Abstract
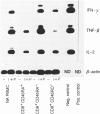
Expression of different isoforms of CD45, the leucocyte common antigen (LCA), on T-cell subsets has permitted distinctions between the functional activities of subpopulations within the major CD4+ T-cell subset. With respect to cytokine production, the expression on CD4+ cells of CD45RA, a high molecular weight isoform, defines a population which produces only interleukin-2 (IL-2) and tumour necrosis factor-beta (TNF-beta) in quantity, with peak production of IL-2 occurring after 24-48 hr stimulation, while the CD4+ population bearing high levels of CD45RO, a low molecular weight isoform, can produce a wide range of cytokines within 24 hr of activation. The literature is conflicting on the capacities for cytokine production of CD8+ subsets divided on the basis of either CD45RA or CD45RO expression. The aim of this study was to attempt to clarify this area by determining the amount and kinetics of production of IL-2, interferon-gamma (IFN-gamma) and TNF-beta in CD8+ cells separated on the basis of both CD45RA and CD45RO isoform expression. The results showed that CD8+ CD45RA- and CD8+ CD45RO+ T lymphocytes produce significantly more of all three cytokines than do CD8+ CD45RA+ or CD8+ CD45RO- T cells. The kinetics for IFN-gamma and TNF-beta production were similar for both subsets, while IL-2 production was delayed by approximately 3 hr in the CD8+ CD45RO- population as compared to the CD8+ CD45RO+ subset. It is suggested that some of the confusion over cytokine production by these CD8+ subsets may be attributable to different conditions for isolation causing pre-activation of positively selected populations. It is also suggested that while CD8+ CD45RA+ cells are shown to acquire CD45RO upon activation, as do CD4+ CD45RA+ cells, the results of the present study argue for a different relationship between CD8+ subsets separated on the basis of CD45 isoform expression than between the corresponding CD4+ subsets.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akbar A. N., Amlot P. L., Timms A., Lombardi G., Lechler R., Janossy G. The development of primed/memory CD8+ lymphocytes in vitro and in rejecting kidneys after transplantation. Clin Exp Immunol. 1990 Aug;81(2):225–231. doi: 10.1111/j.1365-2249.1990.tb03322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbar A. N., Timms A., Janossy G. Cellular events during memory T-cell activation in vitro: the UCHL1 (180,000 MW) determinant is newly synthesized after mitosis. Immunology. 1989 Feb;66(2):213–218. [PMC free article] [PubMed] [Google Scholar]
- Cooley M. A., McLachlan K., Atkinson K. Cytokine activity after human bone marrow transplantation. III. Defect in IL2 production by peripheral blood mononuclear cells is not corrected by stimulation with Ca++ ionophore plus phorbol ester. Br J Haematol. 1989 Nov;73(3):341–347. doi: 10.1111/j.1365-2141.1989.tb07750.x. [DOI] [PubMed] [Google Scholar]
- Deans J. P., Boyd A. W., Pilarski L. M. Transitions from high to low molecular weight isoforms of CD45 (T200) involve rapid activation of alternate mRNA splicing and slow turnover of surface CD45R. J Immunol. 1989 Aug 15;143(4):1233–1238. [PubMed] [Google Scholar]
- Dianzani U., Redoglia V., Malavasi F., Bragardo M., Pileri A., Janeway C. A., Jr, Bottomly K. Isoform-specific associations of CD45 with accessory molecules in human T lymphocytes. Eur J Immunol. 1992 Feb;22(2):365–371. doi: 10.1002/eji.1830220212. [DOI] [PubMed] [Google Scholar]
- Dohlsten M., Hedlund G., Sjögren H. O., Carlsson R. Two subsets of human CD4+ T helper cells differing in kinetics and capacities to produce interleukin 2 and interferon-gamma can be defined by the Leu-18 and UCHL1 monoclonal antibodies. Eur J Immunol. 1988 Aug;18(8):1173–1178. doi: 10.1002/eji.1830180805. [DOI] [PubMed] [Google Scholar]
- Ehlers S., Smith K. A. Differentiation of T cell lymphokine gene expression: the in vitro acquisition of T cell memory. J Exp Med. 1991 Jan 1;173(1):25–36. doi: 10.1084/jem.173.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer H., Dohlsten M., Andersson U., Hedlund G., Ericsson P., Hansson J., Sjögren H. O. Production of TNF-alpha and TNF-beta by staphylococcal enterotoxin A activated human T cells. J Immunol. 1990 Jun 15;144(12):4663–4669. [PubMed] [Google Scholar]
- Hirohata S. T8 cell regulation of human B cell responsiveness: regulatory influences of CD45RA+ and CD45RA- T8 cell subsets. Cell Immunol. 1991 Mar;133(1):15–26. doi: 10.1016/0008-8749(91)90176-c. [DOI] [PubMed] [Google Scholar]
