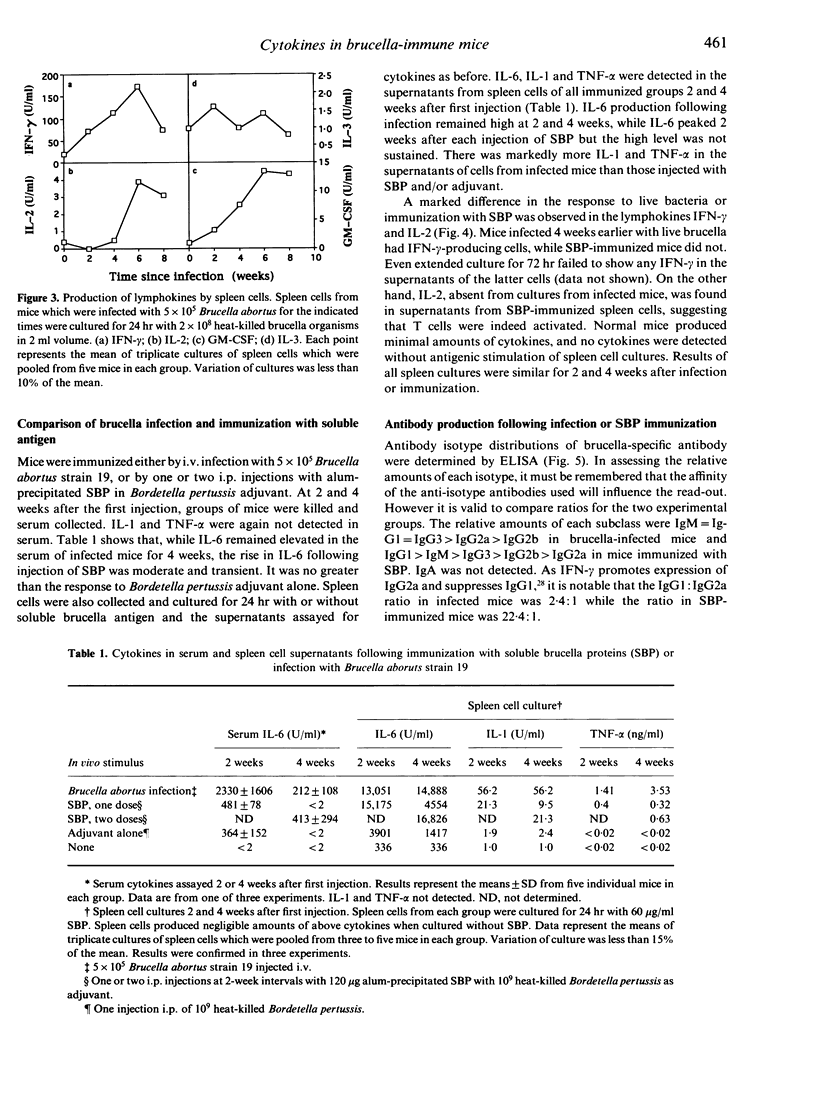
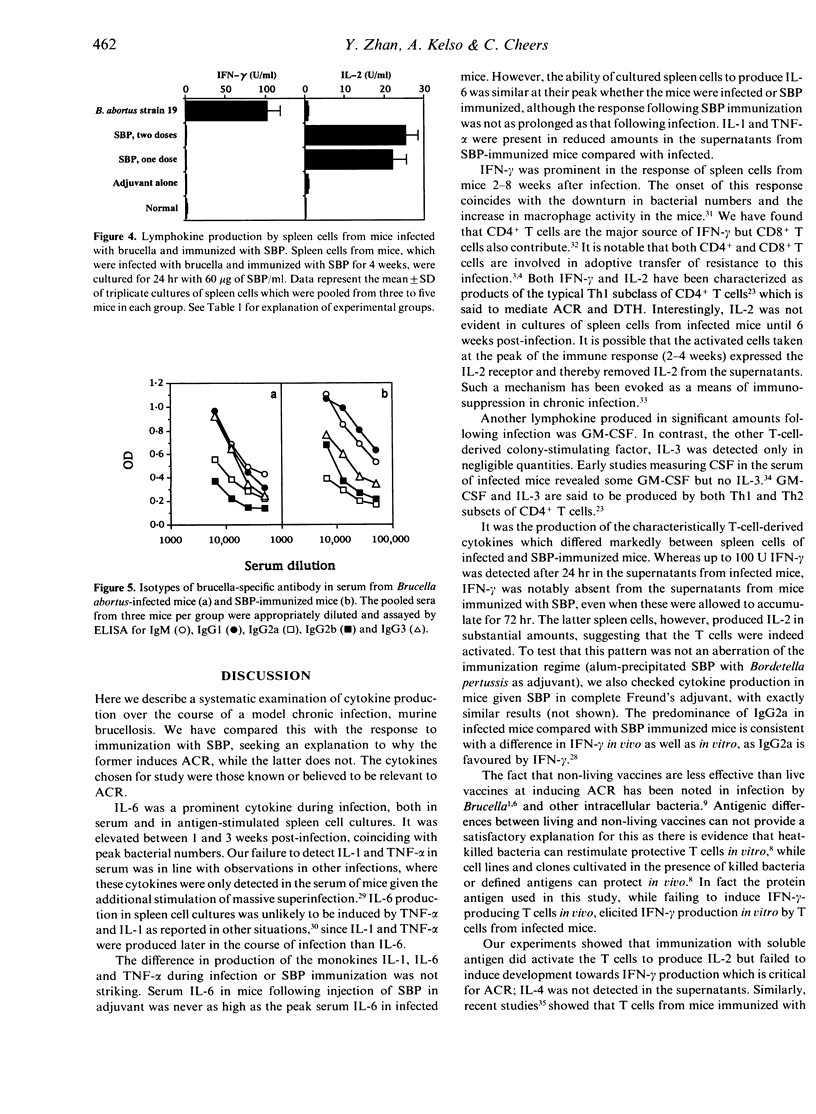
Abstract
In order to induce acquired cellular resistance (ACR) to facultative intracellular bacterial pathogens, infection with live organisms is required. It is possible that different cytokine responses to live bacteria or their extracted antigens could account for their different abilities to induce ACR. Therefore, mice were infected with live attenuated Brucella abortus vaccine strain 19, and their ability to produce cytokines, both in vivo and in vitro, was investigated over 12 weeks of infection. This was compared with the response to injection of soluble brucella proteins (SBP). During infection, serum levels of interleukin-6 (IL-6) were markedly increased over a period of 4 weeks during the peak of infection. SBP plus adjuvant induced a transient increase in serum IL-6. IL-1 and tumour necrosis factor-alpha (TNF-alpha) remained undetectable in both instances. Spleen cells taken at intervals after infection and cultured with brucella antigens produced high titres of IL-6, IL-1 and TNF-alpha. Immunization with SBP was less efficient than live infection at inducing these cytokines. Of the characteristically T-cell-derived lymphokines, interferon-gamma (IFN-gamma) production rose 2 weeks after infection, peaking at 6 weeks, while IL-2 was not detected until 6 weeks post-infection. Granulocyte-macrophage colony-stimulating factor (GM-CSF) was produced in substantial amounts, but IL-3 production was minimal. In contrast, spleen cells from mice immunized with SBP produced IL-2 but failed to produce IFN-gamma. The implications of these results for the induction of ACR are discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araya L. N., Elzer P. H., Rowe G. E., Enright F. M., Winter A. J. Temporal development of protective cell-mediated and humoral immunity in BALB/c mice infected with Brucella abortus. J Immunol. 1989 Nov 15;143(10):3330–3337. [PubMed] [Google Scholar]
- Buchmeier N. A., Schreiber R. D. Requirement of endogenous interferon-gamma production for resolution of Listeria monocytogenes infection. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7404–7408. doi: 10.1073/pnas.82.21.7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheers C., Haigh A. M., Kelso A., Metcalf D., Stanley E. R., Young A. M. Production of colony-stimulating factors (CSFs) during infection: separate determinations of macrophage-, granulocyte-, granulocyte-macrophage-, and multi-CSFs. Infect Immun. 1988 Jan;56(1):247–251. doi: 10.1128/iai.56.1.247-251.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheers C., Ho M. Resistance and susceptibility of mice to bacterial infection. IV. Functional specificity in natural resistance to facultative intracellular bacteria. J Reticuloendothel Soc. 1983 Oct;34(4):299–309. [PubMed] [Google Scholar]
