Abstract
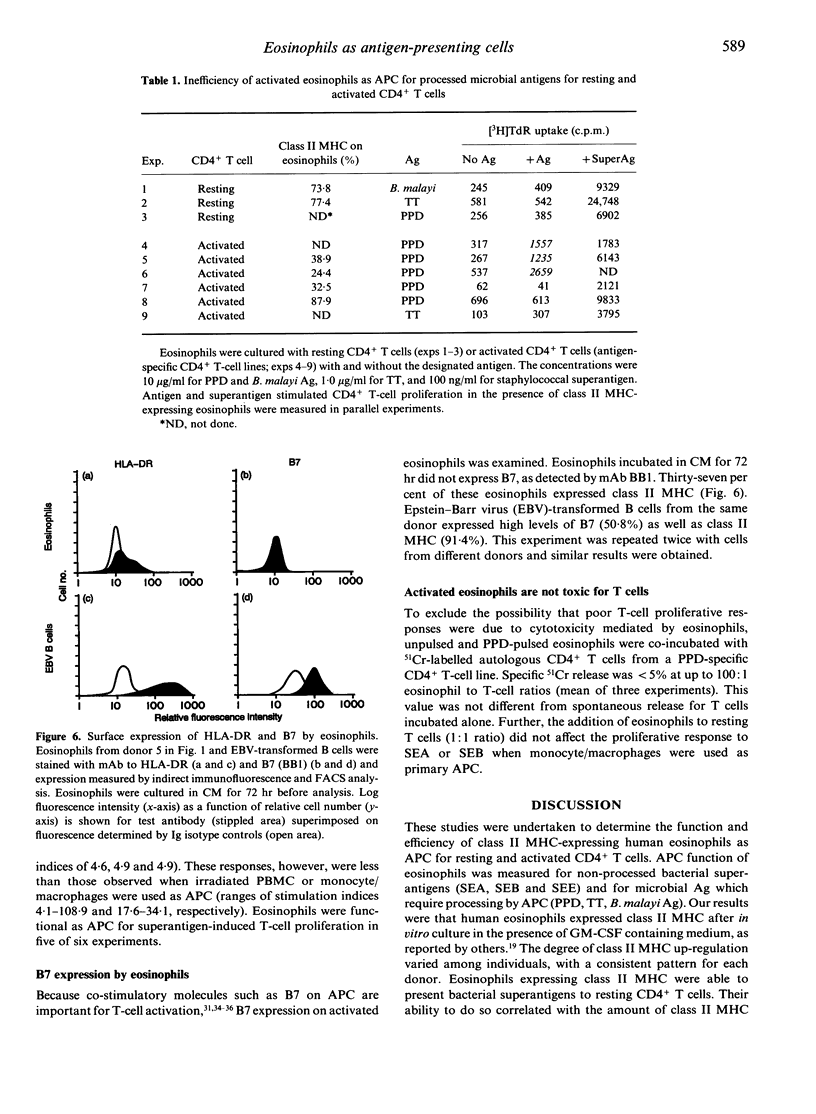
Human eosinophils become hypodense and express class II major histocompatibility (MHC) molecules when activated by granulocyte-macrophage colony-stimulating factor (GM-CSF) in vitro or in vivo in pathological conditions such as allergic disorders. In this study, we examined the capacity of class II MHC-expressing eosinophils to serve as antigen-presenting cells (APC) for resting and activated CD4+ T cells. Eosinophils were isolated from healthy donors and incubated in conditioned medium (CM) containing GM-CSF for 2-4 days, after which 15-92% of the cells expressed class II MHC (HLA-DR). Preincubated eosinophils induced resting T cells to proliferate in response to the staphylococcal superantigens, Staphylococcus enterotoxins A, B and E. Furthermore, superantigen-induced T-cell proliferation correlated with the proportion of eosinophils expressing class II MHC molecules. When eosinophils and macrophages were compared for their ability to act as accessory cells for superantigen-induced T-cell proliferation, macrophages were more efficient than eosinophils. Eosinophils were not effective APC for microbial antigens (Ag), which required processing. Proliferative responses to purified protein derivative, tetanus toxoid, or Brugia malayi antigen were observed in only three of nine studies. The three positive studies included activated CD4+ T cells, whereas no responses were observed with resting CD4+ T cells. Macrophages and mononuclear cells were effective APC for these Ag for both resting and activated CD4+ T cells. These data indicate that although class II MHC-expressing eosinophils can serve as APC, they are relatively inefficient for the activation of CD4+ T cells by Ag, which require processing.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boom W. H., Wallis R. S., Chervenak K. A. Human Mycobacterium tuberculosis-reactive CD4+ T-cell clones: heterogeneity in antigen recognition, cytokine production, and cytotoxicity for mononuclear phagocytes. Infect Immun. 1991 Aug;59(8):2737–2743. doi: 10.1128/iai.59.8.2737-2743.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth A. E., Sturrock R. F., Houba V., Mahmoud A. A., Sher A., Rees P. H. Eosinophils as mediators of antibody-dependent damage to schistosomula. Nature. 1975 Aug 28;256(5520):727–729. doi: 10.1038/256727a0. [DOI] [PubMed] [Google Scholar]
- Carlsson R., Fischer H., Sjögren H. O. Binding of staphylococcal enterotoxin A to accessory cells is a requirement for its ability to activate human T cells. J Immunol. 1988 Apr 15;140(8):2484–2488. [PubMed] [Google Scholar]
- Corrigan C. J., Kay A. B. T cells and eosinophils in the pathogenesis of asthma. Immunol Today. 1992 Dec;13(12):501–507. doi: 10.1016/0167-5699(92)90026-4. [DOI] [PubMed] [Google Scholar]
- Damle N. K., Klussman K., Leytze G., Linsley P. S. Proliferation of human T lymphocytes induced with superantigens is not dependent on costimulation by the CD28 counter-receptor B7. J Immunol. 1993 Feb 1;150(3):726–735. [PubMed] [Google Scholar]
- Damle N. K., Klussman K., Linsley P. S., Aruffo A. Differential costimulatory effects of adhesion molecules B7, ICAM-1, LFA-3, and VCAM-1 on resting and antigen-primed CD4+ T lymphocytes. J Immunol. 1992 Apr 1;148(7):1985–1992. [PubMed] [Google Scholar]
- Del Pozo V., De Andres B., Martin E., Maruri N., Zubeldia J. M., Palomino P., Lahoz C. Murine eosinophils and IL-1: alpha IL-1 mRNA detection by in situ hybridization. Production and release of IL-1 from peritoneal eosinophils. J Immunol. 1990 Apr 15;144(8):3117–3122. [PubMed] [Google Scholar]
