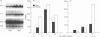Abstract
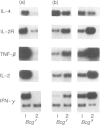
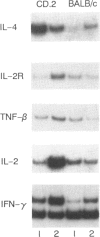
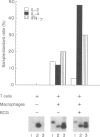
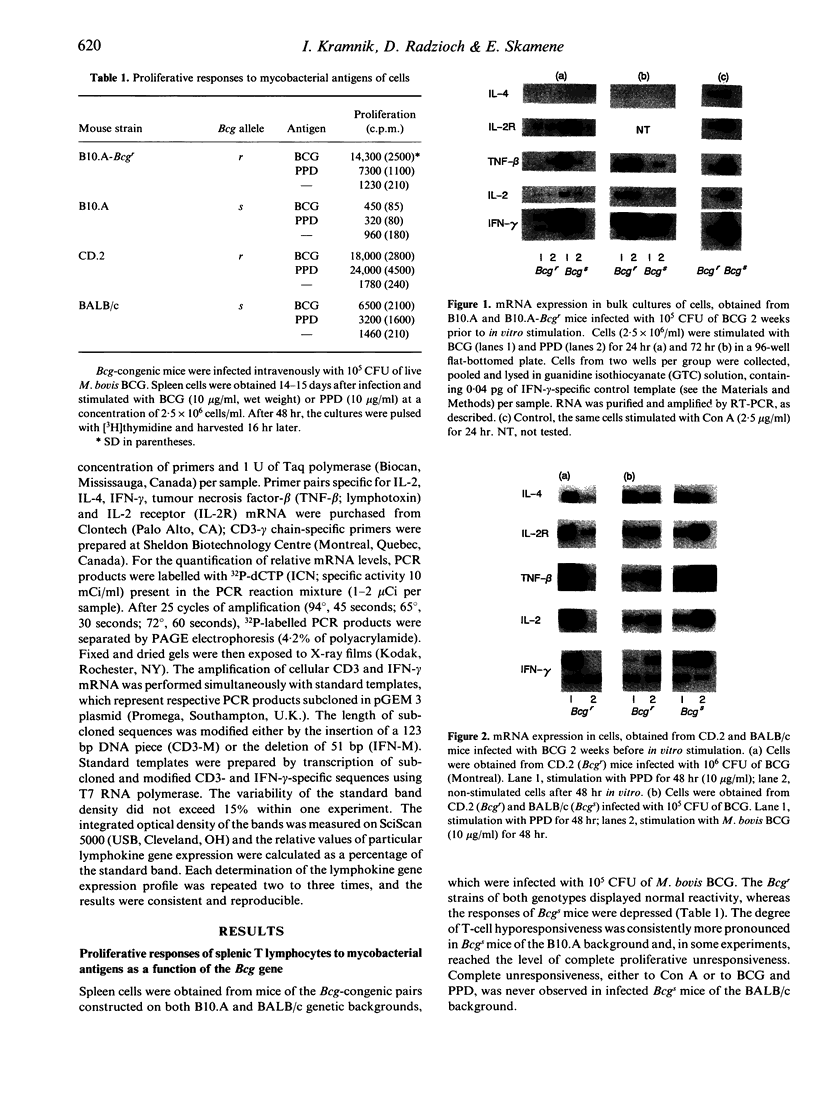
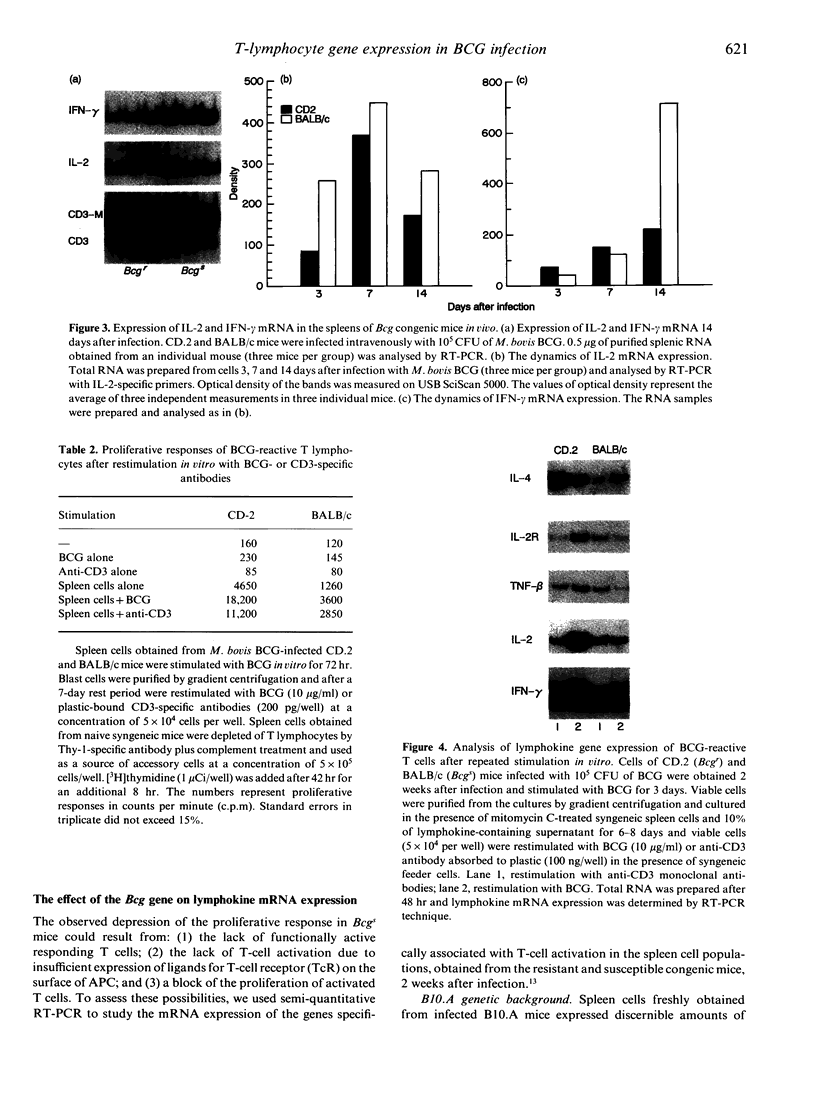
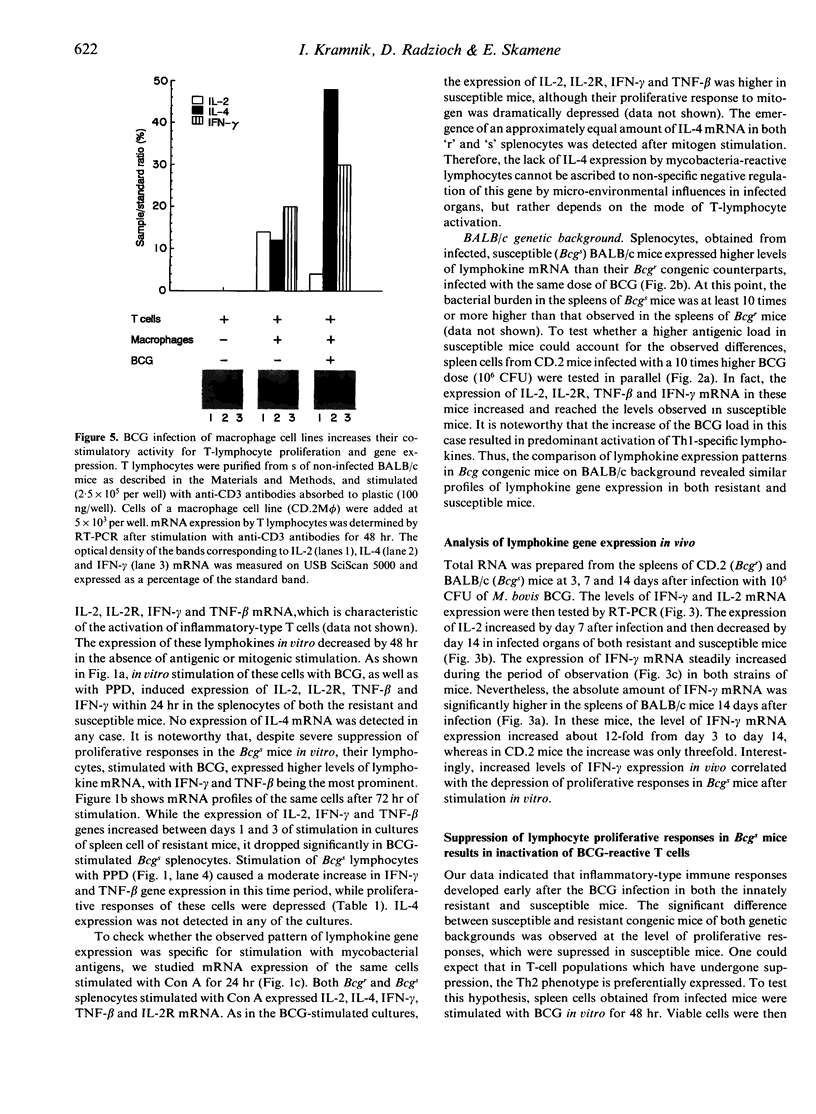
The Bcg gene has been shown to control natural resistance of mice to intravenous infection with low doses of Mycobacterium bovis (bacillus Calmette-Guérin; BCG). In the present study, we evaluated the impact of the Bcg gene on the development of T-cell reactivity during the early stages of infection. Congenic strains of mice, bearing 'r' and 's' alleles of the Bcg gene on B10.A and BALB/c backgrounds, were studied at different time-points for 2 weeks after infection. The in vitro proliferative response of spleen cells, induced by mycobacteria or concanavalin A, was depressed in the Bcgs mice compared to the Bcgr congenic mice 14 days after infection with 10(5) colony-forming units (CFU) of BCG. Polymerase chain reaction (PCR)-based methodology was used to compare the level of lymphokine gene expression in the spleens of infected congenic mice both ex vivo and after in vitro stimulation. In both cases, preferential expression of interferon-gamma (IFN-gamma), lymphotoxin, interleukin-2 (IL-2) and IL-2 receptor genes was observed. The lymphokine gene expression profiles indicated that T lymphocytes activated in the course of the BCG infection preferentially expressed the T-helper 1-specific pattern, irrespective of the allele of the Bcg gene. We showed that this bias in T-cell differentiation could not be attributed to either down-regulation of IL-4 gene expression or modulation of the macrophage co-stimulatory activity by live M. bovis BCG. We conclude that the mechanism of phenotypic expression of the Bcg gene resides in the differential ability of macrophages to be activated by lymphokines produced by protective T cells, rather than in the lack of these lymphokines in susceptible animals.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apt A. S., Kramnik I. B., Moroz A. M. Regulation of T-cell proliferative responses by cells from solid lung tissue of M. tuberculosis-infected mice. Immunology. 1991 Jun;73(2):173–179. [PMC free article] [PubMed] [Google Scholar]
