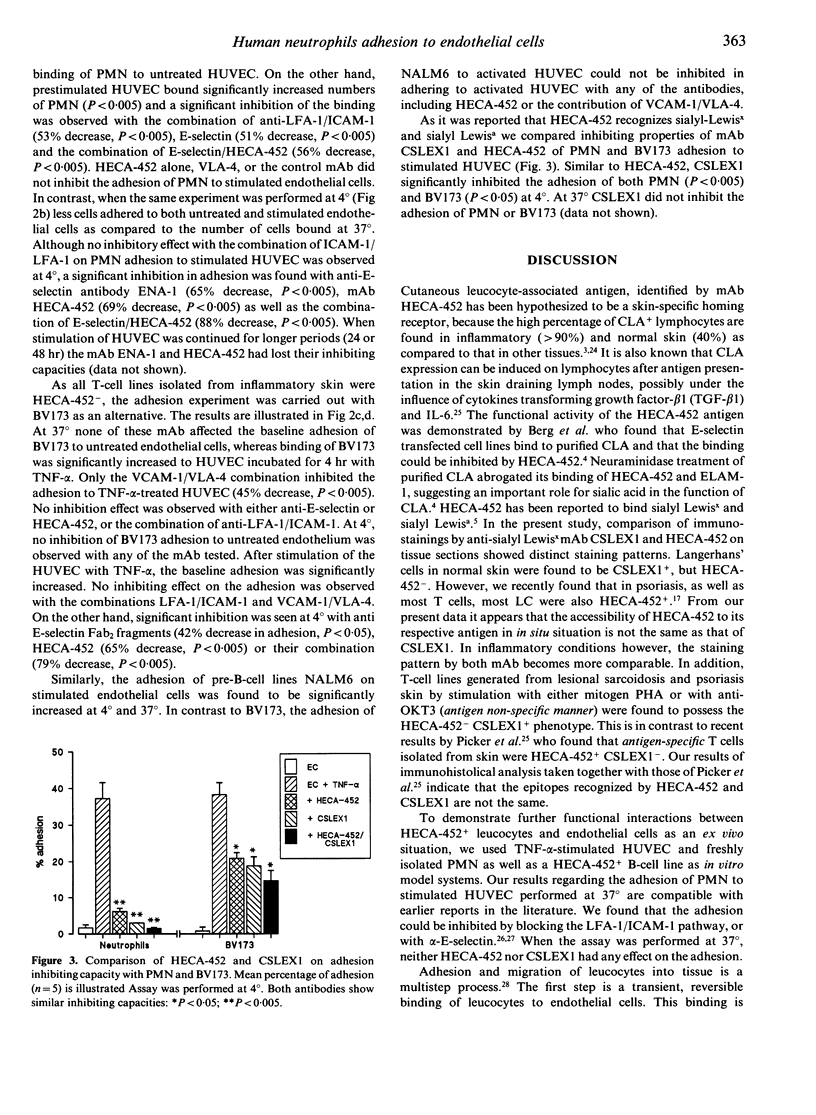
Abstract
The migration of leucocytes into tissues is a process mediated by leucocyte endothelial interactions, in which adhesion receptors play a crucial role. Recently, it was found that 80-90% of T cells in inflammatory skin diseases were reactive to the monoclonal antibody (mAb) HECA-452+ in contrast to inflamed non-cutaneous tissues. It was suggested that the HECA-452 antigen is a homing receptor for lymphocyte migration into skin. This receptor was designated cutaneous lymphocyte-associated antigen or CLA and subsequently identified as a group of related sugar moieties. E-selectin, formerly known as ELAM-1 expressed by the endothelium has been implicated to be a counter-receptor for CLA. In this study, we investigated the adhesion of HECA-452+ leucocytes, i.e. freshly isolated neutrophils and B-cell line BV173 to tumour necrosis factor-alpha (TNF-alpha)-stimulated (E-selectin+) endothelial cells. We found that the adhesion of these cells could be inhibited significantly by mAb HECA-452, in a similar fashion to CSLEX1, a mAb specific for E-selectin ligand sialyl Lewisx. This inhibiting effect of both mAb on the adhesion of polymorphonuclear leucocytes (PMN) and BV173 could only be demonstrated when the assay was performed at 4 degrees, but not at 37 degrees. Furthermore, using immunohistochemical analysis we found that the mAb HECA-452-reactive epitope is different from that recognized by CSLEX1. The present results give direct evidence that the antigen recognized by HECA-452 is involved in the adhesion of leucocytes to endothelial cells, although this antigenic epitope is different from that reactive to CSLEX1.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg E. L., Robinson M. K., Mansson O., Butcher E. C., Magnani J. L. A carbohydrate domain common to both sialyl Le(a) and sialyl Le(X) is recognized by the endothelial cell leukocyte adhesion molecule ELAM-1. J Biol Chem. 1991 Aug 15;266(23):14869–14872. [PubMed] [Google Scholar]
- Berg E. L., Yoshino T., Rott L. S., Robinson M. K., Warnock R. A., Kishimoto T. K., Picker L. J., Butcher E. C. The cutaneous lymphocyte antigen is a skin lymphocyte homing receptor for the vascular lectin endothelial cell-leukocyte adhesion molecule 1. J Exp Med. 1991 Dec 1;174(6):1461–1466. doi: 10.1084/jem.174.6.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Mendrick D. L., Cotran R. S., Gimbrone M. A., Jr Identification of an inducible endothelial-leukocyte adhesion molecule. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9238–9242. doi: 10.1073/pnas.84.24.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochner B. S., Luscinskas F. W., Gimbrone M. A., Jr, Newman W., Sterbinsky S. A., Derse-Anthony C. P., Klunk D., Schleimer R. P. Adhesion of human basophils, eosinophils, and neutrophils to interleukin 1-activated human vascular endothelial cells: contributions of endothelial cell adhesion molecules. J Exp Med. 1991 Jun 1;173(6):1553–1557. doi: 10.1084/jem.173.6.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos J. D., de Boer O. J., Tibosch E., Das P. K., Pals S. T. Skin-homing T lymphocytes: detection of cutaneous lymphocyte-associated antigen (CLA) by HECA-452 in normal human skin. Arch Dermatol Res. 1993;285(4):179–183. doi: 10.1007/BF00372006. [DOI] [PubMed] [Google Scholar]
