Abstract
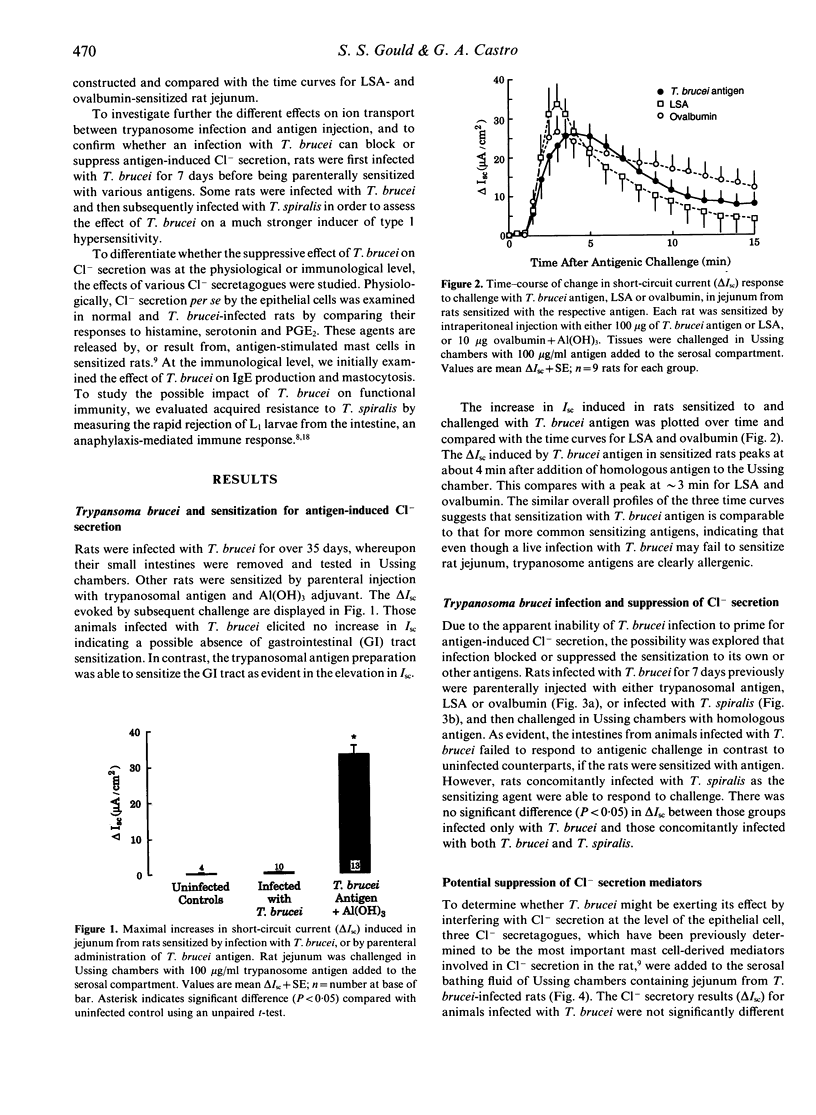
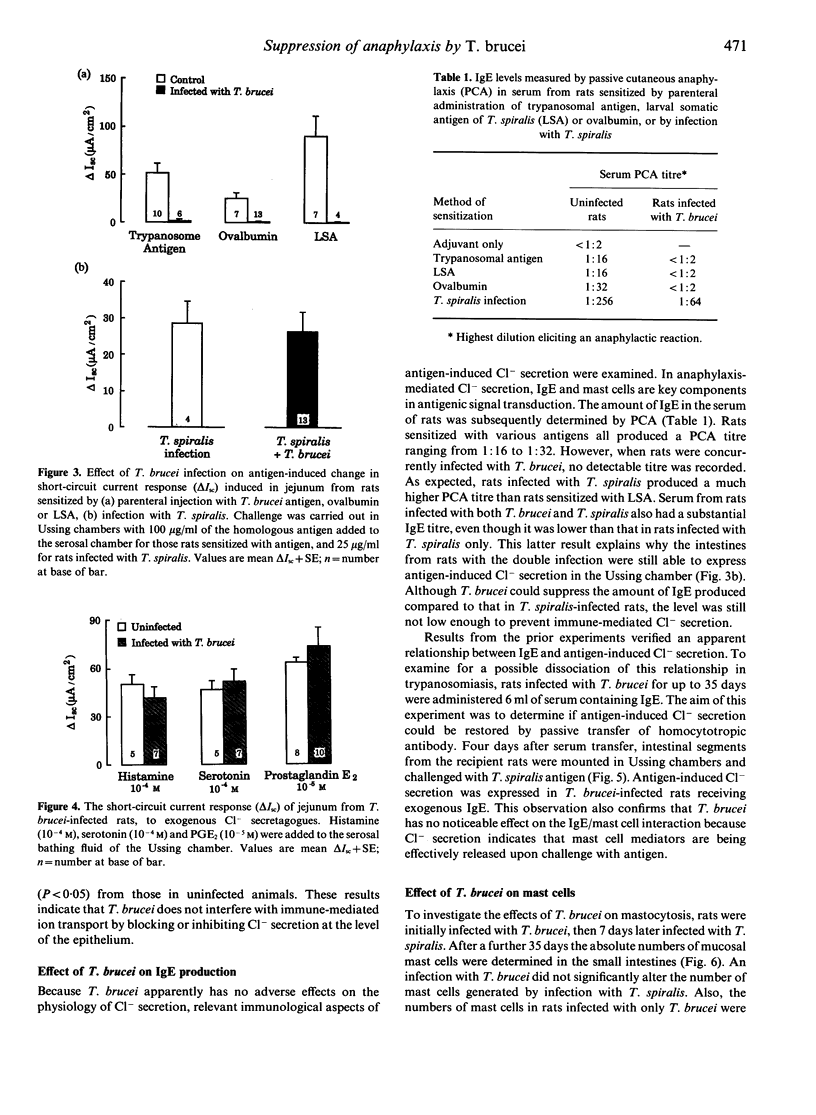
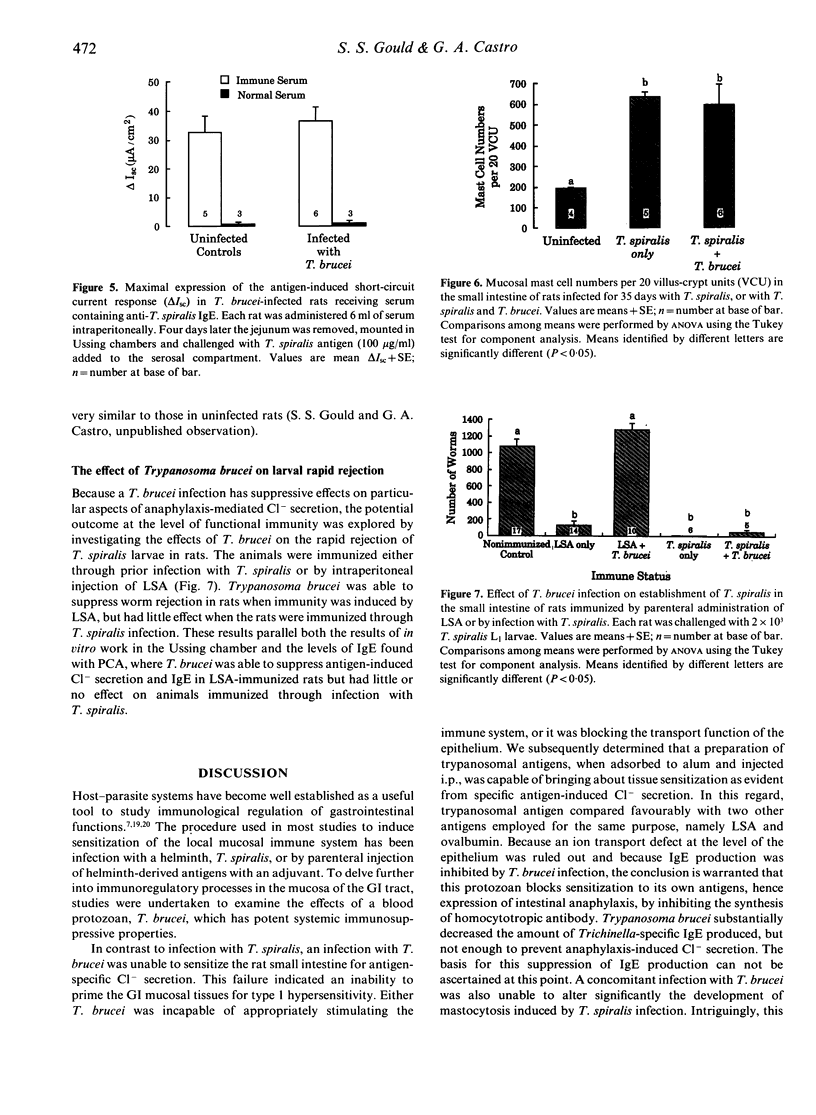
The hypothesis that failure of hosts infected with Trypanosoma brucei to express type 1 hypersensitivity is related to this parasite's ability to down-regulate IgE production, and not to an innate lack of allergenicity of T. brucei antigens, was tested by studying anaphylaxis-induced changes in net epithelial ion transport in rats. Transport changes were quantified electrophysiologically in vitro, as a change in transmural short-circuit current when sensitized intestine was challenged with homologous antigen. Rats injected parenterally with trypanosome antigen elicited intestinal anaphylaxis in response to antigenic challenge, whereas the intestine of rats infected with T. brucei failed to respond. Infection with T. brucei also suppressed the anaphylactic response in rats sensitized to and challenged with ovalbumin and T. spiralis-derived antigens. In these cases suppression was related to the ability of T. brucei to block production of IgE, and not to the physiological failure of the epithelial response. However, in rats sensitized by infection with T. spiralis, neither the anaphylactic response nor IgE production were inhibited by T. brucei. Furthermore, intestinal mastocytosis normally associated with trichinosis was unaffected by the trypanosome infection. Results support the conclusion that the failure to express anaphylaxis in T. brucei-infected rats is due to the inhibition of IgE production and not to the lack of allergenicity of trypanosome antigens.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad A., Wang C. H., Bell R. G. A role for IgE in intestinal immunity. Expression of rapid expulsion of Trichinella spiralis in rats transfused with IgE and thoracic duct lymphocytes. J Immunol. 1991 May 15;146(10):3563–3570. [PubMed] [Google Scholar]
