Abstract
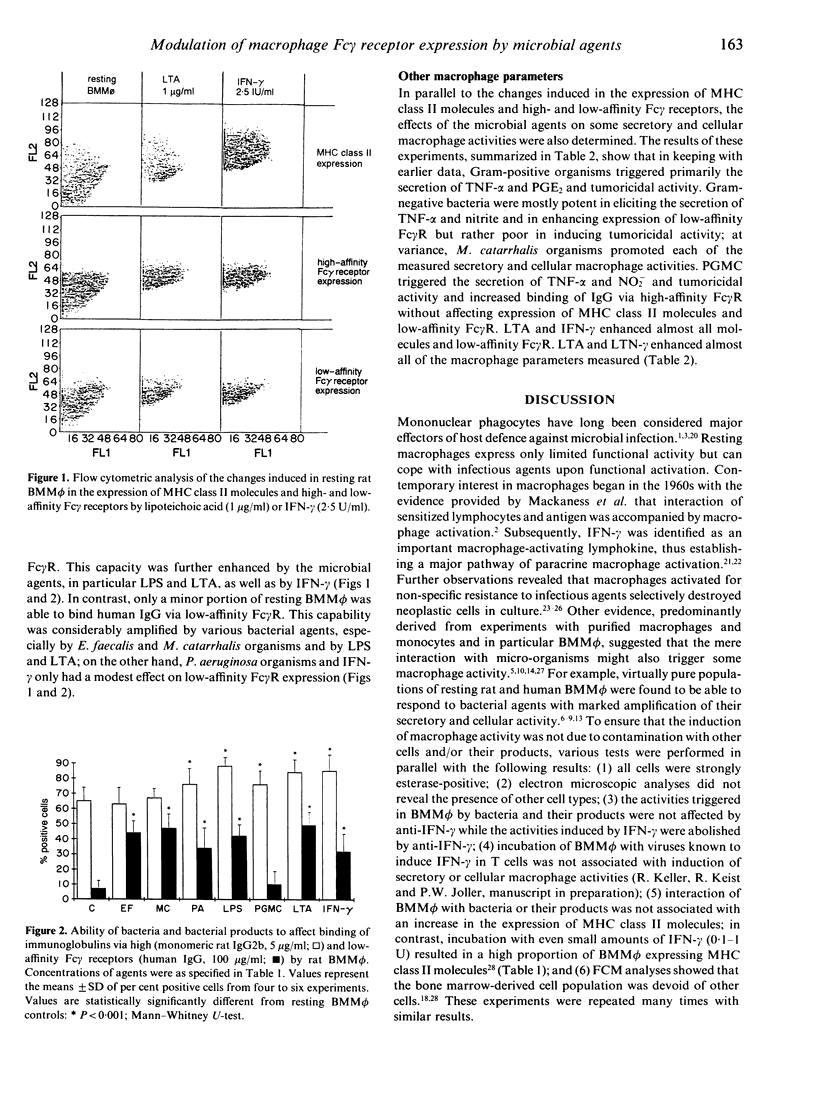
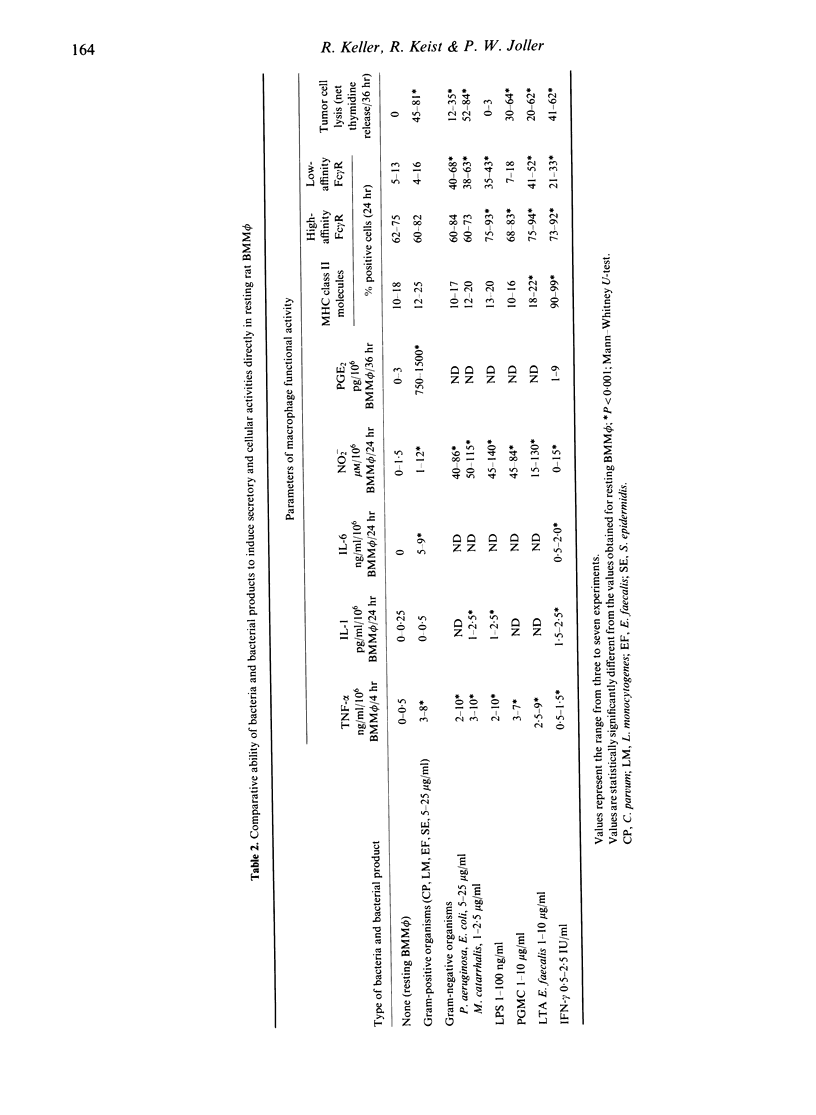
The ability of bacteria and bacterial products to modulate the expression of Fc gamma receptors and major histocompatibility complex (MHC) class II molecules in resting rat bone marrow-derived mononuclear phagocytes (BMM phi) was determined by means of flow cytometry (FCM). Binding of IgG via Fc gamma receptors was considerably enhanced by most microbial agents; bacterial lipopolysaccharide, lipoteichoic acid and some intact bacteria proved to be as active as interferon-gamma (IFN-gamma) and augmented binding of IgG via high- and low-affinity Fc gamma receptors. In contrast, expression of MHC class II molecules by BMM phi was only slightly affected by the microbial agents. Additional findings attest that resting unprimed rat BMM phi are able to respond directly to Gram-negative and Gram-positive bacteria and to some of their products with the expression of marked secretory [in particular tumour necrosis factor-alpha (TNF-alpha) and nitrite] and cellular activities (TNF-alpha-independent tumoricidal activity). This extensive, direct type of macrophage activation may substantially amplify the capability of these cells to cope with these infectious agents in first-line, non-specific host defence.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander P., Evans R. Endotoxin and double stranded RNA render macrophages cytotoxic. Nat New Biol. 1971 Jul 21;232(29):76–78. doi: 10.1038/newbio232076a0. [DOI] [PubMed] [Google Scholar]
- Arenzana-Seisdedos F., Virelizier J. L. Interferons as macrophage-activating factors. II. Enhanced secretion of interleukin 1 by lipopolysaccharide-stimulated human monocytes. Eur J Immunol. 1983 Jun;13(6):437–440. doi: 10.1002/eji.1830130602. [DOI] [PubMed] [Google Scholar]
- Denham S., Barfoot R., Jackson E. A receptor for monomeric IgG2b on rat macrophages. Immunology. 1987 Sep;62(1):69–74. [PMC free article] [PubMed] [Google Scholar]
- Djeu J. Y., Blanchard D. K., Richards A. L., Friedman H. Tumor necrosis factor induction by Candida albicans from human natural killer cells and monocytes. J Immunol. 1988 Dec 1;141(11):4047–4052. [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Lambert L. H., Jr, Remington J. S. Possible role of macrophage mediated nonspecific cytotoxicity in tumour resistance. Nat New Biol. 1972 Jan 12;235(54):48–50. doi: 10.1038/newbio235048a0. [DOI] [PubMed] [Google Scholar]
