Abstract
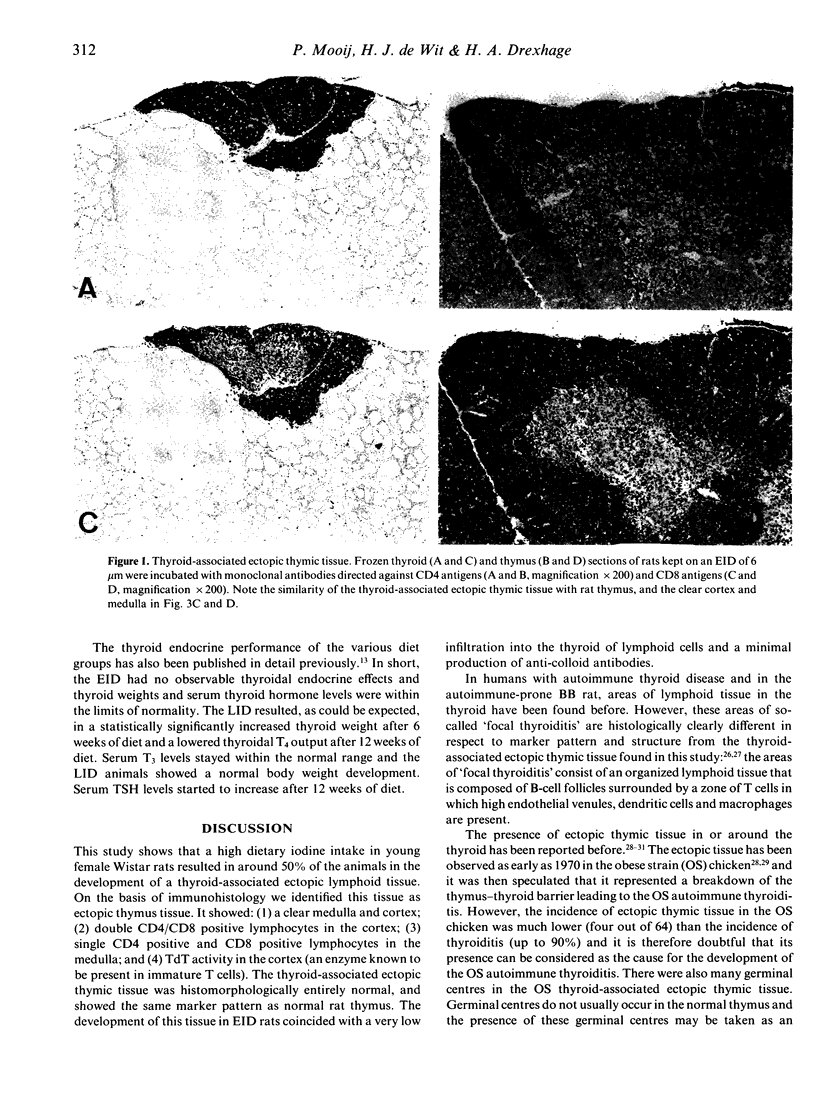
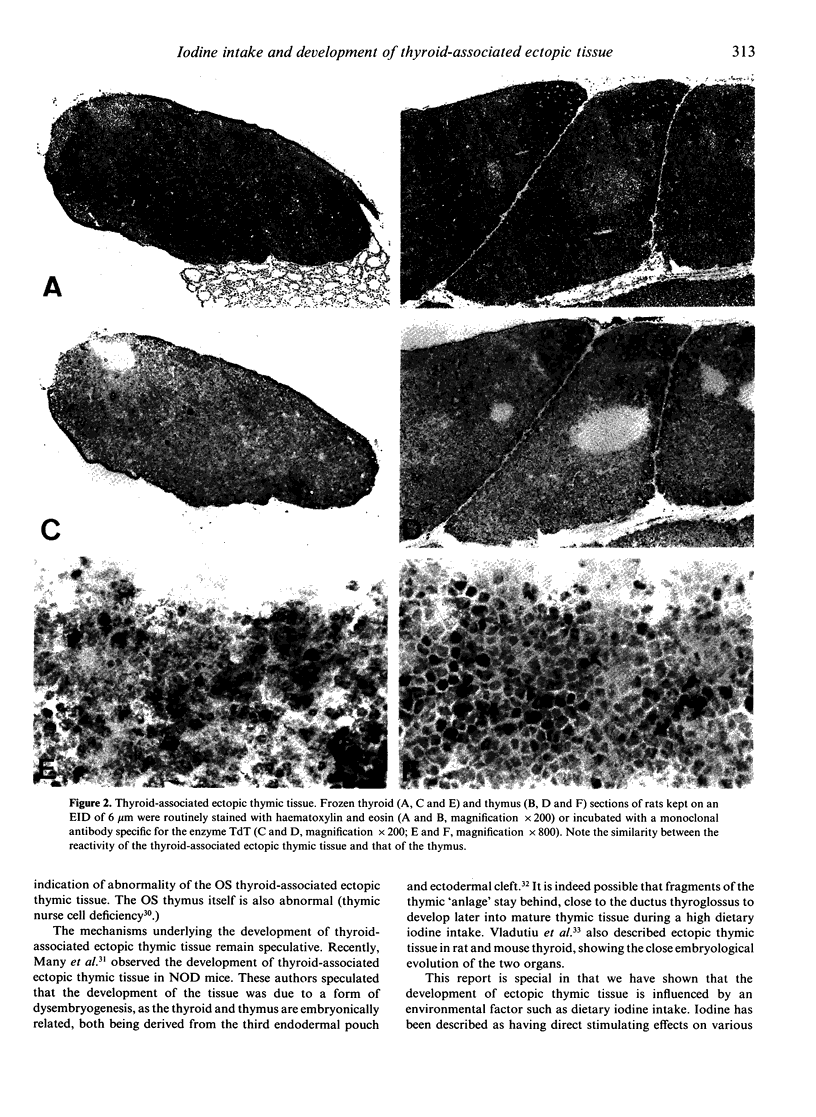
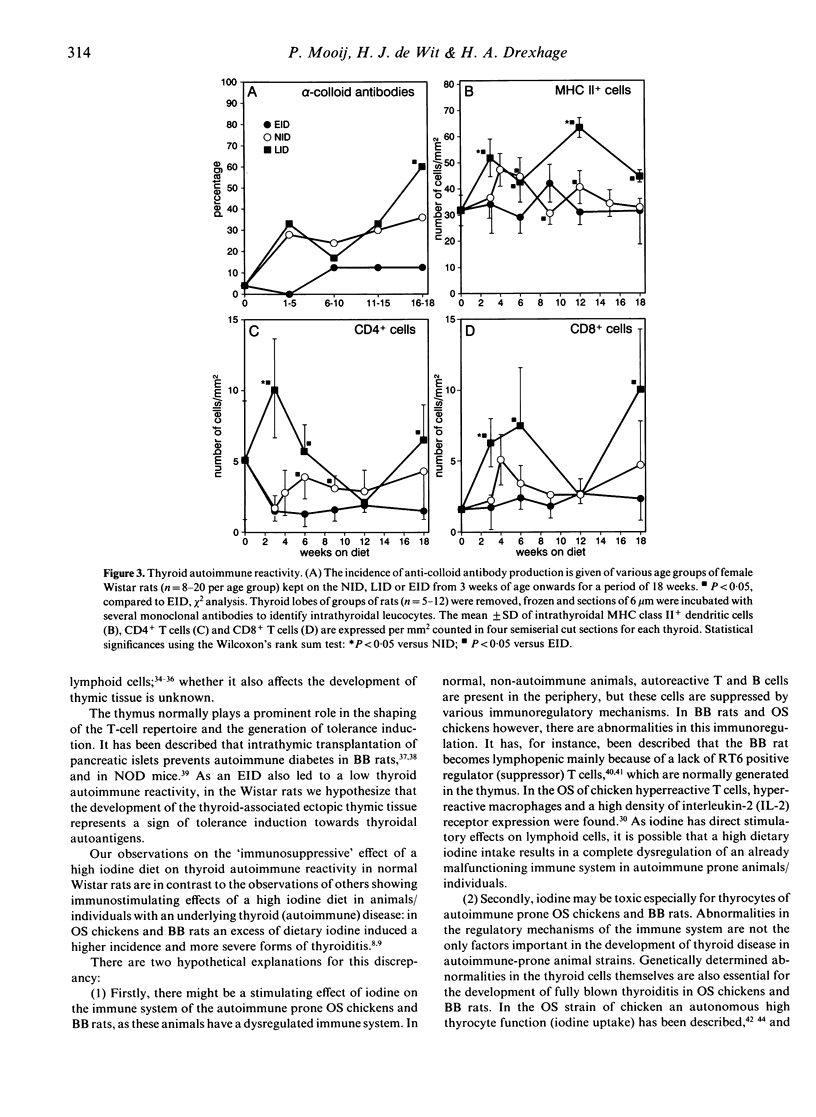
Evidence is accumulating that dietary iodine intake is an important modulator of autoimmune thyroid reactions. To study this role of iodine intake further, female Wistar rats were kept on an enriched iodine diet (EID, iodine intake 100 micrograms iodine/day) for a period of up to 18 weeks. Control rats were either on a normal iodine diet (NID, iodine intake 7 micrograms iodine/day) or a low iodine diet (LID, 2 days of 1% KClO4 followed by iodine-deficient drinking water/pellets). During the first 6 weeks of the EID rats developed a thyroid-associated ectopic thymic tissue (50-57% of the animals on EID versus 7-14% of NID rats and 0% of LID rats). This thyroid-associated ectopic thymic tissue showed a similar histology (cortex and medulla) and a similar marker pattern as normal rat thymus concerning TdT expression (positive cells in the cortex) and CD4/CD8 positivity (double-positive cells in the cortex, single-positive cells in the medulla). The excessive iodine diet also resulted in a lowered thyroid autoimmune reactivity as compared to the NID and LID, viz. (1) in a lower incidence of anti-colloid antibodies in serum (12.5% positivity in EID rats versus 36% in NID and 60% in LID rats at 18 weeks) and (2) lower numbers of intrathyroidal lymphoid cells, viz. lower numbers of dendritic cells and lower numbers of CD4 and CD8 positive lymphocytes. It is hypothesized that the development of the thyroid-associated ectopic thymic tissue in the EID rats is related to their low thyroid autoimmune responsiveness; the tissue might play a role in tolerance induction to thyroidal autoantigens.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen E. M., Appel M. C., Braverman L. E. The effect of iodide ingestion on the development of spontaneous lymphocytic thyroiditis in the diabetes-prone BB/W rat. Endocrinology. 1986 May;118(5):1977–1981. doi: 10.1210/endo-118-5-1977. [DOI] [PubMed] [Google Scholar]