- Alizadeh H., Wakelin D. Comparison of rapid expulsion of Trichinella spiralis in mice and rats. Int J Parasitol. 1982 Feb;12(1):65–73. doi: 10.1016/0020-7519(82)90097-2. [DOI] [PubMed] [Google Scholar]
- Askonas B. A. Macrophages as mediators of immunosuppression in murine African trypanosomiasis. Curr Top Microbiol Immunol. 1985;117:119–127. doi: 10.1007/978-3-642-70538-0_6. [DOI] [PubMed] [Google Scholar]
- Bancroft G. J., Sutton C. J., Morris A. G., Askonas B. A. Production of interferons during experimental African trypanosomiasis. Clin Exp Immunol. 1983 Apr;52(1):135–143. [PMC free article] [PubMed] [Google Scholar]
- Castro G. A., Fairbairn D. Effect of immune serum on glucose absorption and infectivity of Trichinella spiralis. J Parasitol. 1969 Feb;55(1):59–66. [PubMed] [Google Scholar]
- Castro G. A., Harari Y., Russell D. Mediators of anaphylaxis-induced ion transport changes in small intestine. Am J Physiol. 1987 Oct;253(4 Pt 1):G540–G548. doi: 10.1152/ajpgi.1987.253.4.G540. [DOI] [PubMed] [Google Scholar]
- Castro G. A. Immunophysiology of enteric parasitism. Parasitol Today. 1989 Jan;5(1):11–19. doi: 10.1016/0169-4758(89)90217-2. [DOI] [PubMed] [Google Scholar]
- Dempsey W. L., Mansfield J. M. Lymphocyte function in experimental African trypanosomiasis. VI. Parasite-specific immunosuppression. J Immunol. 1983 Jun;130(6):2896–2898. [PubMed] [Google Scholar]
- Desowitz R. S. Plasmodium-specific immunoglobulin E in sera from an area of holoendemic malaria. Trans R Soc Trop Med Hyg. 1989 Jul-Aug;83(4):478–479. doi: 10.1016/0035-9203(89)90254-x. [DOI] [PubMed] [Google Scholar]