- Butcher E. C. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991 Dec 20;67(6):1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- Carlos T. M., Schwartz B. R., Kovach N. L., Yee E., Rosa M., Osborn L., Chi-Rosso G., Newman B., Lobb R., Rosso M. Vascular cell adhesion molecule-1 mediates lymphocyte adherence to cytokine-activated cultured human endothelial cells. Blood. 1990 Sep 1;76(5):965–970. [PubMed] [Google Scholar]
- Duijvestijn A. M., Horst E., Pals S. T., Rouse B. N., Steere A. C., Picker L. J., Meijer C. J., Butcher E. C. High endothelial differentiation in human lymphoid and inflammatory tissues defined by monoclonal antibody HECA-452. Am J Pathol. 1988 Jan;130(1):147–155. [PMC free article] [PubMed] [Google Scholar]
- Fukushima K., Hirota M., Terasaki P. I., Wakisaka A., Togashi H., Chia D., Suyama N., Fukushi Y., Nudelman E., Hakomori S. Characterization of sialosylated Lewisx as a new tumor-associated antigen. Cancer Res. 1984 Nov;44(11):5279–5285. [PubMed] [Google Scholar]
- Groves R. W., Allen M. H., Barker J. N., Haskard D. O., MacDonald D. M. Endothelial leucocyte adhesion molecule-1 (ELAM-1) expression in cutaneous inflammation. Br J Dermatol. 1991 Feb;124(2):117–123. doi: 10.1111/j.1365-2133.1991.tb00419.x. [DOI] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijpers T. W., Hakkert B. C., Hoogerwerf M., Leeuwenberg J. F., Roos D. Role of endothelial leukocyte adhesion molecule-1 and platelet-activating factor in neutrophil adherence to IL-1-prestimulated endothelial cells. Endothelial leukocyte adhesion molecule-1-mediated CD18 activation. J Immunol. 1991 Aug 15;147(4):1369–1376. [PubMed] [Google Scholar]
- Larkin M., Ahern T. J., Stoll M. S., Shaffer M., Sako D., O'Brien J., Yuen C. T., Lawson A. M., Childs R. A., Barone K. M. Spectrum of sialylated and nonsialylated fuco-oligosaccharides bound by the endothelial-leukocyte adhesion molecule E-selectin. Dependence of the carbohydrate binding activity on E-selectin density. J Biol Chem. 1992 Jul 5;267(19):13661–13668. [PubMed] [Google Scholar]
- Leeuwenberg J. F., Jeunhomme T. M., Buurman W. A. Induction of an activation antigen on human endothelial cells in vitro. Eur J Immunol. 1989 Apr;19(4):715–720. doi: 10.1002/eji.1830190422. [DOI] [PubMed] [Google Scholar]
- Leeuwenberg J. F., Jeunhomme T. M., Buurman W. A. Role of ELAM-1 in adhesion of monocytes to activated human endothelial cells. Scand J Immunol. 1992 Mar;35(3):335–341. doi: 10.1111/j.1365-3083.1992.tb02866.x. [DOI] [PubMed] [Google Scholar]
- Lo S. K., Detmers P. A., Levin S. M., Wright S. D. Transient adhesion of neutrophils to endothelium. J Exp Med. 1989 May 1;169(5):1779–1793. doi: 10.1084/jem.169.5.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay C. R., Marston W. L., Dudler L., Spertini O., Tedder T. F., Hein W. R. Tissue-specific migration pathways by phenotypically distinct subpopulations of memory T cells. Eur J Immunol. 1992 Apr;22(4):887–895. doi: 10.1002/eji.1830220402. [DOI] [PubMed] [Google Scholar]