- Ben-Sasson S. Z., Le Gros G., Conrad D. H., Finkelman F. D., Paul W. E. IL-4 production by T cells from naive donors. IL-2 is required for IL-4 production. J Immunol. 1990 Aug 15;145(4):1127–1136. [PubMed] [Google Scholar]
- Brett S. J., Ivanyi J. Genetic influences on the immune repertoire following tuberculous infection in mice. Immunology. 1990 Sep;71(1):113–119. [PMC free article] [PubMed] [Google Scholar]
- Brett S., Orrell J. M., Swanson Beck J., Ivanyi J. Influence of H-2 genes on growth of Mycobacterium tuberculosis in the lungs of chronically infected mice. Immunology. 1992 May;76(1):129–132. [PMC free article] [PubMed] [Google Scholar]
- Denis M., Forget A., Pelletier M., Skamene E. Pleiotropic effects of the Bcg gene. I. Antigen presentation in genetically susceptible and resistant congenic mouse strains. J Immunol. 1988 Apr 1;140(7):2395–2400. [PubMed] [Google Scholar]
- Flamand V., Abramowicz D., Goldman M., Biernaux C., Huez G., Urbain J., Moser M., Leo O. Anti-CD3 antibodies induce T cells from unprimed animals to secrete IL-4 both in vitro and in vivo. J Immunol. 1990 Apr 15;144(8):2875–2882. [PubMed] [Google Scholar]
- Gajewski T. F., Pinnas M., Wong T., Fitch F. W. Murine Th1 and Th2 clones proliferate optimally in response to distinct antigen-presenting cell populations. J Immunol. 1991 Mar 15;146(6):1750–1758. [PubMed] [Google Scholar]
- Haanen J. B., de Waal Malefijt R., Res P. C., Kraakman E. M., Ottenhoff T. H., de Vries R. R., Spits H. Selection of a human T helper type 1-like T cell subset by mycobacteria. J Exp Med. 1991 Sep 1;174(3):583–592. doi: 10.1084/jem.174.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzel F. P., Sadick M. D., Holaday B. J., Coffman R. L., Locksley R. M. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med. 1989 Jan 1;169(1):59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huygen K., Abramowicz D., Vandenbussche P., Jacobs F., De Bruyn J., Kentos A., Drowart A., Van Vooren J. P., Goldman M. Spleen cell cytokine secretion in Mycobacterium bovis BCG-infected mice. Infect Immun. 