- Cheers C., Pagram F. Macrophage activation during experimental murine brucellosis: a basis for chronic infection. Infect Immun. 1979 Feb;23(2):197–205. doi: 10.1128/iai.23.2.197-205.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colizzi V. In vivo and in vitro administration of interleukin 2-containing preparation reverses T-cell unresponsiveness in Mycobacterium bovis BCG-infected mice. Infect Immun. 1984 Jul;45(1):25–28. doi: 10.1128/iai.45.1.25-28.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Confer A. W., Tabatabai L. B., Deyoe B. L., Oltjen S. L., Hall S. M., Oltjen J. W., Morton R. J., Fulnechek D. L., Smith R. E., Smith R. A. Vaccination of cattle with chemically modified and unmodified salt-extractable proteins from Brucella abortus. Vet Microbiol. 1987 Dec;15(4):325–339. doi: 10.1016/0378-1135(87)90020-4. [DOI] [PubMed] [Google Scholar]
- Espevik T., Nissen-Meyer J. A highly sensitive cell line, WEHI 164 clone 13, for measuring cytotoxic factor/tumor necrosis factor from human monocytes. J Immunol Methods. 1986 Dec 4;95(1):99–105. doi: 10.1016/0022-1759(86)90322-4. [DOI] [PubMed] [Google Scholar]
- Gajewski T. F., Schell S. R., Nau G., Fitch F. W. Regulation of T-cell activation: differences among T-cell subsets. Immunol Rev. 1989 Oct;111:79–110. doi: 10.1111/j.1600-065x.1989.tb00543.x. [DOI] [PubMed] [Google Scholar]
- Gearing A. J., Bird C. R., Bristow A., Poole S., Thorpe R. A simple sensitive bioassay for interleukin-1 which is unresponsive to 10(3) U/ml of interleukin-2. J Immunol Methods. 1987 May 4;99(1):7–11. doi: 10.1016/0022-1759(87)90025-1. [DOI] [PubMed] [Google Scholar]
- Havell E. A. Evidence that tumor necrosis factor has an important role in antibacterial resistance. J Immunol. 1989 Nov 1;143(9):2894–2899. [PubMed] [Google Scholar]
- Havell E. A., Moldawer L. L., Helfgott D., Kilian P. L., Sehgal P. B. Type I IL-1 receptor blockade exacerbates murine listeriosis. J Immunol. 1992 Mar 1;148(5):1486–1492. [PubMed] [Google Scholar]
- Havell E. A. Production of tumor necrosis factor during murine listeriosis. J Immunol. 1987 Dec 15;139(12):4225–4231. [PubMed] [Google Scholar]
