Abstract
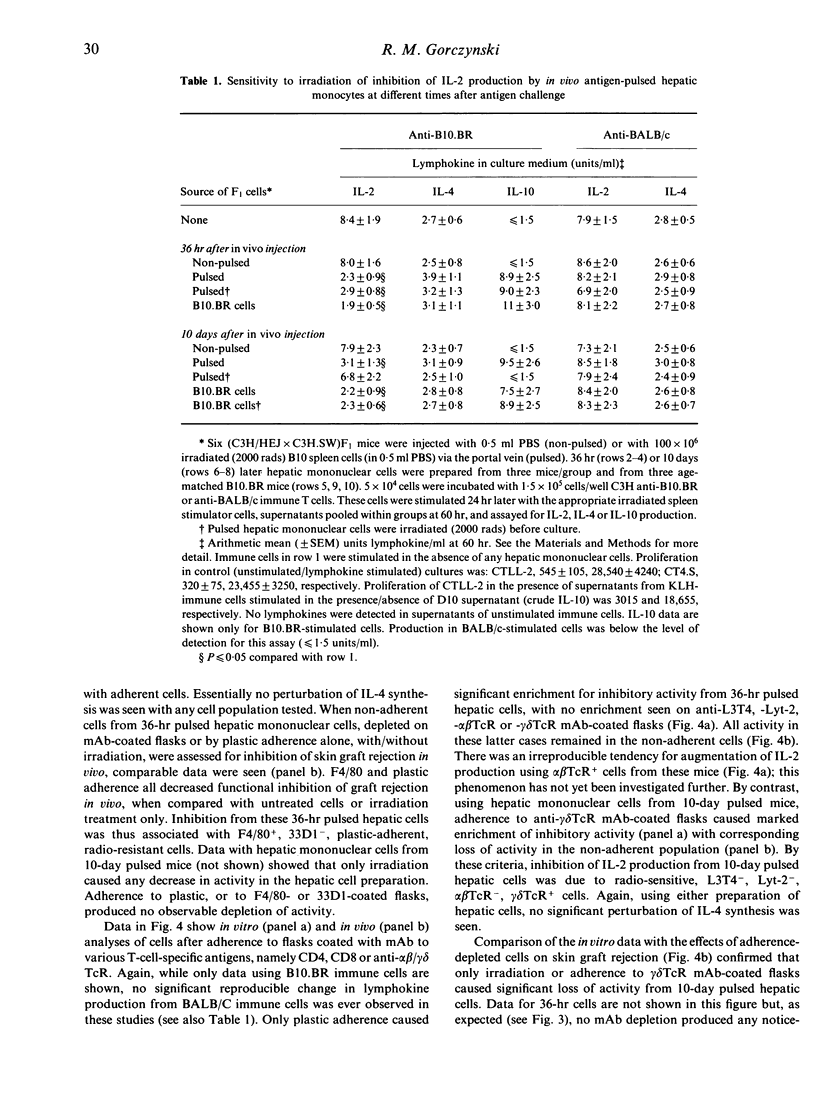
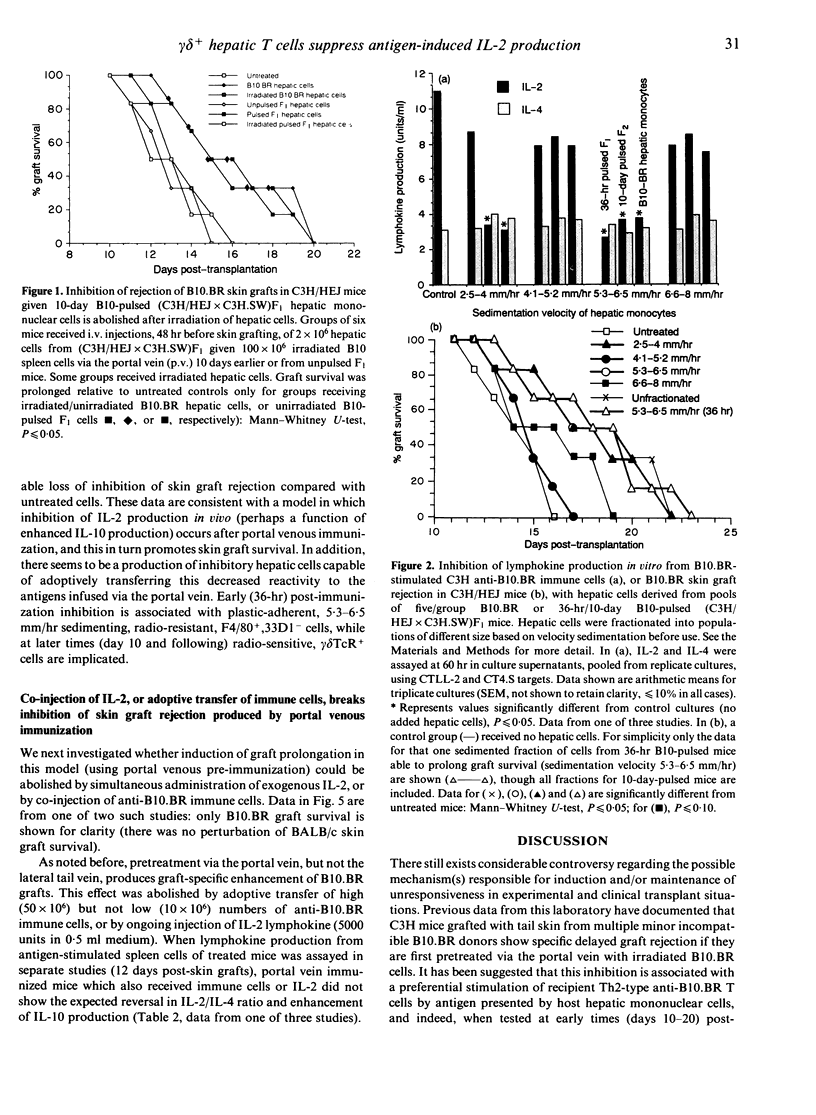
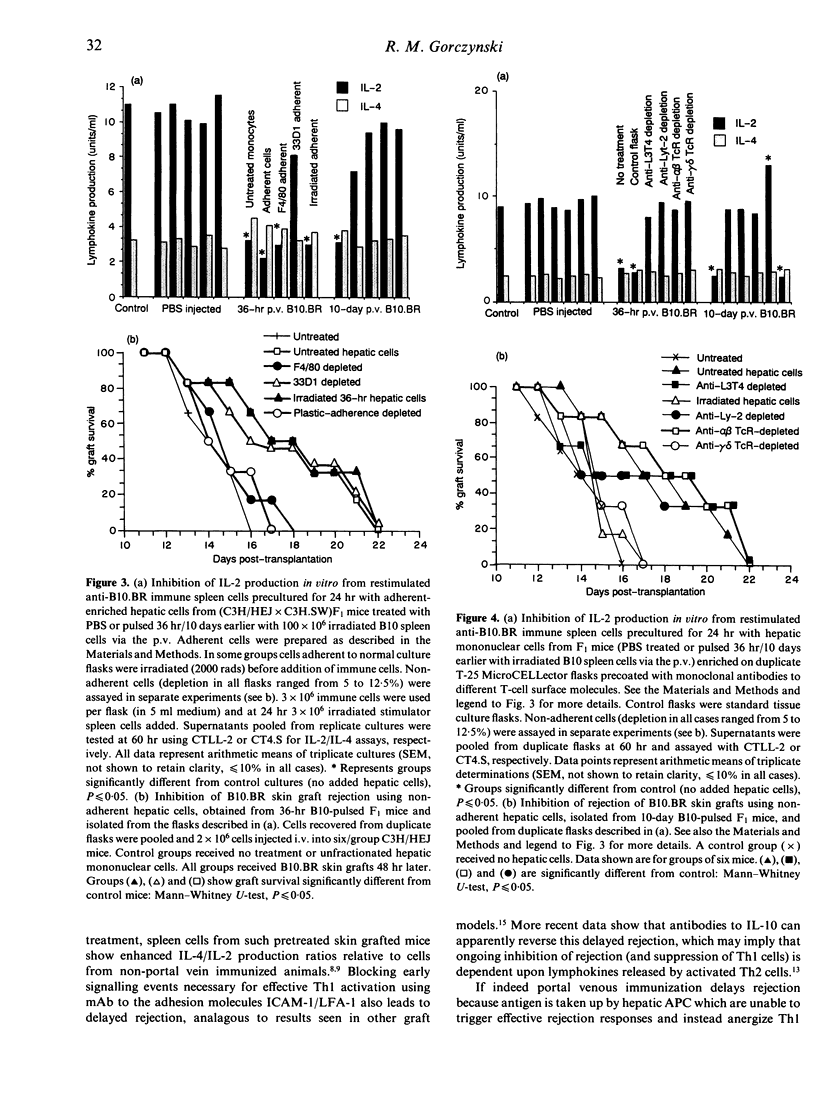
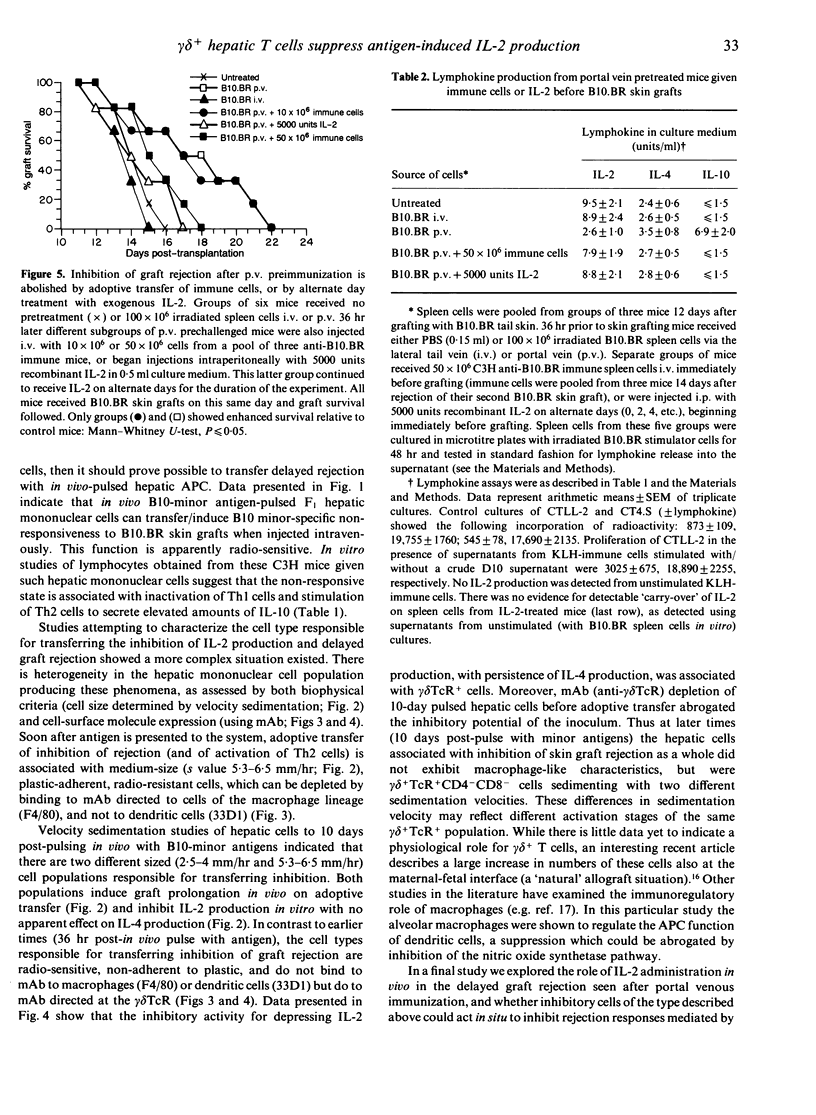
C3H/HEJ mice injected with irradiated multiple minor incompatible B10.BR lymphoid cells via the portal vein showed delayed rejection of subsequent B10.BR skin grafts. Similar delayed rejection was produced by lateral tail vein injection of B10.BR hepatic mononuclear cells or H-2k cells pulsed in vivo with B10 minor histocompatibility antigens. Inhibition of C3H anti-B10.BR immunity in vivo (assessed by delayed graft rejection) and in vitro (assessed by B10.BR-induced lymphokine production) can be transferred by radioresistant, plastic-adherent F4/80+33D1-CD4-CD8-alpha beta TcR-gamma delta TcR- mononuclear hepatic cells from (C3H/HEJ x C3H.SW)F1 mice injected 36 hr earlier with 100 x 10(6) irradiated spleen cells. By 10 days post-injection, cells transferring delayed rejection are radiosensitive, plastic non-adherent, F4/80-33D1-CD4-CD8- alpha beta Tc+- gamma delta TcR+ cells. Injection of interleukin-2 (IL-2) in vivo into mice receiving pretreatment with B10.BR cells via the portal vein, or adoptive transfer into such mice of immune anti-B10.BR lymphoid cells, abolished delayed rejection on subsequent skin grafting. Delayed rejection or modulation of lymphokine production was associated in all cases with suppression of IL-2 production and preferential retention of IL-4 production from cells stimulated in vitro.