Abstract
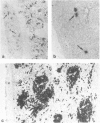
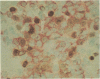
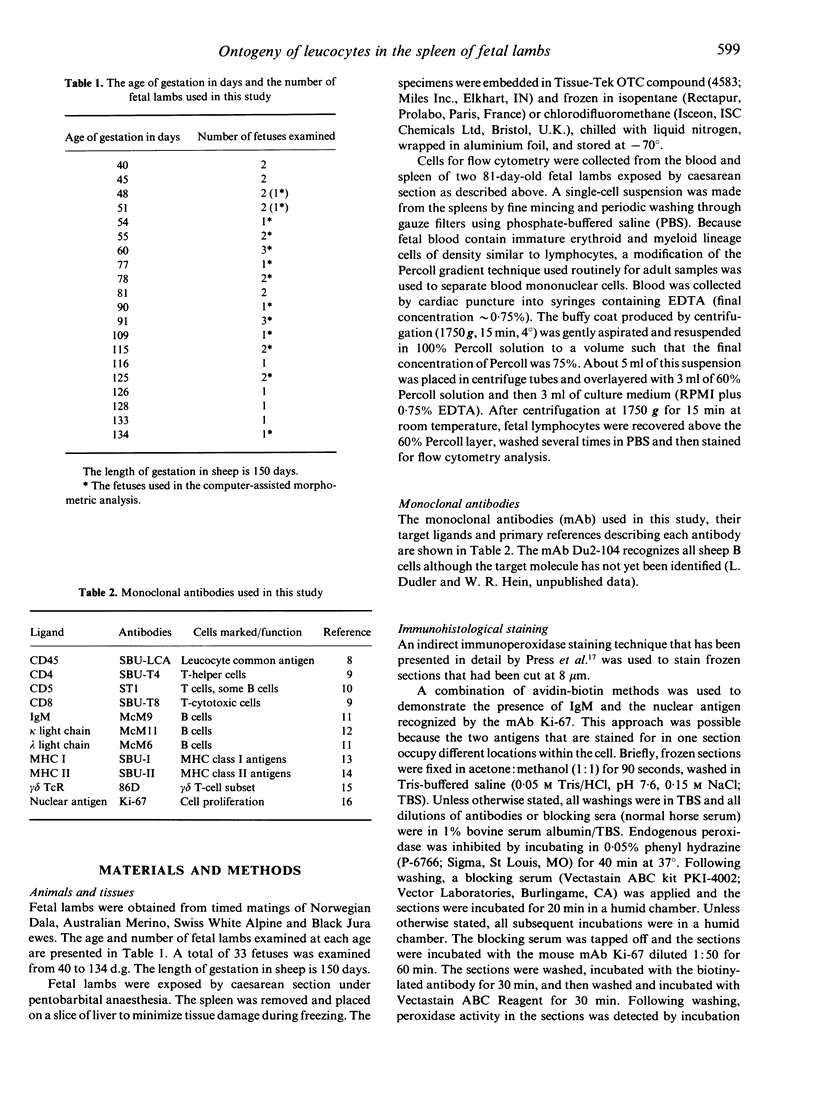
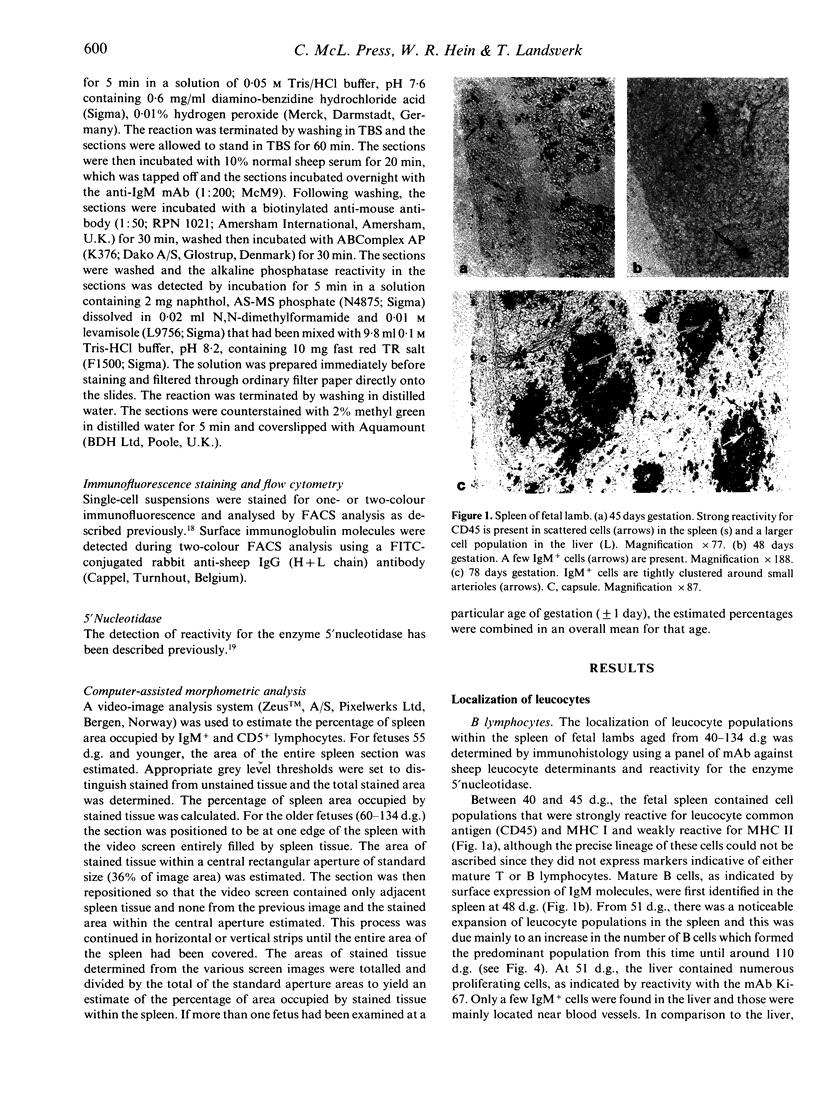
The presence and distribution of B cells and other early leucocyte populations are described in the spleen of fetal lambs from 40 to 134 days of gestation (length of gestation 150 days). Computer-assisted morphometric analysis and flow cytometry were used to quantify the early predominance of B cells in mid-gestation. B cells appeared at about 48 days and increased in number to occupy over 20% of the spleen area at 77 days. All spleens were collected on their respective livers and at no stage did the livers contain more than a few IgM-positive (+) cells, which were usually close to blood vessels. Two-colour flow cytometry demonstrated that only 1-2% of IgM+ cells expressed CD5 at 81 days. Beyond 77 days, with the expanding presence of T cells, the percentage of area occupied by IgM+ cells declined to stabilize at about 7% during late gestation. The conventional organization of the splenic white pulp was observed from 90 days along with 5' nucleotidase-positive primary follicles. Double staining technique using immunohistochemical methods demonstrated that IgM+ cells were proliferating in the spleen from as early as 51 days and that clusters of proliferating IgM+ cells were prominent between 60 and 77 days. The results of the present study suggest that during the ontogeny of fetal lambs the spleen is a site of B-cell development or expansion before colonization of the ileal Peyer's patch and the subsequent generation of the preimmune antibody repertoire.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aleksandersen M., Nicander L., Landsverk T. Ontogeny, distribution and structure of aggregated lymphoid follicles in the large intestine of sheep. Dev Comp Immunol. 1991 Fall;15(4):413–422. doi: 10.1016/0145-305x(91)90033-u. [DOI] [PubMed] [Google Scholar]
- Alt F. W., Blackwell T. K., Yancopoulos G. D. Development of the primary antibody repertoire. Science. 1987 Nov 20;238(4830):1079–1087. doi: 10.1126/science.3317825. [DOI] [PubMed] [Google Scholar]
- Beh K. J. Monoclonal antibodies against sheep immunoglobulin light chain, IgM and IgA. Vet Immunol Immunopathol. 1988 Feb;18(1):19–27. doi: 10.1016/0165-2427(88)90033-5. [DOI] [PubMed] [Google Scholar]