- Heinzel F. P., Sadick M. D., Holaday B. J., Coffman R. L., Locksley R. M. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med. 1989 Jan 1;169(1):59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi K., Mitsuyama M., Muramori K., Tsukada H., Nomoto K. Interleukin-1-induced promotion of T-cell differentiation in mice immunized with killed Listeria monocytogenes. Infect Immun. 1990 Dec;58(12):3973–3979. doi: 10.1128/iai.58.12.3973-3979.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Baldwin C. L. Effects of cytokines on intracellular growth of Brucella abortus. Infect Immun. 1993 Jan;61(1):124–134. doi: 10.1128/iai.61.1.124-134.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura I., Tsukada H., Yoshikawa H., Fujita M., Nomoto K., Mitsuyama M. IFN-gamma-producing ability as a possible marker for the protective T cells against Mycobacterium bovis BCG in mice. J Immunol. 1992 May 1;148(9):2887–2893. [PubMed] [Google Scholar]
- Kelso A. Frequency analysis of lymphokine-secreting CD4+ and CD8+ T cells activated in a graft-versus-host reaction. J Immunol. 1990 Oct 1;145(7):2167–2176. [PubMed] [Google Scholar]
- Kiderlen A. F., Kaufmann S. H., Lohmann-Matthes M. L. Protection of mice against the intracellular bacterium Listeria monocytogenes by recombinant immune interferon. Eur J Immunol. 1984 Oct;14(10):964–967. doi: 10.1002/eji.1830141019. [DOI] [PubMed] [Google Scholar]
- Liu Z., Simpson R. J., Cheers C. Recombinant interleukin-6 protects mice against experimental bacterial infection. Infect Immun. 1992 Oct;60(10):4402–4406. doi: 10.1128/iai.60.10.4402-4406.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee D. M., Wing E. J. Cloned L3T4+ T lymphocytes protect mice against Listeria monocytogenes by secreting IFN-gamma. J Immunol. 1988 Nov 1;141(9):3203–3207. [PubMed] [Google Scholar]
- Mitsuyama M., Igarashi K., Kawamura I., Ohmori T., Nomoto K. Difference in the induction of macrophage interleukin-1 production between viable and killed cells of Listeria monocytogenes. Infect Immun. 1990 May;58(5):1254–1260. doi: 10.1128/iai.58.5.1254-1260.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaraz J. A., Winter A. J., Hunter D. M., Sowa B. A., Wu A. M., Adams L. G. Protection against Brucella abortus in mice with O-polysaccharide-specific monoclonal antibodies. Infect Immun. 1986 Mar;51(3):961–963. doi: 10.1128/iai.51.3.961-963.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Orme I. M. Induction of nonspecific acquired resistance and delayed-type hypersensitivity, but not specific acquired resistance in mice inoculated with killed mycobacterial vaccines. Infect Immun. 1988 Dec;56(12):3310–3312. doi: 10.1128/iai.56.12.3310-3312.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov H., Hogarth M., McKenzie I. F., Cheers C. In vivo and in vitro effects of monoclonal antibody to Ly antigens on immunity to infection. Cell Immunol. 1982 Jul 15;71(1):127–138. doi: 10.1016/0008-8749(82)90502-0. [DOI] [PubMed] [Google Scholar]