- Bagchi N., Brown T. R., Urdanivia E., Sundick R. S. Induction of autoimmune thyroiditis in chickens by dietary iodine. Science. 1985 Oct 18;230(4723):325–327. doi: 10.1126/science.4048936. [DOI] [PubMed] [Google Scholar]
- Barclay A. N. The localization of populations of lymphocytes defined by monoclonal antibodies in rat lymphoid tissues. Immunology. 1981 Apr;42(4):593–600. [PMC free article] [PubMed] [Google Scholar]
- Bollum F. J., Chang L. M. Terminal transferase in normal and leukemic cells. Adv Cancer Res. 1986;47:37–61. doi: 10.1016/s0065-230x(08)60197-9. [DOI] [PubMed] [Google Scholar]
- Bollum F. J. Terminal deoxynucleotidyl transferase as a hematopoietic cell marker. Blood. 1979 Dec;54(6):1203–1215. [PubMed] [Google Scholar]
- Boukis M. A., Koutras D. A., Souvatzoglou A., Evangelopoulou A., Vrontakis M., Moulopoulos S. D. Thyroid hormone and immunological studies in endemic goiter. J Clin Endocrinol Metab. 1983 Oct;57(4):859–862. doi: 10.1210/jcem-57-4-859. [DOI] [PubMed] [Google Scholar]
- Boyages S. C., Bloot A. M., Maberly G. F., Eastman C. J., Li M., Qian Q. D., Liu D. R., van der Gaag R. D., Drexhage H. A. Thyroid autoimmunity in endemic goitre caused by excessive iodine intake. Clin Endocrinol (Oxf) 1989 Oct;31(4):453–465. doi: 10.1111/j.1365-2265.1989.tb01269.x. [DOI] [PubMed] [Google Scholar]
- Brayman K. L., Nakai I., Field J., Lloveras J. J., Farney A., Najarian J. S., Sutherland D. E. Intrathymic islet allografts prevent hyperglycemia and autoimmune beta-cell destruction in BB rats following transplantation in the prediabetic period. Transplant Proc. 1993 Feb;25(1 Pt 1):284–285. [PubMed] [Google Scholar]
- Brideau R. J., Carter P. B., McMaster W. R., Mason D. W., Williams A. F. Two subsets of rat T lymphocytes defined with monoclonal antibodies. Eur J Immunol. 1980 Aug;10(8):609–615. doi: 10.1002/eji.1830100807. [DOI] [PubMed] [Google Scholar]
