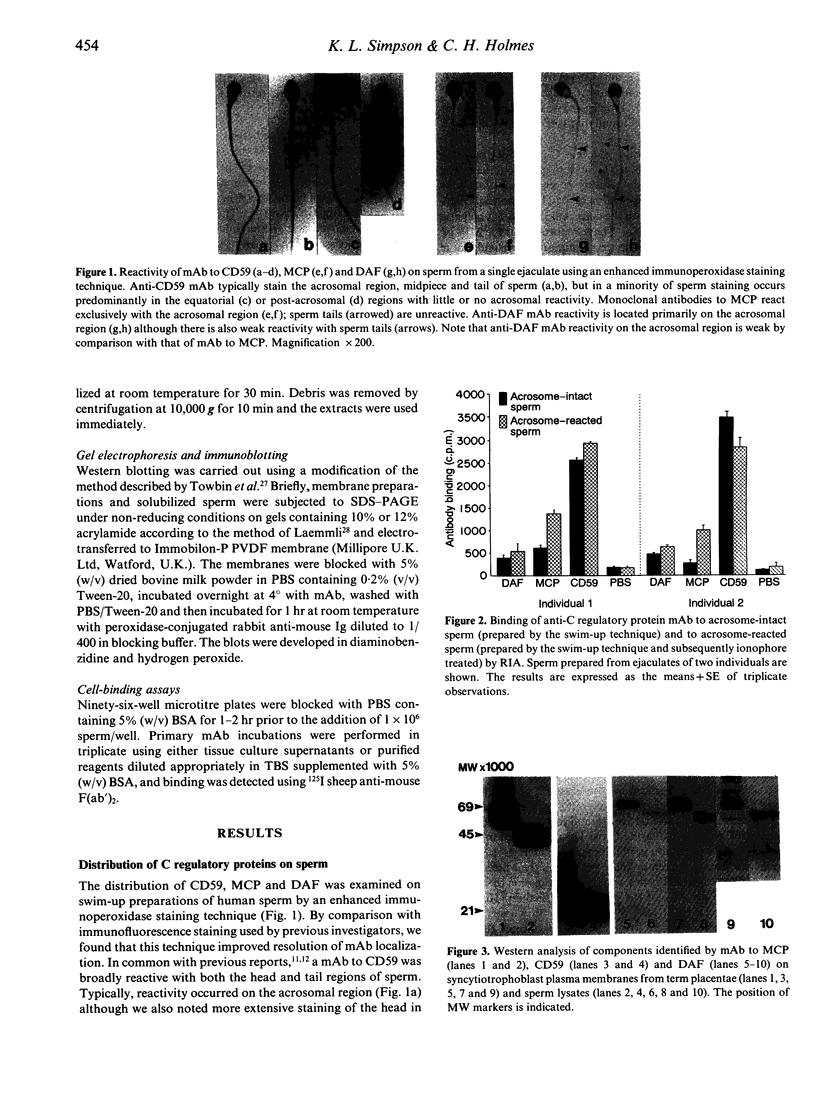
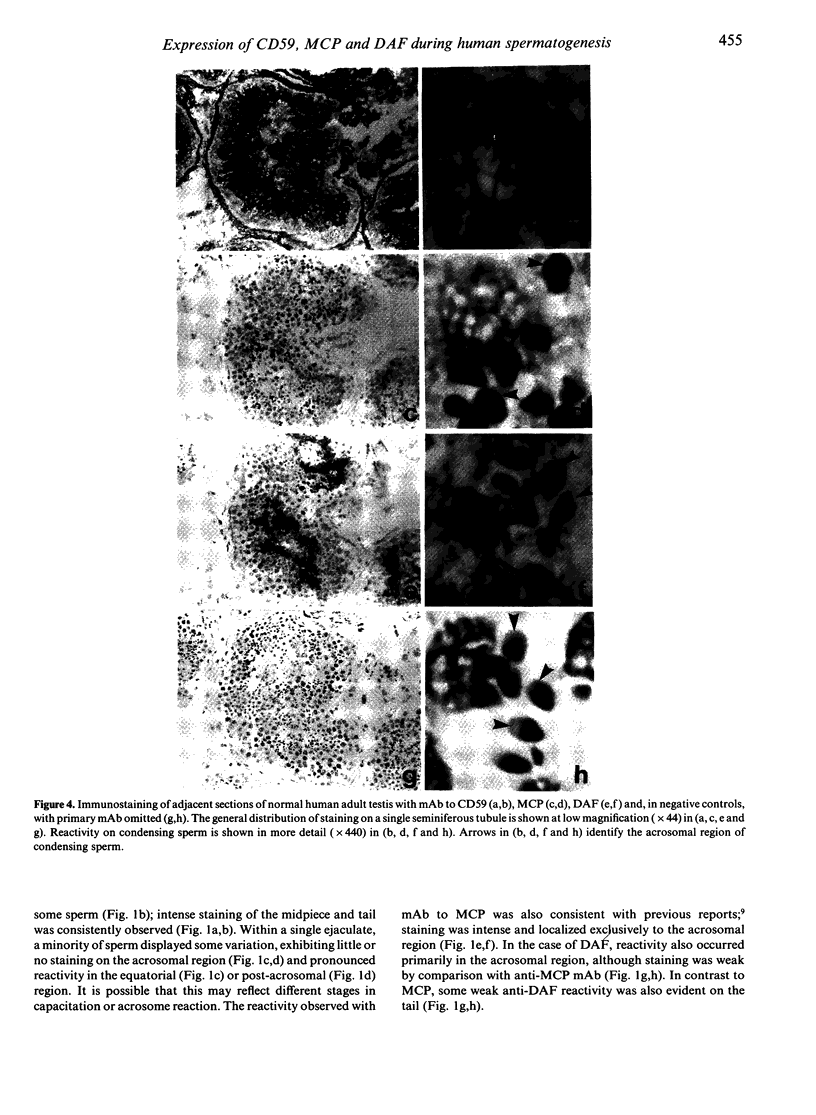
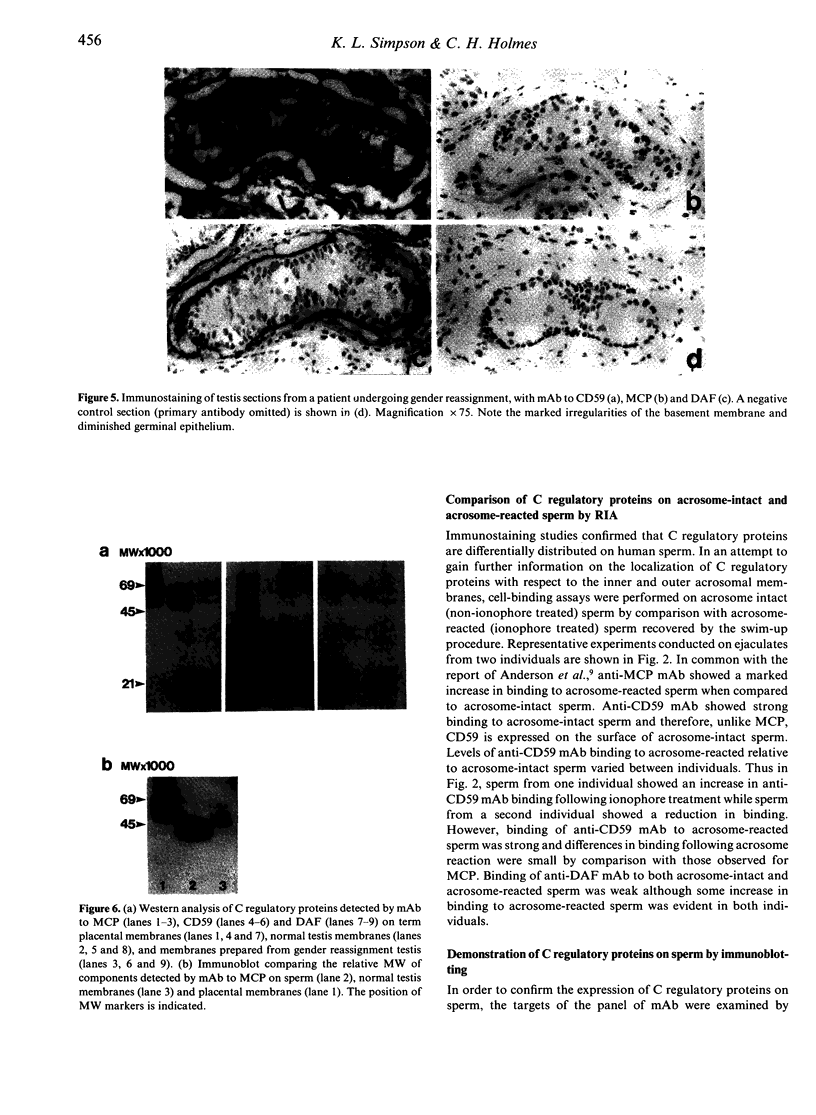
Abstract
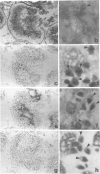
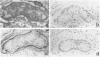
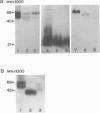
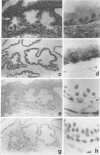
We have examined the distribution of the complement (C) regulatory proteins CD59, membrane cofactor protein (MCP) and decay-accelerating factor (DAF) on mature sperm and compared expression of these proteins in parallel both during spermatogenesis and in the prostate. Enhanced immunoperoxidase staining and radioimmunoassay confirmed that C regulators are differentially expressed on sperm; CD59 was strongly expressed on the surface of acrosome intact sperm while MCP and DAF appear to be located primarily on the inner acrosomal membrane. While the MW of CD59 on sperm is typical of other systems, we confirm that in addition to a novel 40,000-46,000 MW MCP protein, sperm also express a novel 55,000 MW DAF product. Examination of normal testis by immunostaining revealed that although C regulators are differentially expressed within the germinal epithelium, all three proteins were present on the acrosomal region of condensing spermatids. We show that novel, low MW forms of MCP and DAF are expressed in normal testis membranes but are absent from testis membranes obtained from patients undergoing gender reassignment surgery in whom the germinal epithelium is diminished. Novel MW C3 convertase regulators are therefore associated with differentiating germinal epithelium. Typical CD59 components were also present on normal testis membranes confirming that CD59 is acquired during spermatogenesis. We demonstrate that the prostatic epithelium, in addition to MCP, expresses CD59 but not DAF. By comparison with CD59, therefore, our studies suggest that DAF may be acquired only in the testis. Overall, our data suggest that, on leaving the testis, sperm express the repertoire of C regulators required for protection from C during their transit through the male and female reproductive tracts.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. J., Michaelson J. S., Johnson P. M. Trophoblast/leukocyte-common antigen is expressed by human testicular germ cells and appears on the surface of acrosome-reacted sperm. Biol Reprod. 1989 Aug;41(2):285–293. doi: 10.1095/biolreprod41.2.285. [DOI] [PubMed] [Google Scholar]