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batchelor J. R., Lombardi G., Lechler R. I. Speculations on the specificity of suppression. Immunol Today. 1989 Feb;10(2):37–40. doi: 10.1016/0167-5699(89)90301-0. [DOI] [PubMed] [Google Scholar]
- Brochu S., Roy D. C., Perreault C. Tolerance to host minor histocompatibility antigens after allogenic bone marrow transplantation. Specific donor-host unresponsiveness is maintained by peripheral tolerizing cells. J Immunol. 1992 Nov 15;149(10):3135–3141. [PubMed] [Google Scholar]
- Fiorentino D. F., Zlotnik A., Vieira P., Mosmann T. R., Howard M., Moore K. W., O'Garra A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991 May 15;146(10):3444–3451. [PubMed] [Google Scholar]
- Fowell D., McKnight A. J., Powrie F., Dyke R., Mason D. Subsets of CD4+ T cells and their roles in the induction and prevention of autoimmunity. Immunol Rev. 1991 Oct;123:37–64. doi: 10.1111/j.1600-065x.1991.tb00605.x. [DOI] [PubMed] [Google Scholar]
- Gorczynski R. M., Gulay Z., Wojcik D. Differential sensitivity to anti-LFA-1 inhibition of Th1 vs Th2 anti-minor histocompatibility antigen immune T cells after restimulation with antigen on hepatic or splenic APCs. Transplant Proc. 1993 Feb;25(1 Pt 1):807–808. [PubMed] [Google Scholar]
- Gorczynski R. M., Holmes W. Specific manipulation in vivo of immunity to skin grafts bearing multiple minor histocompatibility differences. Immunol Lett. 1991 Feb;27(2):163–171. doi: 10.1016/0165-2478(91)90145-z. [DOI] [PubMed] [Google Scholar]