- Del Pozo V., De Andrés B., Martín E., Cárdaba B., Fernández J. C., Gallardo S., Tramón P., Leyva-Cobian F., Palomino P., Lahoz C. Eosinophil as antigen-presenting cell: activation of T cell clones and T cell hybridoma by eosinophils after antigen processing. Eur J Immunol. 1992 Jul;22(7):1919–1925. doi: 10.1002/eji.1830220736. [DOI] [PubMed] [Google Scholar]
- Dellabona P., Peccoud J., Kappler J., Marrack P., Benoist C., Mathis D. Superantigens interact with MHC class II molecules outside of the antigen groove. Cell. 1990 Sep 21;62(6):1115–1121. doi: 10.1016/0092-8674(90)90388-u. [DOI] [PubMed] [Google Scholar]
- Fischer H. G., Frosch S., Reske K., Reske-Kunz A. B. Granulocyte-macrophage colony-stimulating factor activates macrophages derived from bone marrow cultures to synthesis of MHC class II molecules and to augmented antigen presentation function. J Immunol. 1988 Dec 1;141(11):3882–3888. [PubMed] [Google Scholar]
- Fischer H., Gjörloff A., Hedlund G., Hedman H., Lundgren E., Kalland T., Sjögren H. O., Dohlsten M. Stimulation of human naive and memory T helper cells with bacterial superantigen. Naive CD4+45RA+ T cells require a costimulatory signal mediated through the LFA-1/ICAM-1 pathway. J Immunol. 1992 Apr 1;148(7):1993–1998. [PubMed] [Google Scholar]
- Gimmi C. D., Freeman G. J., Gribben J. G., Sugita K., Freedman A. S., Morimoto C., Nadler L. M. B-cell surface antigen B7 provides a costimulatory signal that induces T cells to proliferate and secrete interleukin 2. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6575–6579. doi: 10.1073/pnas.88.15.6575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleich G. J., Adolphson C. R. The eosinophilic leukocyte: structure and function. Adv Immunol. 1986;39:177–253. doi: 10.1016/s0065-2776(08)60351-x. [DOI] [PubMed] [Google Scholar]
- Gleich G. J. The eosinophil and bronchial asthma: current understanding. J Allergy Clin Immunol. 1990 Feb;85(2):422–436. doi: 10.1016/0091-6749(90)90151-s. [DOI] [PubMed] [Google Scholar]
- Hamann K. J., Gleich G. J., Checkel J. L., Loegering D. A., McCall J. W., Barker R. L. In vitro killing of microfilariae of Brugia pahangi and Brugia malayi by eosinophil granule proteins. J Immunol. 1990 Apr 15;144(8):3166–3173. [PubMed] [Google Scholar]
- Hansel T. T., Braunstein J. B., Walker C., Blaser K., Bruijnzeel P. L., Virchow J. C., Jr, Virchow C., Sr Sputum eosinophils from asthmatics express ICAM-1 and HLA-DR. Clin Exp Immunol. 1991 Nov;86(2):271–277. doi: 10.1111/j.1365-2249.1991.tb05809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansel T. T., De Vries I. J., Carballido J. M., Braun R. K., Carballido-Perrig N., Rihs S., Blaser K., Walker C. Induction and function of eosinophil intercellular adhesion molecule-1 and HLA-DR. J Immunol. 1992 Sep 15;149(6):2130–2136. [PubMed] [Google Scholar]
- Herndon F. J., Kayes S. G. Depletion of eosinophils by anti-IL-5 monoclonal antibody treatment of mice infected with Trichinella spiralis does not alter parasite burden or immunologic resistance to reinfection. J Immunol. 1992 Dec 1;149(11):3642–3647. [PubMed] [Google Scholar]
- June C. H., Ledbetter J. A., Gillespie M. M., Lindsten T., Thompson C. B. T-cell proliferation involving the CD28 pathway is associated with cyclosporine-resistant interleukin 2 gene expression. Mol Cell Biol. 1987 Dec;7(12):4472–4481. doi: 10.1128/mcb.7.12.4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C. L., Kumaraswami V., Poindexter R. W., Kumari S., Jayaraman K., Alling D. W., Ottesen E. A., Nutman T. B. Immunologic tolerance in lymphatic filariasis. Diminished parasite-specific T and B lymphocyte precursor frequency in the microfilaremic state. J Clin Invest. 1992 May;89(5):1403–1410. doi: 10.1172/JCI115729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenderman L., Kok P. T., Hamelink M. L., Verhoeven A. J., Bruijnzeel P. L. An improved method for the isolation of eosinophilic granulocytes from peripheral blood of normal individuals. J Leukoc Biol. 1988 Aug;44(2):79–86. doi: 10.1002/jlb.44.2.79. [DOI] [PubMed] [Google Scholar]