1992 Jul;60(7):2880–2886. doi: 10.1128/iai.60.7.2880-2886.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huygen K., Ljungqvist L., ten Berg R., Van Vooren J. P. Repertoires of antibodies to culture filtrate antigens in different mouse strains infected with Mycobacterium bovis BCG. Infect Immun. 1990 Jul;58(7):2192–2197. doi: 10.1128/iai.58.7.2192-2197.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye P. M., Blackwell J. M. Lsh, antigen presentation and the development of CMI. Res Immunol. 1989 Oct;140(8):810–822. doi: 10.1016/0923-2494(89)90038-2. [DOI] [PubMed] [Google Scholar]
- Kaye P. M., Curry A. J., Blackwell J. M. Differential production of Th1- and Th2-derived cytokines does not determine the genetically controlled or vaccine-induced rate of cure in murine visceral leishmaniasis. J Immunol. 1991 Apr 15;146(8):2763–2770. [PubMed] [Google Scholar]
- Kramnik I., Skamene E., Radzioch D. Assessment of lymphokine profiles in activated lymphocytes by semiquantitative PCR. J Immunol Methods. 1993 Jun 18;162(2):143–153. doi: 10.1016/0022-1759(93)90379-l. [DOI] [PubMed] [Google Scholar]
- Liu Y., Janeway C. A., Jr Interferon gamma plays a critical role in induced cell death of effector T cell: a possible third mechanism of self-tolerance. J Exp Med. 1990 Dec 1;172(6):1735–1739. doi: 10.1084/jem.172.6.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Mueller D. L., Jenkins M. K., Schwartz R. H. Clonal expansion versus functional clonal inactivation: a costimulatory signalling pathway determines the outcome of T cell antigen receptor occupancy. Annu Rev Immunol. 1989;7:445–480. doi: 10.1146/annurev.iy.07.040189.002305. [DOI] [PubMed] [Google Scholar]
- Nesbitt M. N., Skamene E. Recombinant inbred mouse strains derived from A/J and C57BL/6J: a tool for the study of genetic mechanisms in host resistance to infection and malignancy. J Leukoc Biol. 1984 Sep;36(3):357–364. doi: 10.1002/jlb.36.3.357. [DOI] [PubMed] [Google Scholar]