- Gorczynski R. M. Immunosuppression induced by hepatic portal venous immunization spares reactivity in IL-4 producing T lymphocytes. Immunol Lett. 1992 Jun;33(1):67–77. doi: 10.1016/0165-2478(92)90095-6. [DOI] [PubMed] [Google Scholar]
- Gorczynski R. M., Wojcik D. Antigen presentation by murine splenic, but not hepatic, antigen-presenting cells to induce IL-2/IL-4 production from immune T cells is regulated by interactions between LFA-1/ICAM-1. Immunol Lett. 1992 Dec;34(3):177–181. doi: 10.1016/0165-2478(92)90210-f. [DOI] [PubMed] [Google Scholar]
- Heath W. R., Allison J., Hoffmann M. W., Schönrich G., Hämmerling G., Arnold B., Miller J. F. Autoimmune diabetes as a consequence of locally produced interleukin-2. Nature. 1992 Oct 8;359(6395):547–549. doi: 10.1038/359547a0. [DOI] [PubMed] [Google Scholar]
- Heyborne K. D., Cranfill R. L., Carding S. R., Born W. K., O'Brien R. L. Characterization of gamma delta T lymphocytes at the maternal-fetal interface. J Immunol. 1992 Nov 1;149(9):2872–2878. [PubMed] [Google Scholar]
- Holt P. G., Oliver J., Bilyk N., McMenamin C., McMenamin P. G., Kraal G., Thepen T. Downregulation of the antigen presenting cell function(s) of pulmonary dendritic cells in vivo by resident alveolar macrophages. J Exp Med. 1993 Feb 1;177(2):397–407. doi: 10.1084/jem.177.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isobe M., Yagita H., Okumura K., Ihara A. Specific acceptance of cardiac allograft after treatment with antibodies to ICAM-1 and LFA-1. Science. 1992 Feb 28;255(5048):1125–1127. doi: 10.1126/science.1347662. [DOI] [PubMed] [Google Scholar]