- Hogg N. The structure and function of Fc receptors. Immunol Today. 1988 Jul-Aug;9(7-8):185–187. doi: 10.1016/0167-5699(88)91206-6. [DOI] [PubMed] [Google Scholar]
- Keller R. Cytostatic elimination of syngeneic rat tumor cells in vitro by nonspecifically activated macrophages. J Exp Med. 1973 Sep 1;138(3):625–644. doi: 10.1084/jem.138.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R., Fischer W., Keist R., Bassetti S. Macrophage response to bacteria: induction of marked secretory and cellular activities by lipoteichoic acids. Infect Immun. 1992 Sep;60(9):3664–3672. doi: 10.1128/iai.60.9.3664-3672.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R., Gehri R., Keist R., Huf E., Kayser F. H. The interaction of macrophages and bacteria: a comparative study of the induction of tumoricidal activity and of reactive nitrogen intermediates. Cell Immunol. 1991 Apr 15;134(1):249–256. doi: 10.1016/0008-8749(91)90348-f. [DOI] [PubMed] [Google Scholar]
- Keller R., Gehri R., Keist R. The interaction of macrophages and bacteria: Escherichia coli species, bacterial lipopolysaccharide, and lipid A differ in their ability to induce tumoricidal activity and the secretion of reactive nitrogen intermediates in macrophages. Cell Immunol. 1992 Apr 15;141(1):47–58. doi: 10.1016/0008-8749(92)90126-a. [DOI] [PubMed] [Google Scholar]
- Keller R., Gustafson J. E., Keist R. The macrophage response to bacteria. Modulation of macrophage functional activity by peptidoglycan from Moraxella (Branhamella) catarrhalis. Clin Exp Immunol. 1992 Sep;89(3):384–389. doi: 10.1111/j.1365-2249.1992.tb06967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R., Joller P., Keist R., Binz H., van der Meide P. H. Modulation of major histocompatibility complex (MHC) expression by interferons and microbial agents. Independent regulation of MHC class II expression and induction of tumoricidal activity in bone marrow-derived mononuclear phagocytes. Scand J Immunol. 1988 Jul;28(1):113–121. doi: 10.1111/j.1365-3083.1988.tb02422.x. [DOI] [PubMed] [Google Scholar]