- Bozas S. E., Kirszbaum L., Sparrow R. L., Walker I. D. Several vascular complement inhibitors are present on human sperm. Biol Reprod. 1993 Mar;48(3):503–511. doi: 10.1095/biolreprod48.3.503. [DOI] [PubMed] [Google Scholar]
- Campbell R. D., Law S. K., Reid K. B., Sim R. B. Structure, organization, and regulation of the complement genes. Annu Rev Immunol. 1988;6:161–195. doi: 10.1146/annurev.iy.06.040188.001113. [DOI] [PubMed] [Google Scholar]
- Cervoni F., Fenichel P., Akhoundi C., Hsi B. L., Rossi B. Characterization of a cDNA clone coding for human testis membrane cofactor protein (MCP, CD46). Mol Reprod Dev. 1993 Jan;34(1):107–113. doi: 10.1002/mrd.1080340117. [DOI] [PubMed] [Google Scholar]
- Cervoni F., Oglesby T. J., Adams E. M., Milesifluet C., Nickells M., Fenichel P., Atkinson J. P., Hsi B. L. Identification and characterization of membrane cofactor protein of human spermatozoa. J Immunol. 1992 Mar 1;148(5):1431–1437. [PubMed] [Google Scholar]
- Cervoni F., Oglesby T. J., Fénichel P., Dohr G., Rossi B., Atkinson J. P., Hsi B. L. Expression of decay-accelerating factor (CD55) of the complement system on human spermatozoa. J Immunol. 1993 Jul 15;151(2):939–948. [PubMed] [Google Scholar]
- Fletcher A., Bryant J. A., Gardner B., Judson P. A., Spring F. A., Parsons S. F., Mallinson G., Anstee D. J. New monoclonal antibodies in CD59: use for the analysis of peripheral blood cells from paroxysmal nocturnal haemoglobinuria (PNH) patients and for the quantitation of CD59 on normal and decay accelerating factor (DAF)-deficient erythrocytes. Immunology. 1992 Mar;75(3):507–512. [PMC free article] [PubMed] [Google Scholar]
- Ford W. C., McLaughlin E. A., Prior S. M., Rees J. M., Wardle P. G., Hull M. G. The yield, motility and performance in the hamster egg test of human spermatozoa prepared from cryopreserved semen by four different methods. Hum Reprod. 1992 May;7(5):654–659. doi: 10.1093/oxfordjournals.humrep.a137714. [DOI] [PubMed] [Google Scholar]