- Jenkins M. K., Schwartz R. H. Antigen presentation by chemically modified splenocytes induces antigen-specific T cell unresponsiveness in vitro and in vivo. J Exp Med. 1987 Feb 1;165(2):302–319. doi: 10.1084/jem.165.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappler J. W., Roehm N., Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987 Apr 24;49(2):273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- Karasuyama H., Melchers F. Establishment of mouse cell lines which constitutively secrete large quantities of interleukin 2, 3, 4 or 5, using modified cDNA expression vectors. Eur J Immunol. 1988 Jan;18(1):97–104. doi: 10.1002/eji.1830180115. [DOI] [PubMed] [Google Scholar]
- Malkovský M., Medawar P. B., Thatcher D. R., Toy J., Hunt R., Rayfield L. S., Doré C. Acquired immunological tolerance of foreign cells is impaired by recombinant interleukin 2 or vitamin A acetate. Proc Natl Acad Sci U S A. 1985 Jan;82(2):536–538. doi: 10.1073/pnas.82.2.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. F., Morahan G., Allison J., Hoffmann M. A transgenic approach to the study of peripheral T-cell tolerance. Immunol Rev. 1991 Aug;122:103–116. doi: 10.1111/j.1600-065x.1991.tb00599.x. [DOI] [PubMed] [Google Scholar]
