natural polyreactive autoantibodies are encoded by nonmutated germ-line genes. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4624–4628. doi: 10.1073/pnas.86.12.4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler E., West C., Blume K. G. The removal of leukocytes and platelets from whole blood. J Lab Clin Med. 1976 Aug;88(2):328–333. [PubMed] [Google Scholar]
- Bächi T., Dorval G., Wigzell H., Binz H. Staphylococcal protein A in immunoferritin techniques. Scand J Immunol. 1977;6(3):241–246. doi: 10.1111/j.1365-3083.1977.tb00390.x. [DOI] [PubMed] [Google Scholar]
- Ehlenberger A. G., Nussenzweig V. The role of membrane receptors for C3b and C3d in phagocytosis. J Exp Med. 1977 Feb 1;145(2):357–371. doi: 10.1084/jem.145.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasler S., Skvaril F., Lutz H. U. Electrophoretic properties of human IgG and its subclasses on sodium dodecyl-sulfate-polyacrylamide gel electrophoresis and immunoblots. Anal Biochem. 1988 Nov 1;174(2):593–600. doi: 10.1016/0003-2697(88)90061-9. [DOI] [PubMed] [Google Scholar]
- Fries L. F., Gaither T. A., Hammer C. H., Frank M. M. C3b covalently bound to IgG demonstrates a reduced rate of inactivation by factors H and I. J Exp Med. 1984 Dec 1;160(6):1640–1655. doi: 10.1084/jem.160.6.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries L. F., Siwik S. A., Malbran A., Frank M. M. Phagocytosis of target particles bearing C3b-IgG covalent complexes by human monocytes and polymorphonuclear leucocytes. Immunology. 1987 Sep;62(1):45–51. [PMC free article] [PubMed] [Google Scholar]
- Gee A. P., Langone J. J. Immunoassay using 125I- or enzyme-labeled protein A and antigen-coated tubes. Anal Biochem. 1981 Sep 15;116(2):524–530. doi: 10.1016/0003-2697(81)90397-3. [DOI] [PubMed] [Google Scholar]
- Hammer C. H., Wirtz G. H., Renfer L., Gresham H. D., Tack B. F. Large scale isolation of functionally active components of the human complement system. J Biol Chem. 1981 Apr 25;256(8):3995–4006. [PubMed] [Google Scholar]
- Hebbel R. P., Miller W. J. Phagocytosis of sickle erythrocytes: immunologic and oxidative determinants of hemolytic anemia. Blood. 1984 Sep;64(3):733–741. [PubMed] [Google Scholar]
- Heegaard N. H. Immunochemical characterization of interactions between circulating autologous immunoglobulin G and normal human erythrocyte membrane proteins. Biochim Biophys Acta. 1990 Apr 13;1023(2):239–246. doi: 10.1016/0005-2736(90)90419-o. [DOI] [PubMed] [Google Scholar]
- Kay M. M. Mechanism of removal of senescent cells by human macrophages in situ. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3521–3525. doi: 10.1073/pnas.72.9.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay M. M. Role of physiologic autoantibody in the removal of senescent human red cells. J Supramol Struct. 1978;9(4):555–567. doi: 10.1002/jss.400090409. [DOI] [PubMed] [Google Scholar]
- Kulics J., Rajnavölgyi E., Füst G., Gergely J. Interaction of C3 and C3b with immunoglobulin G. Mol Immunol. 1983 Aug;20(8):805–810. doi: 10.1016/0161-5890(83)90076-7. [DOI] [PubMed] [Google Scholar]
- Lukacovic M. F., Feinstein M. B., Sha'afi R. I., Perrie S. Purification of stabilized band 3 protein of the human erythrocyte membrane and its reconstitution into liposomes. Biochemistry. 1981 May 26;20(11):3145–3151. doi: 10.1021/bi00514a025. [DOI] [PubMed] [Google Scholar]
