Abstract
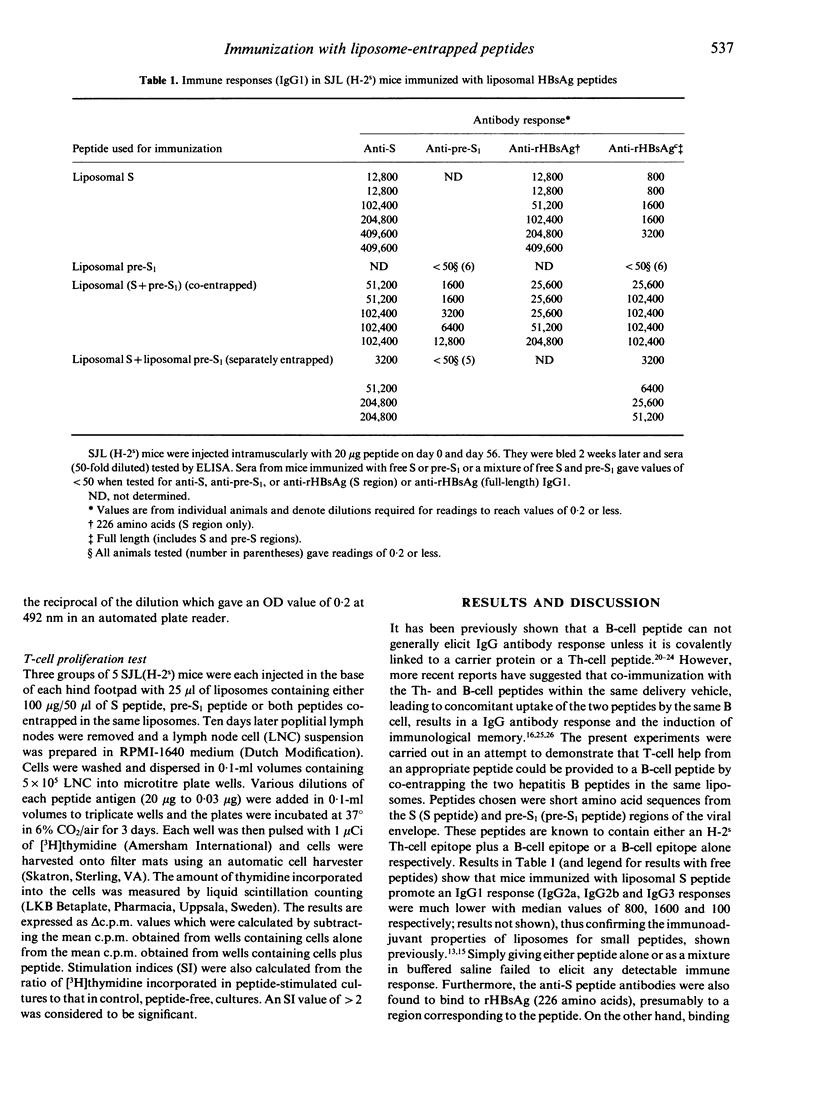
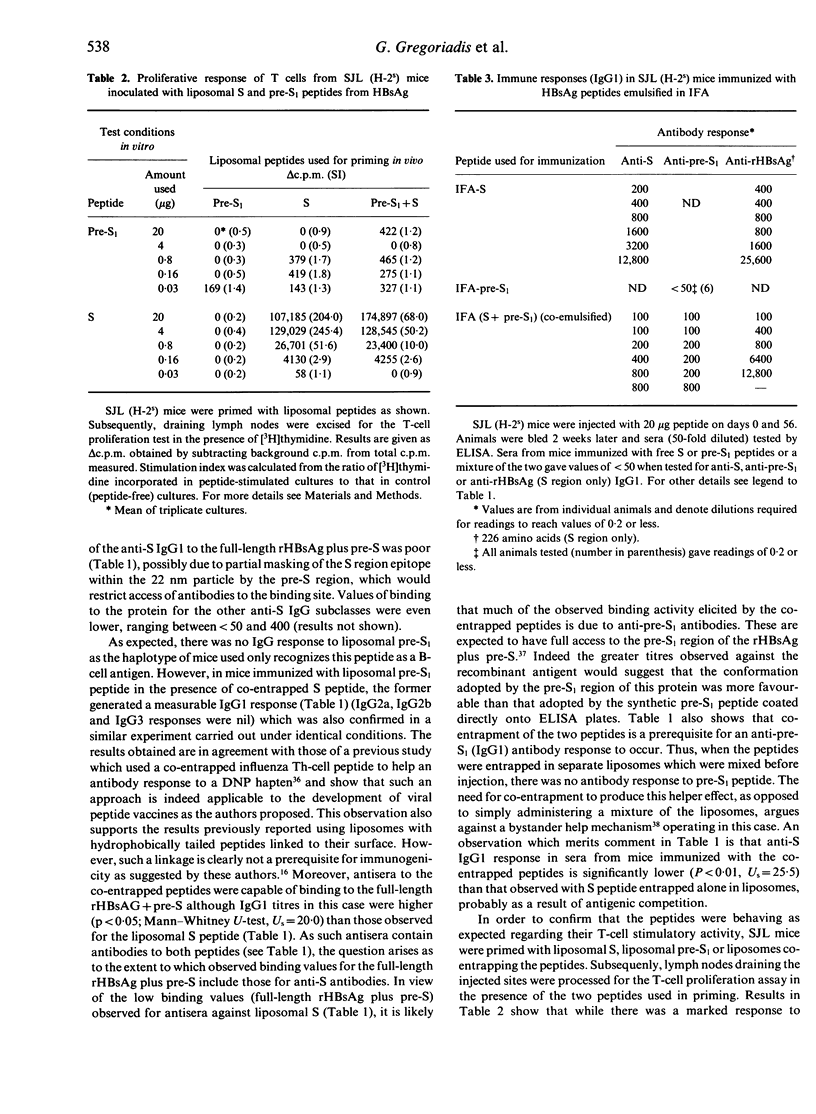
We have investigated the possibility of a T-cell epitope peptide providing help for a B-cell epitope peptide when both peptides are co-entrapped in the same liposomes. Epitope models used were a 28 amino acid peptide from the S region of the hepatitis B surface antigen (HBsAg) (subtype adw) containing an H-2s Th-cell epitope, and a 33 amino acid peptide from the pre-S1 region of the HBsAg (subtype adw) designed to exclude an adjacent H-2s T-cell epitope, the latter (pre-S1) peptide being recognized by SJL (H-2s) mice as a B-cell epitope. SJL(H-2s) mice were immunized twice intramuscularly with S or pre-S1 peptide alone, co-entrapped in the same liposomes or entrapped in separate liposomes which were mixed before injection. Analysis of sera for anti-peptide IgG1 antibodies revealed that the Th-cell peptide provided help for the pre-S1 peptide only when the two peptides were co-entrapped in the same vesicles. This helper effect was found to correlate with the ability of S peptide (co-entrapped with the pre-S1) to stimulate T-cell proliferation in vitro. There was no IgG1 response against pre-S1 peptide in mice immunized with a mixture of the free peptides or a mixture of separately entrapped peptides. A helper effect, albeit much weaker, was also observed in mice immunized with the two peptides emulsified in incomplete Freund's adjuvant. Antisera from mice immunized with both peptides co-entrapped in liposomes were found to bind to full length (pre-S1 containing) recombinant HBsAg. Moreover, binding values were much higher than those seen with antisera from animals immunized with the liposomal S peptide above, presumably because of full access of anti-pre-S1 antibodies to the pre-S1 region of the rHBsAg. It is concluded that liposomes could serve not only as an immunological adjuvant for peptides but also as a carrier for Th- and B-cell epitopes thus eliminating the need for covalent linkage to a carrier protein.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alving C. R. Liposomes as carriers of antigens and adjuvants. J Immunol Methods. 1991 Jun 24;140(1):1–13. doi: 10.1016/0022-1759(91)90120-5. [DOI] [PubMed] [Google Scholar]