- Poston R. M., Kurlander R. J. Cytokine expression in vivo during murine listeriosis. Infection with live, virulent bacteria is required for monokine and lymphokine messenger RNA accumulation in the spleen. J Immunol. 1992 Nov 1;149(9):3040–3044. [PubMed] [Google Scholar]
- Shalaby M. R., Waage A., Aarden L., Espevik T. Endotoxin, tumor necrosis factor-alpha and interleukin 1 induce interleukin 6 production in vivo. Clin Immunol Immunopathol. 1989 Dec;53(3):488–498. doi: 10.1016/0090-1229(89)90010-x. [DOI] [PubMed] [Google Scholar]
- Snapper C. M., Paul W. E. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987 May 22;236(4804):944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- Stabel T. J., Mayfield J. E., Tabatabai L. B., Wannemuehler M. J. Oral immunization of mice with attenuated Salmonella typhimurium containing a recombinant plasmid which codes for production of a 31-kilodalton protein of Brucella abortus. Infect Immun. 1990 Jul;58(7):2048–2055. doi: 10.1128/iai.58.7.2048-2055.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens M. G., Pugh G. W., Jr, Tabatabai L. B. Effects of gamma interferon and indomethacin in preventing Brucella abortus infections in mice. Infect Immun. 1992 Oct;60(10):4407–4409. doi: 10.1128/iai.60.10.4407-4409.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabatabai L. B., Deyoe B. L., Patterson J. M. Immunogenicity of Brucella abortus salt-extractable proteins. Vet Microbiol. 1989 May;20(1):49–58. doi: 10.1016/0378-1135(89)90006-0. [DOI] [PubMed] [Google Scholar]
- Tran H. T., Metcalf D., Cheers C. Anti-bacterial activity of peritoneal cells from transgenic mice producing high levels of GM-CSF. Immunology. 1990 Nov;71(3):377–382. [PMC free article] [PubMed] [Google Scholar]
- Tsukada H., Kawamura I., Arakawa M., Nomoto K., Mitsuyama M. Dissociated development of T cells mediating delayed-type hypersensitivity and protective T cells against Listeria monocytogenes and their functional difference in lymphokine production. Infect Immun. 1991 Oct;59(10):3589–3595. doi: 10.1128/iai.59.10.3589-3595.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Snick J. Interleukin-6: an overview. Annu Rev Immunol. 1990;8:253–278. doi: 10.1146/annurev.iy.08.040190.001345. [DOI] [PubMed] [Google Scholar]
- Zhan Y. F., Stanley E. R., Cheers C. Prophylaxis or treatment of experimental brucellosis with interleukin-1. Infect Immun. 1991 May;59(5):1790–1794. doi: 10.1128/iai.59.5.1790-1794.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan Y., Yang J., Cheers C. Cytokine response of T-cell subsets from Brucella abortus-infected mice to soluble Brucella proteins. Infect Immun. 1993 Jul;61(7):2841–2847. doi: 10.1128/iai.61.7.2841-2847.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]


