Abstract
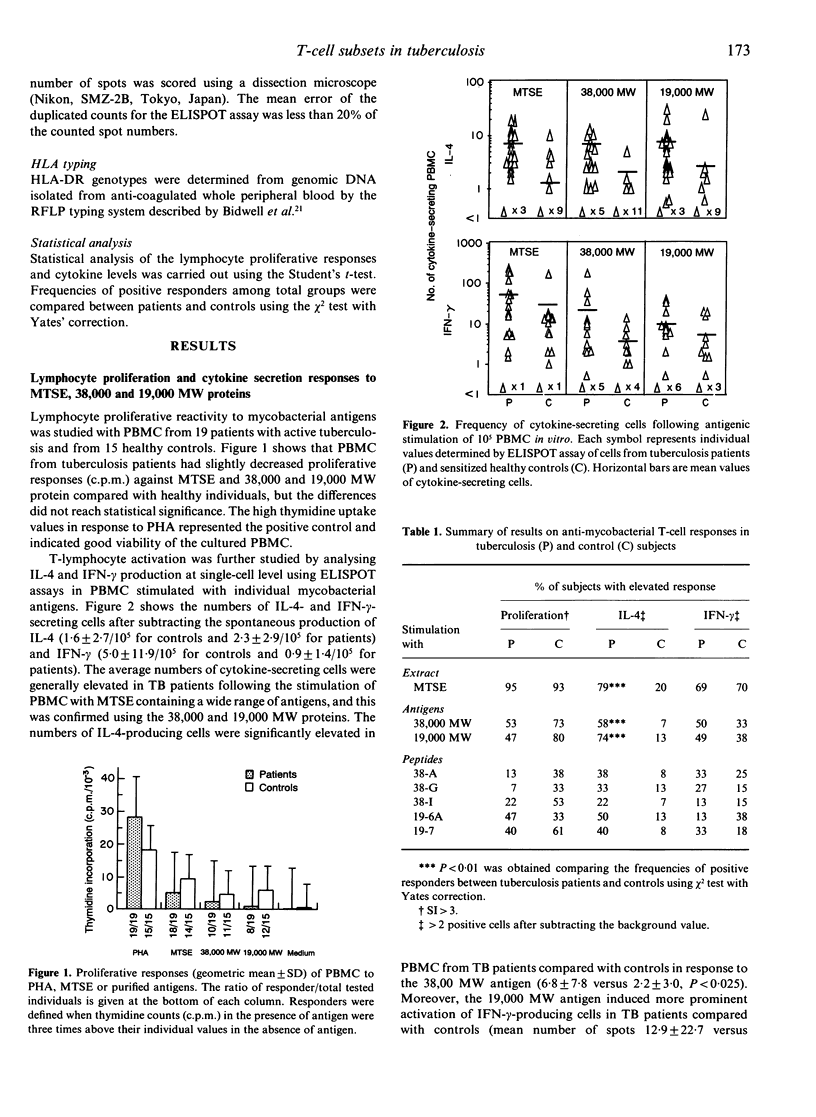
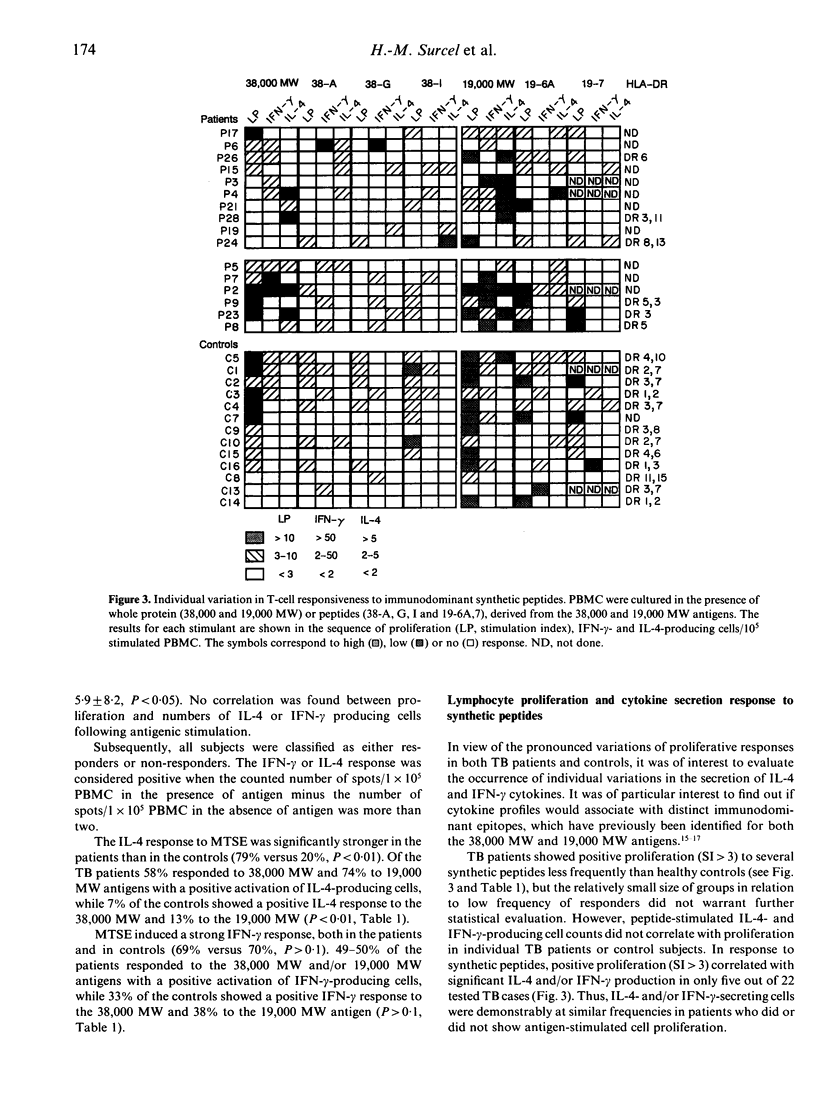
Proliferation and cytokine production profiles by blood mononuclear cells in response to in vitro stimulation with mycobacterial antigens were compared in patients with active tuberculosis and in sensitized healthy controls. Interleukin-4 (IL-4) and interferon-gamma (IFN-gamma) were detected at single-cell level using the ELISPOT assay. Patients showed significantly (P < 0.01) increased numbers of IL-4-secreting cells and decreased thymidine incorporation, but no significant difference in IFN-gamma-producing cells in response to the 38,000 MW or 19,000 MW antigens and their immunodominant peptide epitopes. Pronounced individual variations were found in both patient and control groups, when comparing the responsiveness to the mycobacterial extract, two protein antigens and five synthetic peptides. None of the antigens or peptides tested showed preferential stimulation of either IL-4- or IFN-gamma-secreting T cells, and proliferation was not correlated with either IL-4 or IFN-gamma production. In particular, cytokine responsiveness was of similar frequency in subjects who did or did not show positive proliferation, indicating that the latter test was not fully representative of the active T-cell repertoire. It is concluded that the demonstrated Th2 type of profile in response to two prominent mycobacterial antigens may play a role in the mechanisms of defective host resistance in tuberculosis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abehsira-Amar O., Gibert M., Joliy M., Thèze J., Jankovic D. L. IL-4 plays a dominant role in the differential development of Tho into Th1 and Th2 cells. J Immunol. 1992 Jun 15;148(12):3820–3829. [PubMed] [Google Scholar]
- Appelberg R., Orme I. M., Pinto de Sousa M. I., Silva M. T. In vitro effects of interleukin-4 on interferon-gamma-induced macrophage activation. Immunology. 1992 Aug;76(4):553–559. [PMC free article] [PubMed] [Google Scholar]
- Barnes P. F., Abrams J. S., Lu S., Sieling P. A., Rea T. H., Modlin R. L. Patterns of cytokine production by mycobacterium-reactive human T-cell clones. Infect Immun. 1993 Jan;61(1):197–203. doi: 10.1128/iai.61.1.197-203.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidwell J. L., Bidwell E. A., Savage D. A., Middleton D., Klouda P. T., Bradley B. A. A DNA-RFLP typing system that positively identifies serologically well-defined and ill-defined HLA-DR and DQ alleles, including DRw10. Transplantation. 1988 Mar;45(3):640–646. doi: 10.1097/00007890-198803000-00027. [DOI] [PubMed] [Google Scholar]