- Hara T., Kuriyama S., Kiyohara H., Nagase Y., Matsumoto M., Seya T. Soluble forms of membrane cofactor protein (CD46, MCP) are present in plasma, tears, and seminal fluid in normal subjects. Clin Exp Immunol. 1992 Sep;89(3):490–494. doi: 10.1111/j.1365-2249.1992.tb06986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes C. H., Simpson K. L., Okada H., Okada N., Wainwright S. D., Purcell D. F., Houlihan J. M. Complement regulatory proteins at the feto-maternal interface during human placental development: distribution of CD59 by comparison with membrane cofactor protein (CD46) and decay accelerating factor (CD55). Eur J Immunol. 1992 Jun;22(6):1579–1585. doi: 10.1002/eji.1830220635. [DOI] [PubMed] [Google Scholar]
- Holmes C. H., Simpson K. L., Wainwright S. D., Tate C. G., Houlihan J. M., Sawyer I. H., Rogers I. P., Spring F. A., Anstee D. J., Tanner M. J. Preferential expression of the complement regulatory protein decay accelerating factor at the fetomaternal interface during human pregnancy. J Immunol. 1990 Apr 15;144(8):3099–3105. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lublin D. M., Atkinson J. P. Decay-accelerating factor: biochemistry, molecular biology, and function. Annu Rev Immunol. 1989;7:35–58. doi: 10.1146/annurev.iy.07.040189.000343. [DOI] [PubMed] [Google Scholar]
- Medof M. E., Walter E. I., Rutgers J. L., Knowles D. M., Nussenzweig V. Identification of the complement decay-accelerating factor (DAF) on epithelium and glandular cells and in body fluids. J Exp Med. 1987 Mar 1;165(3):848–864. doi: 10.1084/jem.165.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meri S., Morgan B. P., Davies A., Daniels R. H., Olavesen M. G., Waldmann H., Lachmann P. J. Human protectin (CD59), an 18,000-20,000 MW complement lysis restricting factor, inhibits C5b-8 catalysed insertion of C9 into lipid bilayers. Immunology. 1990 Sep;71(1):1–9. [PMC free article] [PubMed] [Google Scholar]
- Meri S., Waldmann H., Lachmann P. J. Distribution of protectin (CD59), a complement membrane attack inhibitor, in normal human tissues. Lab Invest. 1991 Nov;65(5):532–537. [PubMed] [Google Scholar]
