Abstract
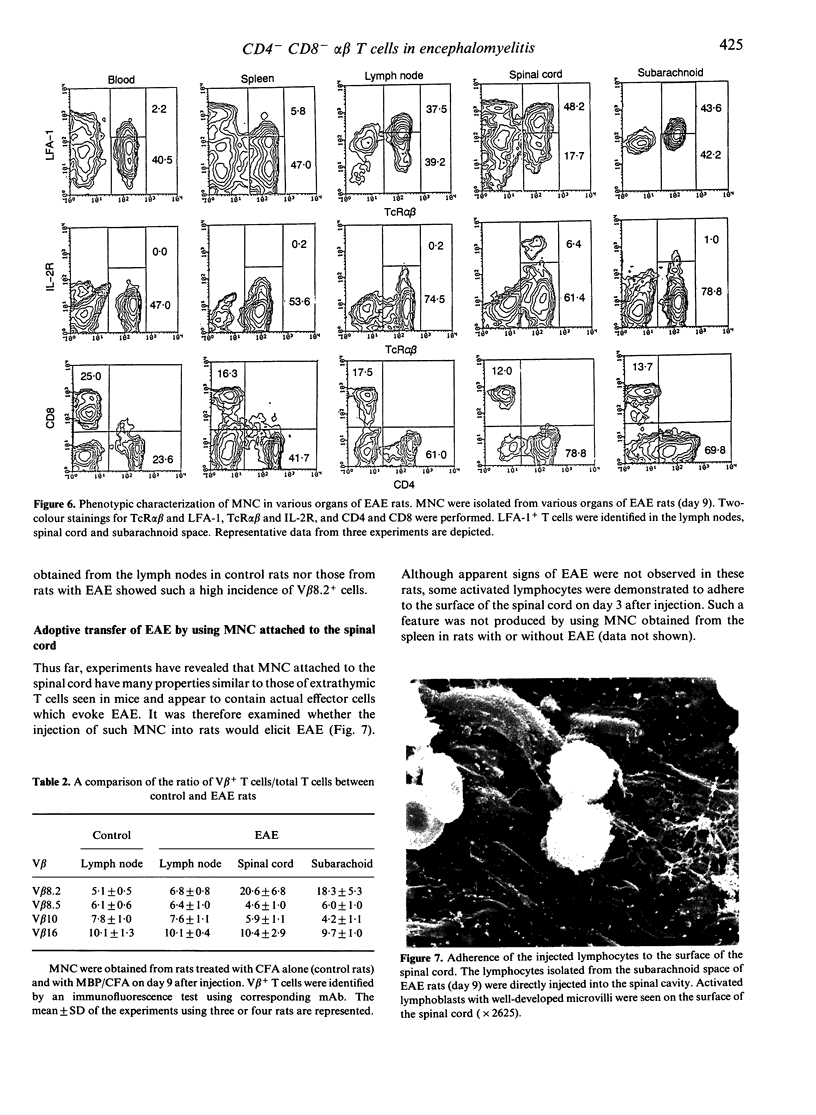
Experimental autoimmune encephalomyelitis (EAE) was induced in Lewis rats to elucidate the origin of effector T cells and the route by which they invade lesions. Since mouse studies have suggested that some autoimmune diseases are induced by extrathymic T cells in the liver, we focused our attention on the properties of mononuclear cells (MNC) isolated from the liver and other organs in rats with EAE. A small but significant proportion of LFA-1+ alpha beta T cells was identified in the liver as early as day 7 after immunization with myelin basic protein (MBP). Such LFA-1+ alpha beta T cells were also abundant among MNC attached to the spinal cord (i.e. subarachnoid space), and MNC infiltrated the spinal cord in rats with EAE (day 12). In electron microscopy, MNC attached to the spinal cord were found to be quite unique in terms of their large cell size with well-developed microvilli. More importantly, they were comprised of a considerably large proportion of double-negative CD4- CD8- T cells as well as single-positive CD4+ T cells. However, the cells which infiltrated the spinal cord were mainly CD4+. The present results raise the possibility that the subarachnoid space might be a major site for the expansion of extrathymic T cells in rats with EAE, and that only a limited population of CD4+ T cells invade the spinal cord directly through the outer layer and elicit EAE.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abo T., Kusumi A., Seki S., Ohteki T., Sugiura K., Masuda T., Rikiishi H., Iiai T., Kumagai K. Activation of extrathymic T cells in the liver and reciprocal inactivation of intrathymic T cells by bacterial stimulation. Cell Immunol. 1992 Jun;142(1):125–136. doi: 10.1016/0008-8749(92)90274-s. [DOI] [PubMed] [Google Scholar]
- Abo T., Ohteki T., Seki S., Koyamada N., Yoshikai Y., Masuda T., Rikiishi H., Kumagai K. The appearance of T cells bearing self-reactive T cell receptor in the livers of mice injected with bacteria. J Exp Med. 1991 Aug 1;174(2):417–424. doi: 10.1084/jem.174.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abo T., Sugawara S., Seki S., Fujii M., Rikiishi H., Takeda K., Kumagai K. Induction of human TCR gamma delta + and TCR gamma delta-CD2+CD3- double negative lymphocytes by bacterial stimulation. Int Immunol. 1990;2(8):775–785. doi: 10.1093/intimm/2.8.775. [DOI] [PubMed] [Google Scholar]
- Fry A. M., Jones L. A., Kruisbeek A. M., Matis L. A. Thymic requirement for clonal deletion during T cell development. Science. 1989 Nov 24;246(4933):1044–1046. doi: 10.1126/science.2511630. [DOI] [PubMed] [Google Scholar]