- Bittle J. L., Houghten R. A., Alexander H., Shinnick T. M., Sutcliffe J. G., Lerner R. A., Rowlands D. J., Brown F. Protection against foot-and-mouth disease by immunization with a chemically synthesized peptide predicted from the viral nucleotide sequence. Nature. 1982 Jul 1;298(5869):30–33. doi: 10.1038/298030a0. [DOI] [PubMed] [Google Scholar]
- Borras-Cuesta F., Petit-Camurdan A., Fedon Y. Engineering of immunogenic peptides by co-linear synthesis of determinants recognized by B and T cells. Eur J Immunol. 1987 Aug;17(8):1213–1215. doi: 10.1002/eji.1830170820. [DOI] [PubMed] [Google Scholar]
- Davis D., Gregoriadis G. Liposomes as adjuvants with immunopurified tetanus toxoid: influence of liposomal characteristics. Immunology. 1987 Jun;61(2):229–234. [PMC free article] [PubMed] [Google Scholar]
- Davis D., Gregoriadis G. Primary immune response to liposomal tetanus toxoid in mice: the effect of mediators. Immunology. 1989 Oct;68(2):277–282. [PMC free article] [PubMed] [Google Scholar]
- Francis M. J., Fry C. M., Rowlands D. J., Brown F., Bittle J. L., Houghten R. A., Lerner R. A. Immunological priming with synthetic peptides of foot-and-mouth disease virus. J Gen Virol. 1985 Nov;66(Pt 11):2347–2354. doi: 10.1099/0022-1317-66-11-2347. [DOI] [PubMed] [Google Scholar]
- Francis M. J., Hastings G. Z., Syred A. D., McGinn B., Brown F., Rowlands D. J. Non-responsiveness to a foot-and-mouth disease virus peptide overcome by addition of foreign helper T-cell determinants. Nature. 1987 Nov 12;330(6144):168–170. doi: 10.1038/330168a0. [DOI] [PubMed] [Google Scholar]
- Frisch B., Muller S., Briand J. P., Van Regenmortel M. H., Schuber F. Parameters affecting the immunogenicity of a liposome-associated synthetic hexapeptide antigen. Eur J Immunol. 1991 Jan;21(1):185–193. doi: 10.1002/eji.1830210128. [DOI] [PubMed] [Google Scholar]
- Garcon N. M., Six H. R. Universal vaccine carrier. Liposomes that provide T-dependent help to weak antigens. J Immunol. 1991 Jun 1;146(11):3697–3702. [PubMed] [Google Scholar]
- Garcon N., Gregoriadis G., Taylor M., Summerfield J. Mannose-mediated targeted immunoadjuvant action of liposomes. Immunology. 1988 Aug;64(4):743–745. [PMC free article] [PubMed] [Google Scholar]