- Ninomiya H., Stewart B. H., Rollins S. A., Zhao J., Bothwell A. L., Sims P. J. Contribution of the N-linked carbohydrate of erythrocyte antigen CD59 to its complement-inhibitory activity. J Biol Chem. 1992 Apr 25;267(12):8404–8410. [PubMed] [Google Scholar]
- Nose M., Katoh M., Okada N., Kyogoku M., Okada H. Tissue distribution of HRF20, a novel factor preventing the membrane attack of homologous complement, and its predominant expression on endothelial cells in vivo. Immunology. 1990 Jun;70(2):145–149. [PMC free article] [PubMed] [Google Scholar]
- Perricone R., Pasetto N., De Carolis C., Vaquero E., Piccione E., Baschieri L., Fontana L. Functionally active complement is present in human ovarian follicular fluid and can be activated by seminal plasma. Clin Exp Immunol. 1992 Jul;89(1):154–157. doi: 10.1111/j.1365-2249.1992.tb06895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesando J. M., Stucki M., Hoffman P. Altered expression of surface antigens with appearance of HLA class II molecules on a malignant human B-cell line. Hum Immunol. 1987 Aug;19(4):235–243. doi: 10.1016/0198-8859(87)90041-3. [DOI] [PubMed] [Google Scholar]
- Price R. J., Boettcher B. The presence of complement in human cervical mucus and its possible relevance to infertility in women with complement-dependent sperm-immobilizing antibodies. Fertil Steril. 1979 Jul;32(1):61–66. doi: 10.1016/s0015-0282(16)44117-8. [DOI] [PubMed] [Google Scholar]
- Rollins S. A., Sims P. J. The complement-inhibitory activity of CD59 resides in its capacity to block incorporation of C9 into membrane C5b-9. J Immunol. 1990 May 1;144(9):3478–3483. [PubMed] [Google Scholar]
- Rollins S. A., Zhao J., Ninomiya H., Sims P. J. Inhibition of homologous complement by CD59 is mediated by a species-selective recognition conferred through binding to C8 within C5b-8 or C9 within C5b-9. J Immunol. 1991 Apr 1;146(7):2345–2351. [PubMed] [Google Scholar]
- Rooney I. A., Atkinson J. P., Krul E. S., Schonfeld G., Polakoski K., Saffitz J. E., Morgan B. P. Physiologic relevance of the membrane attack complex inhibitory protein CD59 in human seminal plasma: CD59 is present on extracellular organelles (prostasomes), binds cell membranes, and inhibits complement-mediated lysis. J Exp Med. 1993 May 1;177(5):1409–1420. doi: 10.1084/jem.177.5.