- Connolly R. J., Vidor G. I., Stewart J. C. Increase in thyrotoxicosis in endemic goitre area after iodation of bread. Lancet. 1970 Mar 7;1(7645):500–502. doi: 10.1016/s0140-6736(70)91582-5. [DOI] [PubMed] [Google Scholar]
- Cordier A. C., Haumont S. M. Development of thymus, parathyroids, and ultimo-branchial bodies in NMRI and nude mice. Am J Anat. 1980 Mar;157(3):227–263. doi: 10.1002/aja.1001570303. [DOI] [PubMed] [Google Scholar]
- FOLLIS R. H., Jr FURTHER OBSERVATIONS ON THYROIDITIS AND COLLOID ACCUMULATION IN HYPERPLASTIC THYROID GLANDS OF HAMSTERS RECEIVING EXCESS IODINE. Lab Invest. 1964 Dec;13:1590–1599. [PubMed] [Google Scholar]
- Ferguson M. M., Alexander W. D., Connell J. M., Lappin A. G., McCruden D. C., MacLure R., Mairs R. J., Younger A. Peroxidase activity in relation to iodide, 17 beta-oestradiol and thioureylene drug uptake in human polymorphoneutrophils. Biochem Pharmacol. 1984 Mar 1;33(5):757–762. doi: 10.1016/0006-2952(84)90459-3. [DOI] [PubMed] [Google Scholar]
- Fradkin J. E., Wolff J. Iodide-induced thyrotoxicosis. Medicine (Baltimore) 1983 Jan;62(1):1–20. doi: 10.1097/00005792-198301000-00001. [DOI] [PubMed] [Google Scholar]
- Green M. A., Sviland L., Malcolm A. J., Pearson A. D. Improved method for immunoperoxidase detection of membrane antigens in frozen sections. J Clin Pathol. 1989 Aug;42(8):875–880. doi: 10.1136/jcp.42.8.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoire K. E., Goldschneider I., Barton R. W., Bollum F. J. Intracellular distribution of terminal deoxynucleotidyl transferase in rat bone marrow and thymus. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3993–3996. doi: 10.1073/pnas.74.9.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiner D. L., Handler E. S., Nakano K., Mordes J. P., Rossini A. A. Absence of the RT-6 T cell subset in diabetes-prone BB/W rats. J Immunol. 1986 Jan;136(1):148–151. [PubMed] [Google Scholar]