- Gajewski T. F., Fitch F. W. Anti-proliferative effect of IFN-gamma in immune regulation. I. IFN-gamma inhibits the proliferation of Th2 but not Th1 murine helper T lymphocyte clones. J Immunol. 1988 Jun 15;140(12):4245–4252. [PubMed] [Google Scholar]
- Gazzinelli R. T., Hakim F. T., Hieny S., Shearer G. M., Sher A. Synergistic role of CD4+ and CD8+ T lymphocytes in IFN-gamma production and protective immunity induced by an attenuated Toxoplasma gondii vaccine. J Immunol. 1991 Jan 1;146(1):286–292. [PubMed] [Google Scholar]
- Harari Y., Russell D. A., Castro G. A. Anaphylaxis-mediated epithelial Cl- secretion and parasite rejection in rat intestine. J Immunol. 1987 Feb 15;138(4):1250–1255. [PubMed] [Google Scholar]
- Harris W. G., Friedman M. J., Bray R. S. Serial measurement of total and parasite-specific IgE in an African population infected with Entamoeba histolytica. Trans R Soc Trop Med Hyg. 1978;72(4):427–430. doi: 10.1016/0035-9203(78)90143-8. [DOI] [PubMed] [Google Scholar]
- Heinzel F. P., Sadick M. D., Holaday B. J., Coffman R. L., Locksley R. M. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med. 1989 Jan 1;169(1):59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson K. M., Byner C., Freeman J., Terry R. J. Immunodepression, high IgM levels and evasion of the immune response in murine trypanosomiasis. Nature. 1976 Nov 18;264(5583):256–258. doi: 10.1038/264256a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lanham S. M., Godfrey D. G. Isolation of salivarian trypanosomes from man and other mammals using DEAE-cellulose. Exp Parasitol. 1970 Dec;28(3):521–534. doi: 10.1016/0014-4894(70)90120-7. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. Heterogeneity of cytokine secretion patterns and functions of helper T cells. Adv Immunol. 1989;46:111–147. doi: 10.1016/s0065-2776(08)60652-5. [DOI] [PubMed] [Google Scholar]