- Lanzavecchia A. Receptor-mediated antigen uptake and its effect on antigen presentation to class II-restricted T lymphocytes. Annu Rev Immunol. 1990;8:773–793. doi: 10.1146/annurev.iy.08.040190.004013. [DOI] [PubMed] [Google Scholar]
- Linsley P. S., Brady W., Grosmaire L., Aruffo A., Damle N. K., Ledbetter J. A. Binding of the B cell activation antigen B7 to CD28 costimulates T cell proliferation and interleukin 2 mRNA accumulation. J Exp Med. 1991 Mar 1;173(3):721–730. doi: 10.1084/jem.173.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucey D. R., Nicholson-Weller A., Weller P. F. Mature human eosinophils have the capacity to express HLA-DR. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1348–1351. doi: 10.1073/pnas.86.4.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller D. L., Jenkins M. K., Schwartz R. H. Clonal expansion versus functional clonal inactivation: a costimulatory signalling pathway determines the outcome of T cell antigen receptor occupancy. Annu Rev Immunol. 1989;7:445–480. doi: 10.1146/annurev.iy.07.040189.002305. [DOI] [PubMed] [Google Scholar]
- Nutman T. B., Cohen S. G., Ottesen E. A. The eosinophil, eosinophilia, and eosinophil-related disorders. II. Eosinophil infiltration and function. Allergy Proc. 1988 Nov-Dec;9(6):641–647. doi: 10.2500/108854188778965456. [DOI] [PubMed] [Google Scholar]
- Roberts R. L., Ank B. J., Salusky I. B., Stiehm E. R. Purification and properties of peritoneal eosinophils from pediatric dialysis patients. J Immunol Methods. 1990 Feb 9;126(2):205–211. doi: 10.1016/0022-1759(90)90152-l. [DOI] [PubMed] [Google Scholar]
- Rothenberg M. E., Owen W. F., Jr, Silberstein D. S., Soberman R. J., Austen K. F., Stevens R. L. Eosinophils cocultured with endothelial cells have increased survival and functional properties. Science. 1987 Aug 7;237(4815):645–647. doi: 10.1126/science.3110954. [DOI] [PubMed] [Google Scholar]
- Rothenberg M. E., Petersen J., Stevens R. L., Silberstein D. S., McKenzie D. T., Austen K. F., Owen W. F., Jr IL-5-dependent conversion of normodense human eosinophils to the hypodense phenotype uses 3T3 fibroblasts for enhanced viability, accelerated hypodensity, and sustained antibody-dependent cytotoxicity. J Immunol. 1989 Oct 1;143(7):2311–2316. [PubMed] [Google Scholar]
- Schwartz R. H. A cell culture model for T lymphocyte clonal anergy. Science. 1990 Jun 15;248(4961):1349–1356. doi: 10.1126/science.2113314. [DOI] [PubMed] [Google Scholar]
- Stadecker M. J., Kamisato J. K., Chikunguwo S. M. Induction of T helper cell unresponsiveness to antigen by macrophages from schistosomal egg granulomas. A basis for immunomodulation in schistosomiasis? J Immunol. 1990 Oct 15;145(8):2697–2700. [PubMed] [Google Scholar]
- Wallen N., Kita H., Weiler D., Gleich G. J. Glucocorticoids inhibit cytokine-mediated eosinophil survival. J Immunol. 1991 Nov 15;147(10):3490–3495. [PubMed] [Google Scholar]
- Weller P. F., Rand T. H., Barrett T., Elovic A., Wong D. T., Finberg R. W. Accessory cell function of human eosinophils. HLA-DR-dependent, MHC-restricted antigen-presentation and IL-1 alpha expression. J Immunol. 1993 Mar 15;150(6):2554–2562. [PubMed] [Google Scholar]