- Kabel P. J., Voorbij H. A., De Haan M., van der Gaag R. D., Drexhage H. A. Intrathyroidal dendritic cells. J Clin Endocrinol Metab. 1988 Jan;66(1):199–207. doi: 10.1210/jcem-66-1-199. [DOI] [PubMed] [Google Scholar]
- Kroese F. G., Opstelten D., Wubbena A. S., Deenen G. J., Aten J., Schwander E. H., de Leij L., Nieuwenhuis P. Monoclonal antibodies to rat B lymphocyte (sub-)populations. Adv Exp Med Biol. 1985;186:81–89. doi: 10.1007/978-1-4613-2463-8_10. [DOI] [PubMed] [Google Scholar]
- Kroese F. G., Wubbena A. S., Opstelten D., Deenen G. J., Schwander E. H., De Leij L., Vos H., Poppema S., Volberda J., Nieuwenhuis P. B lymphocyte differentiation in the rat: production and characterization of monoclonal antibodies to B lineage-associated antigens. Eur J Immunol. 1987 Jul;17(7):921–928. doi: 10.1002/eji.1830170705. [DOI] [PubMed] [Google Scholar]
- Mahmoud I., Colin I., Many M. C., Denef J. F. Direct toxic effect of iodide in excess on iodine-deficient thyroid glands: epithelial necrosis and inflammation associated with lipofuscin accumulation. Exp Mol Pathol. 1986 Jun;44(3):259–271. doi: 10.1016/0014-4800(86)90040-7. [DOI] [PubMed] [Google Scholar]
- Many M. C., Denef J. F., Hamudi S., Cornette C., Haumont S., Beckers C. Effects of iodide and thyroxine on iodine-deficient mouse thyroid: a morphological and functional study. J Endocrinol. 1986 Aug;110(2):203–210. doi: 10.1677/joe.0.1100203. [DOI] [PubMed] [Google Scholar]
- Many M. C., Drexhage H. A., Denef J. F. High frequency of thymic ectopy in thyroids from autoimmune prone nonobese diabetic female mice. Lab Invest. 1993 Sep;69(3):364–367. [PubMed] [Google Scholar]
- McCaffrey R., Harrison T. A., Parkman R., Baltimore D. Terminal deoxynucleotidyl transferase activity in human leukemic cells and in normal human thymocytes. N Engl J Med. 1975 Apr 10;292(15):775–780. doi: 10.1056/NEJM197504102921504. [DOI] [PubMed] [Google Scholar]