- Guy-Grand D., Cerf-Bensussan N., Malissen B., Malassis-Seris M., Briottet C., Vassalli P. Two gut intraepithelial CD8+ lymphocyte populations with different T cell receptors: a role for the gut epithelium in T cell differentiation. J Exp Med. 1991 Feb 1;173(2):471–481. doi: 10.1084/jem.173.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy-Grand D., Malassis-Seris M., Briottet C., Vassalli P. Cytotoxic differentiation of mouse gut thymodependent and independent intraepithelial T lymphocytes is induced locally. Correlation between functional assays, presence of perforin and granzyme transcripts, and cytoplasmic granules. J Exp Med. 1991 Jun 1;173(6):1549–1552. doi: 10.1084/jem.173.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanawa H., Tsuchida M., Matsumoto Y., Watanabe H., Abo T., Sekikawa H., Kodama M., Zhang S., Izumi T., Shibata A. Characterization of T cells infiltrating the heart in rats with experimental autoimmune myocarditis. Their similarity to extrathymic T cells in mice and the site of proliferation. J Immunol. 1993 Jun 15;150(12):5682–5695. [PubMed] [Google Scholar]
- Hodes R. J., Sharrow S. O., Solomon A. Failure of T cell receptor V beta negative selection in an athymic environment. Science. 1989 Nov 24;246(4933):1041–1044. doi: 10.1126/science.2587987. [DOI] [PubMed] [Google Scholar]
- Iiai T., Watanabe H., Seki S., Sugiura K., Hirokawa K., Utsuyama M., Takahashi-Iwanaga H., Iwanaga T., Ohteki T., Abo T. Ontogeny and development of extrathymic T cells in mouse liver. Immunology. 1992 Dec;77(4):556–563. [PMC free article] [PubMed] [Google Scholar]
- Kimura M., Watanabe H., Ohtsuka K., Iiai T., Tsuchida M., Sato S., Abo T. Radioresistance of intermediate TCR cells and their localization in the body of mice revealed by irradiation. Microbiol Immunol. 1993;37(8):641–652. doi: 10.1111/j.1348-0421.1993.tb01687.x. [DOI] [PubMed] [Google Scholar]
- Masuda T., Ohteki T., Abo T., Seki S., Nose S., Nagura H., Kumagai K. Expansion of the population of double negative CD4-8- T alpha beta-cells in the liver is a common feature of autoimmune mice. J Immunol. 1991 Nov 1;147(9):2907–2912. [PubMed] [Google Scholar]
- Matsumoto Y., Hara N., Tanaka R., Fujiwara M. Immunohistochemical analysis of the rat central nervous system during experimental allergic encephalomyelitis, with special reference to Ia-positive cells with dendritic morphology. J Immunol. 1986 May 15;136(10):3668–3676. [PubMed] [Google Scholar]
- Matsumoto Y., Kawai K., Fujiwara M. Analysis of the T cell repertoire for myelin basic protein in thymus-grafted and other types of chimera: evidence that major histocompatibility complex molecules on accessory cells rather than T cell specificity mainly regulate susceptibility to autoimmune encephalomyelitis. Eur J Immunol. 1990 Sep;20(9):2119–2126. doi: 10.1002/eji.1830200934. [DOI] [PubMed] [Google Scholar]