- Boom W. H., Wallis R. S., Chervenak K. A. Human Mycobacterium tuberculosis-reactive CD4+ T-cell clones: heterogeneity in antigen recognition, cytokine production, and cytotoxicity for mononuclear phagocytes. Infect Immun. 1991 Aug;59(8):2737–2743. doi: 10.1128/iai.59.8.2737-2743.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothamley G. H., Rudd R., Festenstein F., Ivanyi J. Clinical value of the measurement of Mycobacterium tuberculosis specific antibody in pulmonary tuberculosis. Thorax. 1992 Apr;47(4):270–275. doi: 10.1136/thx.47.4.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigan C. J., Kay A. B. T cells and eosinophils in the pathogenesis of asthma. Immunol Today. 1992 Dec;13(12):501–507. doi: 10.1016/0167-5699(92)90026-4. [DOI] [PubMed] [Google Scholar]
- Del Prete G. F., De Carli M., Mastromauro C., Biagiotti R., Macchia D., Falagiani P., Ricci M., Romagnani S. Purified protein derivative of Mycobacterium tuberculosis and excretory-secretory antigen(s) of Toxocara canis expand in vitro human T cells with stable and opposite (type 1 T helper or type 2 T helper) profile of cytokine production. J Clin Invest. 1991 Jul;88(1):346–350. doi: 10.1172/JCI115300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espitia C., Cervera I., González R., Mancilla R. A 38-kD Mycobacterium tuberculosis antigen associated with infection. Its isolation and serologic evaluation. Clin Exp Immunol. 1989 Sep;77(3):373–377. [PMC free article] [PubMed] [Google Scholar]
- Faith A., Moreno C., Lathigra R., Roman E., Fernandez M., Brett S., Mitchell D. M., Ivanyi J., Rees A. D. Analysis of human T-cell epitopes in the 19,000 MW antigen of Mycobacterium tuberculosis: influence of HLA-DR. Immunology. 1991 Sep;74(1):1–7. [PMC free article] [PubMed] [Google Scholar]
- Garbe T., Harris D., Vordermeier M., Lathigra R., Ivanyi J., Young D. Expression of the Mycobacterium tuberculosis 19-kilodalton antigen in Mycobacterium smegmatis: immunological analysis and evidence of glycosylation. Infect Immun. 1993 Jan;61(1):260–267. doi: 10.1128/iai.61.1.260-267.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haanen J. B., de Waal Malefijt R., Res P. C., Kraakman E. M., Ottenhoff T. H., de Vries R. R., Spits H. Selection of a human T helper type 1-like T cell subset by mycobacteria. J Exp Med. 1991 Sep 1;174(3):583–592. doi: 10.1084/jem.174.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris D. P., Vordermeier H. M., Friscia G., Román E., Surcel H. M., Pasvol G., Moreno C., Ivanyi J. Genetically permissive recognition of adjacent epitopes from the 19-kDa antigen of Mycobacterium tuberculosis by human and murine T cells. J Immunol. 1993 Jun 1;150(11):5041–5050. [PubMed] [Google Scholar]