- Lutz H. U., Bussolino F., Flepp R., Fasler S., Stammler P., Kazatchkine M. D., Arese P. Naturally occurring anti-band-3 antibodies and complement together mediate phagocytosis of oxidatively stressed human erythrocytes. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7368–7372. doi: 10.1073/pnas.84.21.7368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz H. U., Fasler S., Stammler P., Bussolino F., Arese P. Naturally occurring anti-band 3 antibodies and complement in phagocytosis of oxidatively-stressed and in clearance of senescent red cells. Blood Cells. 1988;14(1):175–203. [PubMed] [Google Scholar]
- Lutz H. U., Flepp R., Stringaro-Wipf G. Naturally occurring autoantibodies to exoplasmic and cryptic regions of band 3 protein, the major integral membrane protein of human red blood cells. J Immunol. 1984 Nov;133(5):2610–2618. [PubMed] [Google Scholar]
- Lutz H. U., Stammler P., Fasler S., Ingold M., Fehr J. Density separation of human red blood cells on self forming Percoll gradients: correlation with cell age. Biochim Biophys Acta. 1992 Mar 5;1116(1):1–10. doi: 10.1016/0304-4165(92)90120-j. [DOI] [PubMed] [Google Scholar]
- Lutz H. U., Stammler P., Fischer E. A. Covalent binding of detergent-solubilized membrane glycoproteins to 'Chemobond' plates for ELISA. J Immunol Methods. 1990 May 25;129(2):211–220. doi: 10.1016/0022-1759(90)90441-w. [DOI] [PubMed] [Google Scholar]
- Lutz H. U., Wipf G. Naturally occurring autoantibodies to skeletal proteins from human red blood cells. J Immunol. 1982 Apr;128(4):1695–1699. [PubMed] [Google Scholar]
- McNeil H. P., Hunt J. E., Krilis S. A. New aspects of anticardiolipin antibodies. Clin Exp Rheumatol. 1990 Nov-Dec;8(6):525–527. [PubMed] [Google Scholar]
- Miller R. D., Calkins C. E. Development of self-tolerance in normal mice. Appearance of suppressor cells that maintain adult self-tolerance follows the neonatal autoantibody response. J Immunol. 1988 Oct 1;141(7):2206–2210. [PubMed] [Google Scholar]
- Morgan A. C., Rossen R. D., McCormick K. J., Stehlin J. S., Jr, Giovanella B. C. "Hidden" cytotoxic antibodies that react with allogeneic cultured fetal and tumor cells contained in soluble immune complexes from normal human sera. Cancer Res. 1982 Mar;42(3):881–887. [PubMed] [Google Scholar]
- Müller H., Lutz H. U. Binding of autologous IgG to human red blood cells before and after ATP-depletion. Selective exposure of binding sites (autoantigens) on spectrin-free vesicles. Biochim Biophys Acta. 1983 Apr 6;729(2):249–257. doi: 10.1016/0005-2736(83)90491-1. [DOI] [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- Newman S. L., Johnston R. B., Jr Role of binding through C3b and IgG in polymorphonuclear neutrophil function: studies with trypsin-generated C3b. J Immunol. 1979 Oct;123(4):1839–1846. [PubMed] [Google Scholar]
- Sorette M. P., Galili U., Clark M. R. Comparison of serum anti-band 3 and anti-Gal antibody binding to density-separated human red blood cells. Blood. 1991 Feb 1;77(3):628–636. [PubMed] [Google Scholar]
- Tack B. D., Prahl J. W. Third component of human complement: purification from plasma and physicochemical characterization. Biochemistry. 1976 Oct 5;15(20):4513–4521. doi: 10.1021/bi00665a028. [DOI] [PubMed] [Google Scholar]
- Tomer Y., Shoenfeld Y. The significance of natural autoantibodies. Immunol Invest. 1988 Jul;17(5):389–424. doi: 10.3109/08820138809049846. [DOI] [PubMed] [Google Scholar]