- Nabors G. S., Tarleton R. L. Differential control of IFN-gamma and IL-2 production during Trypanosoma cruzi infection. J Immunol. 1991 May 15;146(10):3591–3598. [PubMed] [Google Scholar]
- Nielsen K., Sheppard J., Holmes W., Tizard I. Experimental bovine trypanosomiasis. Changes in serum immunoglobulins, complement and complement components in infected animals. Immunology. 1978 Nov;35(5):817–826. [PMC free article] [PubMed] [Google Scholar]
- Perito S., Calabresi A., Romani L., Puccetti P., Bistoni F. Involvement of the Th1 subset of CD4+ T cells in acquired immunity to mouse infection with Trypanosoma equiperdum. Cell Immunol. 1992 Sep;143(2):261–271. doi: 10.1016/0008-8749(92)90024-j. [DOI] [PubMed] [Google Scholar]
- Poirriez J., Toubas D., Marx-Chemla C., Leroux B., Dupouy D., Talmud M., Pinon J. M. Isotypic characterization of anti-Toxoplasma gondii antibodies in 18 cases of congenital toxoplasmic chorioretinitis. Acta Ophthalmol (Copenh) 1989 Apr;67(2):164–168. doi: 10.1111/j.1755-3768.1989.tb00747.x. [DOI] [PubMed] [Google Scholar]
- Russell D. A., Castro G. A. Anaphylactic-like reaction of small intestinal epithelium in parasitized guinea-pigs. Immunology. 1985 Mar;54(3):573–579. [PMC free article] [PubMed] [Google Scholar]
- Watanabe N., Ovary Z. Antigen and antibody detection by in vivo methods; a reevaluation of passive cutaneous anaphylactic reactions. J Immunol Methods. 1977;14(3-4):381–390. doi: 10.1016/0022-1759(77)90149-1. [DOI] [PubMed] [Google Scholar]