- Good M. F., Maloy W. L., Lunde M. N., Margalit H., Cornette J. L., Smith G. L., Moss B., Miller L. H., Berzofsky J. A. Construction of synthetic immunogen: use of new T-helper epitope on malaria circumsporozoite protein. Science. 1987 Feb 27;235(4792):1059–1062. doi: 10.1126/science.2434994. [DOI] [PubMed] [Google Scholar]
- Goodman-Snitkoff G., Eisele L. E., Heimer E. P., Felix A. M., Andersen T. T., Fuerst T. R., Mannino R. J. Defining minimal requirements for antibody production to peptide antigens. Vaccine. 1990 Jun;8(3):257–262. doi: 10.1016/0264-410x(90)90055-q. [DOI] [PubMed] [Google Scholar]
- Gregoriadis G., Florence A. T. Liposomes in drug delivery. Clinical, diagnostic and ophthalmic potential. Drugs. 1993 Jan;45(1):15–28. doi: 10.2165/00003495-199345010-00003. [DOI] [PubMed] [Google Scholar]
- Gregoriadis G., Garcon N., da Silva H., Sternberg B. Coupling of ligands to liposomes independently of solute entrapment: observations on the formed vesicles. Biochim Biophys Acta. 1993 Apr 22;1147(2):185–193. doi: 10.1016/0005-2736(93)90003-i. [DOI] [PubMed] [Google Scholar]
- Gregoriadis G. Immunological adjuvants: a role for liposomes. Immunol Today. 1990 Mar;11(3):89–97. doi: 10.1016/0167-5699(90)90034-7. [DOI] [PubMed] [Google Scholar]
- Jensen P. E., Kapp J. A. Bystander help in primary immune responses in vivo. J Exp Med. 1986 Sep 1;164(3):841–854. doi: 10.1084/jem.164.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby C., Clarke J., Gregoriadis G. Effect of the cholesterol content of small unilamellar liposomes on their stability in vivo and in vitro. Biochem J. 1980 Feb 15;186(2):591–598. doi: 10.1042/bj1860591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latif N. A., Bachhawat B. K. The effect of surface-coupled antigen of liposomes in immunopotentiation. Immunol Lett. 1987 May;15(1):45–51. doi: 10.1016/0165-2478(87)90075-7. [DOI] [PubMed] [Google Scholar]
- Leclerc C., Przewlocki G., Schutze M. P., Chedid L. A synthetic vaccine constructed by copolymerization of B and T cell determinants. Eur J Immunol. 1987 Feb;17(2):269–273. doi: 10.1002/eji.1830170218. [DOI] [PubMed] [Google Scholar]