- Ohteki T., Abo T., Seki S., Kobata T., Yagita H., Okumura K., Kumagai K. Predominant appearance of gamma/delta T lymphocytes in the liver of mice after birth. Eur J Immunol. 1991 Jul;21(7):1733–1740. doi: 10.1002/eji.1830210722. [DOI] [PubMed] [Google Scholar]
- Ohteki T., Okuyama R., Seki S., Abo T., Sugiura K., Kusumi A., Ohmori T., Watanabe H., Kumagai K. Age-dependent increase of extrathymic T cells in the liver and their appearance in the periphery of older mice. J Immunol. 1992 Sep 1;149(5):1562–1570. [PubMed] [Google Scholar]
- Ohteki T., Seki S., Abo T., Kumagai K. Liver is a possible site for the proliferation of abnormal CD3+4-8- double-negative lymphocytes in autoimmune MRL-lpr/lpr mice. J Exp Med. 1990 Jul 1;172(1):7–12. doi: 10.1084/jem.172.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuyama R., Abo T., Seki S., Ohteki T., Sugiura K., Kusumi A., Kumagai K. Estrogen administration activates extrathymic T cell differentiation in the liver. J Exp Med. 1992 Mar 1;175(3):661–669. doi: 10.1084/jem.175.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki S., Abo T., Masuda T., Ohteki T., Kanno A., Takeda K., Rikiishi H., Nagura H., Kumagai K. Identification of activated T cell receptor gamma delta lymphocytes in the liver of tumor-bearing hosts. J Clin Invest. 1990 Aug;86(2):409–415. doi: 10.1172/JCI114726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki S., Abo T., Ohteki T., Sugiura K., Kumagai K. Unusual alpha beta-T cells expanded in autoimmune lpr mice are probably a counterpart of normal T cells in the liver. J Immunol. 1991 Aug 15;147(4):1214–1221. [PubMed] [Google Scholar]
- Seki S., Abo T., Sakihara H., Sugiura K., Kanno A., Rikiishi H., Masuda T., Kumagai K. An appropriate in vitro culture condition for the induction of human TCR gamma delta + cells by heat-killed bacteria. J Immunol Methods. 1991 May 17;139(1):31–40. doi: 10.1016/0022-1759(91)90348-j. [DOI] [PubMed] [Google Scholar]
- Seki S., Abo T., Sugiura K., Ohteki T., Kobata T., Yagita H., Okumura K., Rikiishi H., Masuda T., Kumagai K. Reciprocal T cell responses in the liver and thymus of mice injected with syngeneic tumor cells. Cell Immunol. 1991 Oct 1;137(1):46–60. doi: 10.1016/0008-8749(91)90055-g. [DOI] [PubMed] [Google Scholar]
- Watanabe H., Ohtsuka K., Kimura M., Ikarashi Y., Ohmori K., Kusumi A., Ohteki T., Seki S., Abo T. Details of an isolation method for hepatic lymphocytes in mice. J Immunol Methods. 1992 Feb 5;146(2):145–154. doi: 10.1016/0022-1759(92)90223-g. [DOI] [PubMed] [Google Scholar]