- Orme I. M., Collins F. M. Immune response to atypical mycobacteria: immunocompetence of heavily infected mice measured in vivo fails to substantiate immunosuppression data obtained in vitro. Infect Immun. 1984 Jan;43(1):32–37. doi: 10.1128/iai.43.1.32-37.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter M., O'Brien A. D., Skamene E., Gros P., Forget A., Kongshavn P. A., Wax J. S. A BALB/c congenic strain of mice that carries a genetic locus (Ityr) controlling resistance to intracellular parasites. Infect Immun. 1983 Jun;40(3):1234–1235. doi: 10.1128/iai.40.3.1234-1235.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radzioch D., Hudson T., Boulé M., Barrera L., Urbance J. W., Varesio L., Skamene E. Genetic resistance/susceptibility to mycobacteria: phenotypic expression in bone marrow derived macrophage lines. J Leukoc Biol. 1991 Sep;50(3):263–272. doi: 10.1002/jlb.50.3.263. [DOI] [PubMed] [Google Scholar]
- Salgame P., Abrams J. S., Clayberger C., Goldstein H., Convit J., Modlin R. L., Bloom B. R. Differing lymphokine profiles of functional subsets of human CD4 and CD8 T cell clones. Science. 1991 Oct 11;254(5029):279–282. doi: 10.1126/science.254.5029.279. [DOI] [PubMed] [Google Scholar]
- Skamene E., Gros P., Forget A., Kongshavn P. A., St Charles C., Taylor B. A. Genetic regulation of resistance to intracellular pathogens. Nature. 1982 Jun 10;297(5866):506–509. doi: 10.1038/297506a0. [DOI] [PubMed] [Google Scholar]
- Stach J. L., Gros P., Forget A., Skamene E. Phenotypic expression of genetically-controlled natural resistance to Mycobacterium bovis (BCG). J Immunol. 1984 Feb;132(2):888–892. [PubMed] [Google Scholar]
- Vidal S. M., Malo D., Vogan K., Skamene E., Gros P. Natural resistance to infection with intracellular parasites: isolation of a candidate for Bcg. Cell. 1993 May 7;73(3):469–485. doi: 10.1016/0092-8674(93)90135-d. [DOI] [PubMed] [Google Scholar]
- Yamamura M., Uyemura K., Deans R. J., Weinberg K., Rea T. H., Bloom B. R., Modlin R. L. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991 Oct 11;254(5029):277–279. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]