- Beya M. F., Miyasaka M., Dudler L., Ezaki T., Trnka Z. Studies on the differentiation of T lymphocytes in sheep. II. Two monoclonal antibodies that recognize all ovine T lymphocytes. Immunology. 1986 Jan;57(1):115–121. [PMC free article] [PubMed] [Google Scholar]
- Bhat N. M., Kantor A. B., Bieber M. M., Stall A. M., Herzenberg L. A., Teng N. N. The ontogeny and functional characteristics of human B-1 (CD5+ B) cells. Int Immunol. 1992 Feb;4(2):243–252. doi: 10.1093/intimm/4.2.243. [DOI] [PubMed] [Google Scholar]
- Fahey K. J., Morris B. Humoral immune responses in foetal sheep. Immunology. 1978 Oct;35(4):651–661. [PMC free article] [PubMed] [Google Scholar]
- Foley R. C., Raison R. L., Beh K. J. Monoclonal antibody against sheep kappa light chain. Hybridoma. 1991 Aug;10(4):507–515. doi: 10.1089/hyb.1991.10.507. [DOI] [PubMed] [Google Scholar]
- Foy T. M., Lynch R. G., Waldschmidt T. J. Ontogeny and distribution of the murine B cell Fc gamma RII. J Immunol. 1992 Sep 1;149(5):1516–1523. [PubMed] [Google Scholar]
- Friedberg S. H., Weissman I. L. Lymphoid tissue architecture. II. Ontogeny of peripheral T and B cells in mice: evidence against Peyer's patches as the site of generation of B cells. J Immunol. 1974 Nov;113(5):1477–1492. [PubMed] [Google Scholar]
- Gerber H. A., Morris B., Trevella W. The role of gut-associated lymphoid tissues in the generation of immunoglobulin-bearing lymphocytes in sheep. Aust J Exp Biol Med Sci. 1986 Jun;64(Pt 3):201–213. doi: 10.1038/icb.1986.22. [DOI] [PubMed] [Google Scholar]
- Gerdes J., Lemke H., Baisch H., Wacker H. H., Schwab U., Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984 Oct;133(4):1710–1715. [PubMed] [Google Scholar]
- Gogolin-Ewens K. J., Mackay C. R., Mercer W. R., Brandon M. R. Sheep lymphocyte antigens (OLA). I. Major histocompatibility complex class I molecules. Immunology. 1985 Dec;56(4):717–723. [PMC free article] [PubMed] [Google Scholar]
- Halleraker M., Landsverk T., Nicander L. Organization of ruminant Peyer's patches as seen with enzyme histochemical markers of stromal and accessory cells. Vet Immunol Immunopathol. 1990 Sep;26(1):93–104. doi: 10.1016/0165-2427(90)90135-f. [DOI] [PubMed] [Google Scholar]
- Herzenberg L. A., Stall A. M., Lalor P. A., Sidman C., Moore W. A., Parks D. R., Herzenberg L. A. The Ly-1 B cell lineage. Immunol Rev. 1986 Oct;93:81–102. doi: 10.1111/j.1600-065x.1986.tb01503.x. [DOI] [PubMed] [Google Scholar]
- Kantor A. B. The development and repertoire of B-1 cells (CD5 B cells). Immunol Today. 1991 Nov;12(11):389–391. doi: 10.1016/0167-5699(91)90136-H. [DOI] [PubMed] [Google Scholar]