- McMaster W. R., Williams A. F. Identification of Ia glycoproteins in rat thymus and purification from rat spleen. Eur J Immunol. 1979 Jun;9(6):426–433. doi: 10.1002/eji.1830090603. [DOI] [PubMed] [Google Scholar]
- Mooij P., de Wit H. J., Bloot A. M., Wilders-Truschnig M. M., Drexhage H. A. Iodine deficiency induces thyroid autoimmune reactivity in Wistar rats. Endocrinology. 1993 Sep;133(3):1197–1204. doi: 10.1210/endo.133.3.8103449. [DOI] [PubMed] [Google Scholar]
- Nomura Y., Mullen Y., Stein E. Syngeneic islets transplanted into the thymus of newborn mice prevent diabetes and reduce insulitis in the NOD mouse. Transplant Proc. 1993 Feb;25(1 Pt 2):963–964. [PubMed] [Google Scholar]
- Posselt A. M., Barker C. F., Friedman A. L., Koeberlein B., Tomaszewski J. E., Naji A. Intrathymic inoculation of islets at birth prevents autoimmune diabetes and pancreatic insulitis in the BB rat. Transplant Proc. 1993 Feb;25(1 Pt 1):301–302. [PubMed] [Google Scholar]
- Rabinowe S. L., Larsen P. R., Antman E. M., George K. L., Friedman P. L., Jackson R. A., Eisenbarth G. S. Amiodarone therapy and autoimmune thyroid disease. Increase in a new monoclonal antibody-defined T cell subset. Am J Med. 1986 Jul;81(1):53–57. doi: 10.1016/0002-9343(86)90181-6. [DOI] [PubMed] [Google Scholar]
- Sato K., Okamura K., Yoshinari M., Ikenoue H., Kuroda T., Torisu M., Fujishima M. Goitrous hypothyroidism with blocking or stimulating thyrotropin binding inhibitor immunoglobulins. J Clin Endocrinol Metab. 1990 Oct;71(4):855–860. doi: 10.1210/jcem-71-4-855. [DOI] [PubMed] [Google Scholar]
- Vladutiu A. O., Rose N. R. Aberrant thymus tissue in rat and mouse thyroid. Experientia. 1972 Jan 15;28(1):79–81. doi: 10.1007/BF01928275. [DOI] [PubMed] [Google Scholar]
- Voorby H. A., Kabel P. J., de Haan M., Jeucken P. H., van der Gaag R. D., de Baets M. H., Drexhage H. A. Dendritic cells and class II MHC expression on thyrocytes during the autoimmune thyroid disease of the BB rat. Clin Immunol Immunopathol. 1990 Apr;55(1):9–22. doi: 10.1016/0090-1229(90)90065-x. [DOI] [PubMed] [Google Scholar]