- Havlir D. V., Wallis R. S., Boom W. H., Daniel T. M., Chervenak K., Ellner J. J. Human immune response to Mycobacterium tuberculosis antigens. Infect Immun. 1991 Feb;59(2):665–670. doi: 10.1128/iai.59.2.665-670.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt J., Coates A. R., Mitchison D. A., Ivanyi J. The use of murine monoclonal antibodies without purification of antigen in the serodiagnosis of tuberculosis. J Immunol Methods. 1982 Dec 17;55(2):205–211. doi: 10.1016/0022-1759(82)90032-1. [DOI] [PubMed] [Google Scholar]
- Huygen K., Van Vooren J. P., Turneer M., Bosmans R., Dierckx P., De Bruyn J. Specific lymphoproliferation, gamma interferon production, and serum immunoglobulin G directed against a purified 32 kDa mycobacterial protein antigen (P32) in patients with active tuberculosis. Scand J Immunol. 1988 Feb;27(2):187–194. doi: 10.1111/j.1365-3083.1988.tb02338.x. [DOI] [PubMed] [Google Scholar]
- Jackett P. S., Bothamley G. H., Batra H. V., Mistry A., Young D. B., Ivanyi J. Specificity of antibodies to immunodominant mycobacterial antigens in pulmonary tuberculosis. J Clin Microbiol. 1988 Nov;26(11):2313–2318. doi: 10.1128/jcm.26.11.2313-2318.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabilan L., Andersson G., Lolli F., Ekre H. P., Olsson T., Troye-Blomberg M. Detection of intracellular expression and secretion of interferon-gamma at the single-cell level after activation of human T cells with tetanus toxoid in vitro. Eur J Immunol. 1990 May;20(5):1085–1089. doi: 10.1002/eji.1830200521. [DOI] [PubMed] [Google Scholar]
- Liew F. Y., Li Y., Severn A., Millott S., Schmidt J., Salter M., Moncada S. A possible novel pathway of regulation by murine T helper type-2 (Th2) cells of a Th1 cell activity via the modulation of the induction of nitric oxide synthase on macrophages. Eur J Immunol. 1991 Oct;21(10):2489–2494. doi: 10.1002/eji.1830211027. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Onwubalili J. K., Scott G. M., Robinson J. A. Deficient immune interferon production in tuberculosis. Clin Exp Immunol. 1985 Feb;59(2):405–413. [PMC free article] [PubMed] [Google Scholar]
- Orme I. M., Miller E. S., Roberts A. D., Furney S. K., Griffin J. P., Dobos K. M., Chi D., Rivoire B., Brennan P. J. T lymphocytes mediating protection and cellular cytolysis during the course of Mycobacterium tuberculosis infection. Evidence for different kinetics and recognition of a wide spectrum of protein antigens. J Immunol. 1992 Jan 1;148(1):189–196. [PubMed] [Google Scholar]
- Pfeiffer C., Murray J., Madri J., Bottomly K. Selective activation of Th1- and Th2-like cells in vivo--response to human collagen IV. Immunol Rev. 1991 Oct;123:65–84. doi: 10.1111/j.1600-065x.1991.tb00606.x. [DOI] [PubMed] [Google Scholar]