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney I. A., Davies A., Morgan B. P. Membrane attack complex (MAC)-mediated damage to spermatozoa: protection of the cells by the presence on their membranes of MAC inhibitory proteins. Immunology. 1992 Mar;75(3):499–506. [PMC free article] [PubMed] [Google Scholar]
- Seya T., Atkinson J. P. Functional properties of membrane cofactor protein of complement. Biochem J. 1989 Dec 1;264(2):581–588. doi: 10.1042/bj2640581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seya T., Hara T., Matsumoto M., Kiyohara H., Nakanishi I., Kinouchi T., Okabe M., Shimizu A., Akedo H. Membrane cofactor protein (MCP, CD46) in seminal plasma and on spermatozoa in normal and "sterile" subjects. Eur J Immunol. 1993 Jun;23(6):1322–1327. doi: 10.1002/eji.1830230620. [DOI] [PubMed] [Google Scholar]
- Simpson K. L., Houlihan J. M., Holmes C. H. Complement regulatory proteins in early human fetal life: CD59, membrane co-factor protein (MCP) and decay-accelerating factor (DAF) are differentially expressed in the developing liver. Immunology. 1993 Oct;80(2):183–190. [PMC free article] [PubMed] [Google Scholar]
- Smith N. C., Brush M. G., Luckett S. Preparation of human placental villous surface membrane. Nature. 1974 Nov 22;252(5481):302–303. doi: 10.1038/252302b0. [DOI] [PubMed] [Google Scholar]
- Spring F. A., Judson P. A., Daniels G. L., Parsons S. F., Mallinson G., Anstee D. J. A human cell-surface glycoprotein that carries Cromer-related blood group antigens on erythrocytes and is also expressed on leucocytes and platelets. Immunology. 1987 Oct;62(2):307–313. [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainwright S. D., Holmes C. H. Distribution of Fc gamma receptors on trophoblast during human placental development: an immunohistochemical and immunoblotting study. Immunology. 1993 Nov;80(3):343–351. [PMC free article] [PubMed] [Google Scholar]
- de Kretser D. M., Kerr J. B., Paulsen C. A. The peritubular tissue in the normal and pathological human testis. An ultrastructural study. Biol Reprod. 1975 Apr;12(3):317–324. doi: 10.1095/biolreprod12.3.317. [DOI] [PubMed] [Google Scholar]