- Mbawuike I. N., Wyde P. R., Anderson P. M. Enhancement of the protective efficacy of inactivated influenza A virus vaccine in aged mice by IL-2 liposomes. Vaccine. 1990 Aug;8(4):347–352. doi: 10.1016/0264-410x(90)90093-2. [DOI] [PubMed] [Google Scholar]
- Milich D. R. Genetic and molecular basis for T- and B-cell recognition of hepatitis B viral antigens. Immunol Rev. 1987 Oct;99:71–103. doi: 10.1111/j.1600-065x.1987.tb01173.x. [DOI] [PubMed] [Google Scholar]
- Milich D. R., McLachlan A., Moriarty A., Thornton G. B. A single 10-residue pre-S(1) peptide can prime T cell help for antibody production to multiple epitopes within the pre-S(1), pre-S(2), and S regions of HBsAg. J Immunol. 1987 Jun 15;138(12):4457–4465. [PubMed] [Google Scholar]
- Neurath A. R., Jameson B. A., Huima T. Hepatitis B virus proteins eliciting protective immunity. Microbiol Sci. 1987 Feb;4(2):45–51. [PubMed] [Google Scholar]
- Neurath A. R., Kent S. B., Strick N. Location and chemical synthesis of a pre-S gene coded immunodominant epitope of hepatitis B virus. Science. 1984 Apr 27;224(4647):392–395. doi: 10.1126/science.6200931. [DOI] [PubMed] [Google Scholar]
- Neurath A. R., Seto B., Strick N. Antibodies to synthetic peptides from the preS1 region of the hepatitis B virus (HBV) envelope (env) protein are virus-neutralizing and protective. Vaccine. 1989 Jun;7(3):234–236. doi: 10.1016/0264-410x(89)90235-1. [DOI] [PubMed] [Google Scholar]
- Partidos C. D., Obeid O. E., Steward M. W. Antibody responses to non-immunogenic synthetic peptides induced by co-immunization with immunogenic peptides. Immunology. 1992 Oct;77(2):262–266. [PMC free article] [PubMed] [Google Scholar]
- Richards R. L., Hayre M. D., Hockmeyer W. T., Alving C. R. Liposomes, lipid A, and aluminum hydroxide enhance the immune response to a synthetic malaria sporozoite antigen. Infect Immun. 1988 Mar;56(3):682–686. doi: 10.1128/iai.56.3.682-686.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarobe P., Lasarte J. J., Golvano J., Gullón A., Civeira M. P., Prieto J., Borrás-Cuesta F. Induction of antibodies against a peptide hapten does not require covalent linkage between the hapten and a class II presentable T helper peptide. Eur J Immunol. 1991 Jun;21(6):1555–1558. doi: 10.1002/eji.1830210633. [DOI] [PubMed] [Google Scholar]