- Ribera E., Español T., Martinez-Vazquez J. M., Ocaña I., Encabo G. Lymphocyte proliferation and gamma-interferon production after "in vitro" stimulation with PPD. Differences between tuberculous and nontuberculous pleurisy in patients with positive tuberculin skin test. Chest. 1990 Jun;97(6):1381–1385. doi: 10.1378/chest.97.6.1381. [DOI] [PubMed] [Google Scholar]
- Sher A., Gazzinelli R. T., Oswald I. P., Clerici M., Kullberg M., Pearce E. J., Berzofsky J. A., Mosmann T. R., James S. L., Morse H. C., 3rd Role of T-cell derived cytokines in the downregulation of immune responses in parasitic and retroviral infection. Immunol Rev. 1992 Jun;127:183–204. doi: 10.1111/j.1600-065x.1992.tb01414.x. [DOI] [PubMed] [Google Scholar]
- Street N. E., Schumacher J. H., Fong T. A., Bass H., Fiorentino D. F., Leverah J. A., Mosmann T. R. Heterogeneity of mouse helper T cells. Evidence from bulk cultures and limiting dilution cloning for precursors of Th1 and Th2 cells. J Immunol. 1990 Mar 1;144(5):1629–1639. [PubMed] [Google Scholar]
- Toossi Z., Kleinhenz M. E., Ellner J. J. Defective interleukin 2 production and responsiveness in human pulmonary tuberculosis. J Exp Med. 1986 May 1;163(5):1162–1172. doi: 10.1084/jem.163.5.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troye-Blomberg M., Riley E. M., Kabilan L., Holmberg M., Perlmann H., Andersson U., Heusser C. H., Perlmann P. Production by activated human T cells of interleukin 4 but not interferon-gamma is associated with elevated levels of serum antibodies to activating malaria antigens. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5484–5488. doi: 10.1073/pnas.87.14.5484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vordermeier H. M., Harris D. P., Friscia G., Román E., Surcel H. M., Moreno C., Pasvol G., Ivanyi J. T cell repertoire in tuberculosis: selective anergy to an immunodominant epitope of the 38-kDa antigen in patients with active disease. Eur J Immunol. 1992 Oct;22(10):2631–2637. doi: 10.1002/eji.1830221024. [DOI] [PubMed] [Google Scholar]
- Yamamura M., Uyemura K., Deans R. J., Weinberg K., Rea T. H., Bloom B. R., Modlin R. L. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991 Oct 11;254(5029):277–279. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]
- Young D. B., Garbe T. R. Lipoprotein antigens of Mycobacterium tuberculosis. Res Microbiol. 1991 Jan;142(1):55–65. doi: 10.1016/0923-2508(91)90097-t. [DOI] [PubMed] [Google Scholar]
- van der Pouw-Kraan T., de Jong R., Aarden L. Development of human Th1 and Th2 cytokine responses: the cytokine production profile of T cells is dictated by the primary in vitro stimulus. Eur J Immunol. 1993 Jan;23(1):1–5. doi: 10.1002/eji.1830230102. [DOI] [PubMed] [Google Scholar]