- Kantor A. A new nomenclature for B cells. Immunol Today. 1991 Nov;12(11):388–388. doi: 10.1016/0167-5699(91)90135-G. [DOI] [PubMed] [Google Scholar]
- Mackay C. R., Beya M. F., Matzinger P. Gamma/delta T cells express a unique surface molecule appearing late during thymic development. Eur J Immunol. 1989 Aug;19(8):1477–1483. doi: 10.1002/eji.1830190820. [DOI] [PubMed] [Google Scholar]
- Mackay C. R., Marston W. L., Dudler L., Spertini O., Tedder T. F., Hein W. R. Tissue-specific migration pathways by phenotypically distinct subpopulations of memory T cells. Eur J Immunol. 1992 Apr;22(4):887–895. doi: 10.1002/eji.1830220402. [DOI] [PubMed] [Google Scholar]
- Maddox J. F., Mackay C. R., Brandon M. R. Ontogeny of ovine lymphocytes. I. An immunohistological study on the development of T lymphocytes in the sheep embryo and fetal thymus. Immunology. 1987 Sep;62(1):97–105. [PMC free article] [PubMed] [Google Scholar]
- Maddox J. F., Mackay C. R., Brandon M. R. Ontogeny of ovine lymphocytes. II. An immunohistological study on the development of T lymphocytes in the sheep fetal spleen. Immunology. 1987 Sep;62(1):107–112. [PMC free article] [PubMed] [Google Scholar]
- Maddox J. F., Mackay C. R., Brandon M. R. Ontogeny of ovine lymphocytes. III. An immunohistological study on the development of T lymphocytes in sheep fetal lymph nodes. Immunology. 1987 Sep;62(1):113–118. [PMC free article] [PubMed] [Google Scholar]
- Maddox J. F., Mackay C. R., Brandon M. R. Surface antigens, SBU-T4 and SBU-T8, of sheep T lymphocyte subsets defined by monoclonal antibodies. Immunology. 1985 Aug;55(4):739–748. [PMC free article] [PubMed] [Google Scholar]
- Maddox J. F., Mackay C. R., Brandon M. R. The sheep analogue of leucocyte common antigen (LCA). Immunology. 1985 Jun;55(2):347–353. [PMC free article] [PubMed] [Google Scholar]
- Miyasaka M., Morris B. The ontogeny of the lymphoid system and immune responsiveness in sheep. Prog Vet Microbiol Immunol. 1988;4:21–55. [PubMed] [Google Scholar]
- Namikawa R., Mizuno T., Matsuoka H., Fukami H., Ueda R., Itoh G., Matsuyama M., Takahashi T. Ontogenic development of T and B cells and non-lymphoid cells in the white pulp of human spleen. Immunology. 1986 Jan;57(1):61–69. [PMC free article] [PubMed] [Google Scholar]
- Press C. M., Halleraker M., Landsverk T. Ontogeny of leukocyte populations in the ileal Peyer's patch of sheep. Dev Comp Immunol. 1992 Mar-Jun;16(2-3):229–241. doi: 10.1016/0145-305x(92)90022-5. [DOI] [PubMed] [Google Scholar]