- Ho M. Role of specific cytotoxic lymphocytes in cellular immunity against murine cytomegalovirus. Infect Immun. 1980 Mar;27(3):767–776. doi: 10.1128/iai.27.3.767-776.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., Tonks N. K., Fischer E. H., Clark E. A. CD45 regulates signal transduction and lymphocyte activation by specific association with receptor molecules on T or B cells. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8628–8632. doi: 10.1073/pnas.85.22.8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightstone E. B., Wyllie D., Marvel J. In the mouse the maturation stage of the peripheral CD4+ CD45RA+ subset is different from that of the CD8+ CD45RA+ subset. Eur J Immunol. 1991 Sep;21(9):2161–2165. doi: 10.1002/eji.1830210926. [DOI] [PubMed] [Google Scholar]
- Mestan J., Brockhaus M., Kirchner H., Jacobsen H. Antiviral activity of tumour necrosis factor. Synergism with interferons and induction of oligo-2',5'-adenylate synthetase. J Gen Virol. 1988 Dec;69(Pt 12):3113–3120. doi: 10.1099/0022-1317-69-12-3113. [DOI] [PubMed] [Google Scholar]
- Morimoto C., Letvin N. L., Distaso J. A., Aldrich W. R., Schlossman S. F. The isolation and characterization of the human suppressor inducer T cell subset. J Immunol. 1985 Mar;134(3):1508–1515. [PubMed] [Google Scholar]
- Salmon M., Kitas G. D., Bacon P. A. Production of lymphokine mRNA by CD45R+ and CD45R- helper T cells from human peripheral blood and by human CD4+ T cell clones. J Immunol. 1989 Aug 1;143(3):907–912. [PubMed] [Google Scholar]
- Schofield L., Villaquiran J., Ferreira A., Schellekens H., Nussenzweig R., Nussenzweig V. Gamma interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature. 1987 Dec 17;330(6149):664–666. doi: 10.1038/330664a0. [DOI] [PubMed] [Google Scholar]
- Schraven B., Roux M., Hutmacher B., Meuer S. C. Triggering of the alternative pathway of human T cell activation involves members of the T 200 family of glycoproteins. Eur J Immunol. 1989 Feb;19(2):397–403. doi: 10.1002/eji.1830190226. [DOI] [PubMed] [Google Scholar]
- Sewell W. A., Valentine J. E., Cooley M. A. Expression of interleukin 5 by the CD4+CD45R0+ subset of human T cells. Growth Factors. 1992;6(4):295–302. doi: 10.3109/08977199209021541. [DOI] [PubMed] [Google Scholar]
- Smith S. H., Brown M. H., Rowe D., Callard R. E., Beverley P. C. Functional subsets of human helper-inducer cells defined by a new monoclonal antibody, UCHL1. Immunology. 1986 May;58(1):63–70. [PMC free article] [PubMed] [Google Scholar]
- Sohen S., Rothstein D. M., Tallman T., Gaudette D., Schlossman S. F., Morimoto C. The functional heterogeneity of CD8+ cells defined by anti-CD45RA (2H4) and anti-CD29 (4B4) antibodies. Cell Immunol. 1990 Jun;128(1):314–328. doi: 10.1016/0008-8749(90)90028-p. [DOI] [PubMed] [Google Scholar]
- Stamenkovic I., Sgroi D., Aruffo A., Sy M. S., Anderson T. The B lymphocyte adhesion molecule CD22 interacts with leukocyte common antigen CD45RO on T cells and alpha 2-6 sialyltransferase, CD75, on B cells. Cell. 1991 Sep 20;66(6):1133–1144. doi: 10.1016/0092-8674(91)90036-x. [DOI] [PubMed] [Google Scholar]
- Streuli M., Hall L. R., Saga Y., Schlossman S. F., Saito H. Differential usage of three exons generates at least five different mRNAs encoding human leukocyte common antigens. J Exp Med. 1987 Nov 1;166(5):1548–1566. doi: 10.1084/jem.166.5.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M. L. The leukocyte common antigen family. Annu Rev Immunol. 1989;7:339–369. doi: 10.1146/annurev.iy.07.040189.002011. [DOI] [PubMed] [Google Scholar]
- Tonks N. K., Charbonneau H., Diltz C. D., Fischer E. H., Walsh K. A. Demonstration that the leukocyte common antigen CD45 is a protein tyrosine phosphatase. Biochemistry. 1988 Nov 29;27(24):8695–8701. doi: 10.1021/bi00424a001. [DOI] [PubMed] [Google Scholar]
- Tonks N. K., Diltz C. D., Fischer E. H. CD45, an integral membrane protein tyrosine phosphatase. Characterization of enzyme activity. J Biol Chem. 1990 Jun 25;265(18):10674–10680. [PubMed] [Google Scholar]
- Woollett G. R., Barclay A. N., Puklavec M., Williams A. F. Molecular and antigenic heterogeneity of the rat leukocyte-common antigen from thymocytes and T and B lymphocytes. Eur J Immunol. 1985 Feb;15(2):168–173. doi: 10.1002/eji.1830150211. [DOI] [PubMed] [Google Scholar]
- de Jong R., Brouwer M., Miedema F., van Lier R. A. Human CD8+ T lymphocytes can be divided into CD45RA+ and CD45RO+ cells with different requirements for activation and differentiation. J Immunol. 1991 Apr 1;146(7):2088–2094. [PubMed] [Google Scholar]