- Moore K. L., Varki A., McEver R. P. GMP-140 binds to a glycoprotein receptor on human neutrophils: evidence for a lectin-like interaction. J Cell Biol. 1991 Feb;112(3):491–499. doi: 10.1083/jcb.112.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noorduyn L. A., Beljaards R. C., Pals S. T., van Heerde P., Radaszkiewicz T., Willemze R., Meijer C. J. Differential expression of the HECA-452 antigen (cutaneous lymphocyte associated antigen, CLA) in cutaneous and non-cutaneous T-cell lymphomas. Histopathology. 1992 Jul;21(1):59–64. doi: 10.1111/j.1365-2559.1992.tb00343.x. [DOI] [PubMed] [Google Scholar]
- Phillips M. L., Nudelman E., Gaeta F. C., Perez M., Singhal A. K., Hakomori S., Paulson J. C. ELAM-1 mediates cell adhesion by recognition of a carbohydrate ligand, sialyl-Lex. Science. 1990 Nov 23;250(4984):1130–1132. doi: 10.1126/science.1701274. [DOI] [PubMed] [Google Scholar]
- Phillips M. L., Nudelman E., Gaeta F. C., Perez M., Singhal A. K., Hakomori S., Paulson J. C. ELAM-1 mediates cell adhesion by recognition of a carbohydrate ligand, sialyl-Lex. Science. 1990 Nov 23;250(4984):1130–1132. doi: 10.1126/science.1701274. [DOI] [PubMed] [Google Scholar]
- Picker L. J., Butcher E. C. Physiological and molecular mechanisms of lymphocyte homing. Annu Rev Immunol. 1992;10:561–591. doi: 10.1146/annurev.iy.10.040192.003021. [DOI] [PubMed] [Google Scholar]
- Picker L. J., Michie S. A., Rott L. S., Butcher E. C. A unique phenotype of skin-associated lymphocytes in humans. Preferential expression of the HECA-452 epitope by benign and malignant T cells at cutaneous sites. Am J Pathol. 1990 May;136(5):1053–1068. [PMC free article] [PubMed] [Google Scholar]
- Picker L. J., Treer J. R., Ferguson-Darnell B., Collins P. A., Bergstresser P. R., Terstappen L. W. Control of lymphocyte recirculation in man. II. Differential regulation of the cutaneous lymphocyte-associated antigen, a tissue-selective homing receptor for skin-homing T cells. J Immunol. 1993 Feb 1;150(3):1122–1136. [PubMed] [Google Scholar]
- Picker L. J., Warnock R. A., Burns A. R., Doerschuk C. M., Berg E. L., Butcher E. C. The neutrophil selectin LECAM-1 presents carbohydrate ligands to the vascular selectins ELAM-1 and GMP-140. Cell. 1991 Sep 6;66(5):921–933. doi: 10.1016/0092-8674(91)90438-5. [DOI] [PubMed] [Google Scholar]
- Sánchez-Madrid F., De Landázuri M. O., Morago G., Cebrián M., Acevedo A., Bernabeu C. VLA-3: a novel polypeptide association within the VLA molecular complex: cell distribution and biochemical characterization. Eur J Immunol. 1986 Nov;16(11):1343–1349. doi: 10.1002/eji.1830161106. [DOI] [PubMed] [Google Scholar]
- Yuen C. T., Lawson A. M., Chai W., Larkin M., Stoll M. S., Stuart A. C., Sullivan F. X., Ahern T. J., Feizi T. Novel sulfated ligands for the cell adhesion molecule E-selectin revealed by the neoglycolipid technology among O-linked oligosaccharides on an ovarian cystadenoma glycoprotein. Biochemistry. 1992 Sep 29;31(38):9126–9131. doi: 10.1021/bi00153a003. [DOI] [PubMed] [Google Scholar]
- van Dinther-Janssen A. C., Pals S. T., Scheper R., Breedveld F., Meijer C. J. Dendritic cells and high endothelial venules in the rheumatoid synovial membrane. J Rheumatol. 1990 Jan;17(1):11–17. [PubMed] [Google Scholar]