- Press C., McClure S., Landsverk T. Computer-assisted morphometric analysis of absorptive and follicle-associated epithelia of Peyer's patches in sheep foetuses and lambs indicates the presence of distinct T- and B-cell components. Immunology. 1991 Mar;72(3):386–392. [PMC free article] [PubMed] [Google Scholar]
- Puri N. K., Mackay C. R., Brandon M. R. Sheep lymphocyte antigens (OLA). II. Major histocompatibility complex class II molecules. Immunology. 1985 Dec;56(4):725–733. [PMC free article] [PubMed] [Google Scholar]
- Reynaud C. A., Anquez V., Grimal H., Weill J. C. A hyperconversion mechanism generates the chicken light chain preimmune repertoire. Cell. 1987 Feb 13;48(3):379–388. doi: 10.1016/0092-8674(87)90189-9. [DOI] [PubMed] [Google Scholar]
- Reynaud C. A., Dahan A., Anquez V., Weill J. C. Somatic hyperconversion diversifies the single Vh gene of the chicken with a high incidence in the D region. Cell. 1989 Oct 6;59(1):171–183. doi: 10.1016/0092-8674(89)90879-9. [DOI] [PubMed] [Google Scholar]
- Reynaud C. A., Imhof B. A., Anquez V., Weill J. C. Emergence of committed B lymphoid progenitors in the developing chicken embryo. EMBO J. 1992 Dec;11(12):4349–4358. doi: 10.1002/j.1460-2075.1992.tb05534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynaud C. A., Mackay C. R., Müller R. G., Weill J. C. Somatic generation of diversity in a mammalian primary lymphoid organ: the sheep ileal Peyer's patches. Cell. 1991 Mar 8;64(5):995–1005. doi: 10.1016/0092-8674(91)90323-q. [DOI] [PubMed] [Google Scholar]
- Reynolds J. D., Morris B. The evolution and involution of Peyer's patches in fetal and postnatal sheep. Eur J Immunol. 1983 Aug;13(8):627–635. doi: 10.1002/eji.1830130805. [DOI] [PubMed] [Google Scholar]
- SILVERSTEIN A. M., UHR J. W., KRANER K. L., LUKES R. J. Fetal response to antigenic stimulus. II. Antibody production by the fetal lamb. J Exp Med. 1963 May 1;117:799–812. doi: 10.1084/jem.117.5.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons D. B., Binns R. M. Immunoglobulin-bearing lymphocytes: their demonstration in adult sheep and ontogeny in the sheep fetus. Int Arch Allergy Appl Immunol. 1975;49(5):658–669. doi: 10.1159/000231448. [DOI] [PubMed] [Google Scholar]
- Timens W., Rozeboom T., Poppema S. Fetal and neonatal development of human spleen: an immunohistological study. Immunology. 1987 Apr;60(4):603–609. [PMC free article] [PubMed] [Google Scholar]