- Keller R., Jones V. E. Role of activated macrophages and antibody in inhibition and enhancement of tumour growth in rats. Lancet. 1971 Oct 16;2(7729):847–849. doi: 10.1016/s0140-6736(71)90222-4. [DOI] [PubMed] [Google Scholar]
- Keller R., Keist R., Bazin H., Joller P., Van der Meide P. H. Binding of monomeric immunoglobulins by bone marrow-derived mononuclear phagocytes; its modulation by interferon-gamma. Eur J Immunol. 1990 Sep;20(9):2137–2140. doi: 10.1002/eji.1830200937. [DOI] [PubMed] [Google Scholar]
- Keller R., Keist R., Frei K. Lymphokines and bacteria, that induce tumoricidal activity, trigger a different secretory response in macrophages. Eur J Immunol. 1990 Mar;20(3):695–698. doi: 10.1002/eji.1830200334. [DOI] [PubMed] [Google Scholar]
- Keller R., Keist R. Induction of interleukin 1 secretion and of tumoricidal activity in macrophages are not closely related phenomena. Eur J Immunol. 1987 Nov;17(11):1665–1668. doi: 10.1002/eji.1830171124. [DOI] [PubMed] [Google Scholar]
- Keller R., Keist R., Joller P., Groscurth P. Mononuclear phagocytes from human bone marrow progenitor cells; morphology, surface phenotype, and functional properties of resting and activated cells. Clin Exp Immunol. 1993 Jan;91(1):176–182. doi: 10.1111/j.1365-2249.1993.tb03375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R., Keist R., Van der Meide P. H., Groscurth P., Aguet M., Leist T. P. Induction, maintenance, and reinduction of tumoricidal activity in bone marrow-derived mononuclear phagocytes by Corynebacterium parvum. Evidence for the involvement of a T cell- and interferon-gamma-independent pathway of macrophage activation. J Immunol. 1987 Apr 1;138(7):2366–2371. [PubMed] [Google Scholar]
- Mackaness G. B. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969 May 1;129(5):973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai U. E., Perez-Perez G. I., Wahl L. M., Wahl S. M., Blaser M. J., Smith P. D. Soluble surface proteins from Helicobacter pylori activate monocytes/macrophages by lipopolysaccharide-independent mechanism. J Clin Invest. 1991 Mar;87(3):894–900. doi: 10.1172/JCI115095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overton W. R. Modified histogram subtraction technique for analysis of flow cytometry data. Cytometry. 1988 Nov;9(6):619–626. doi: 10.1002/cyto.990090617. [DOI] [PubMed] [Google Scholar]