- Weetman A. P., McGregor A. M., Campbell H., Lazarus J. H., Ibbertson H. K., Hall R. Iodide enhances IgG synthesis by human peripheral blood lymphocytes in vitro. Acta Endocrinol (Copenh) 1983 Jun;103(2):210–215. doi: 10.1530/acta.0.1030210. [DOI] [PubMed] [Google Scholar]
- Wick G., Brezinschek H. P., Hála K., Dietrich H., Wolf H., Kroemer G. The obese strain of chickens: an animal model with spontaneous autoimmune thyroiditis. Adv Immunol. 1989;47:433–500. doi: 10.1016/s0065-2776(08)60666-5. [DOI] [PubMed] [Google Scholar]
- Wick G. Concept of a multigenic basis for the pathogenesis of spontaneous autoimmune thyroiditis. Acta Endocrinol Suppl (Copenh) 1987;281:63–69. doi: 10.1530/acta.0.114s063. [DOI] [PubMed] [Google Scholar]
- Wick G., Kite J. H., Jr, Witebsky E. Spontaneous thyroiditis in the obese strain of chickens. IV. The effect of thymectomy and thymo-bursectomy on the development of the disease. J Immunol. 1970 Jan;104(1):54–62. [PubMed] [Google Scholar]
- Wick G., Kite J. H., Jr, Witebsky E. Spontaneous thyroiditis in the obese strain of chickens. V. The effect of sublethal total body X-irradiation on the development of the disease. J Immunol. 1970 Feb;104(2):344–352. [PubMed] [Google Scholar]
- Wick G., Müller P. U., Kuhn L., Lefkovits I. Molecular analysis of genetically determined target organ abnormalities in spontaneous autoimmune thyroiditis. Immunobiology. 1990 Nov;181(4-5):414–429. doi: 10.1016/S0171-2985(11)80510-3. [DOI] [PubMed] [Google Scholar]
- Wilders-Truschnig M. M., Drexhage H. A., Leb G., Eber O., Brezinschek H. P., Dohr G., Lanzer G., Krejs G. J. Chromatographically purified immunoglobulin G of endemic and sporadic goiter patients stimulates FRTL5 cell growth in a mitotic arrest assay. J Clin Endocrinol Metab. 1990 Feb;70(2):444–452. doi: 10.1210/jcem-70-2-444. [DOI] [PubMed] [Google Scholar]