- Miller R. G., Phillips R. A. Separation of cells by velocity sedimentation. J Cell Physiol. 1969 Jun;73(3):191–201. doi: 10.1002/jcp.1040730305. [DOI] [PubMed] [Google Scholar]
- Mueller D. L., Jenkins M. K., Schwartz R. H. Clonal expansion versus functional clonal inactivation: a costimulatory signalling pathway determines the outcome of T cell antigen receptor occupancy. Annu Rev Immunol. 1989;7:445–480. doi: 10.1146/annurev.iy.07.040189.002305. [DOI] [PubMed] [Google Scholar]
- Powell T. J., Jr, Streilein J. W. Neonatal tolerance induction by class II alloantigens activates IL-4-secreting, tolerogen-responsive T cells. J Immunol. 1990 Feb 1;144(3):854–859. [PubMed] [Google Scholar]
- Qian J., Hashimoto T., Fujiwara H., Hamaoka T. Studies on the induction of tolerance to alloantigens. I. The abrogation of potentials for delayed-type-hypersensitivity response to alloantigens by portal venous inoculation with allogeneic cells. J Immunol. 1985 Jun;134(6):3656–3661. [PubMed] [Google Scholar]
- Rocha B., Tanchot C., Von Boehmer H. Clonal anergy blocks in vivo growth of mature T cells and can be reversed in the absence of antigen. J Exp Med. 1993 May 1;177(5):1517–1521. doi: 10.1084/jem.177.5.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starzl T. E., Demetris A. J., Murase N., Ildstad S., Ricordi C., Trucco M. Cell migration, chimerism, and graft acceptance. Lancet. 1992 Jun 27;339(8809):1579–1582. doi: 10.1016/0140-6736(92)91840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


