Abstract
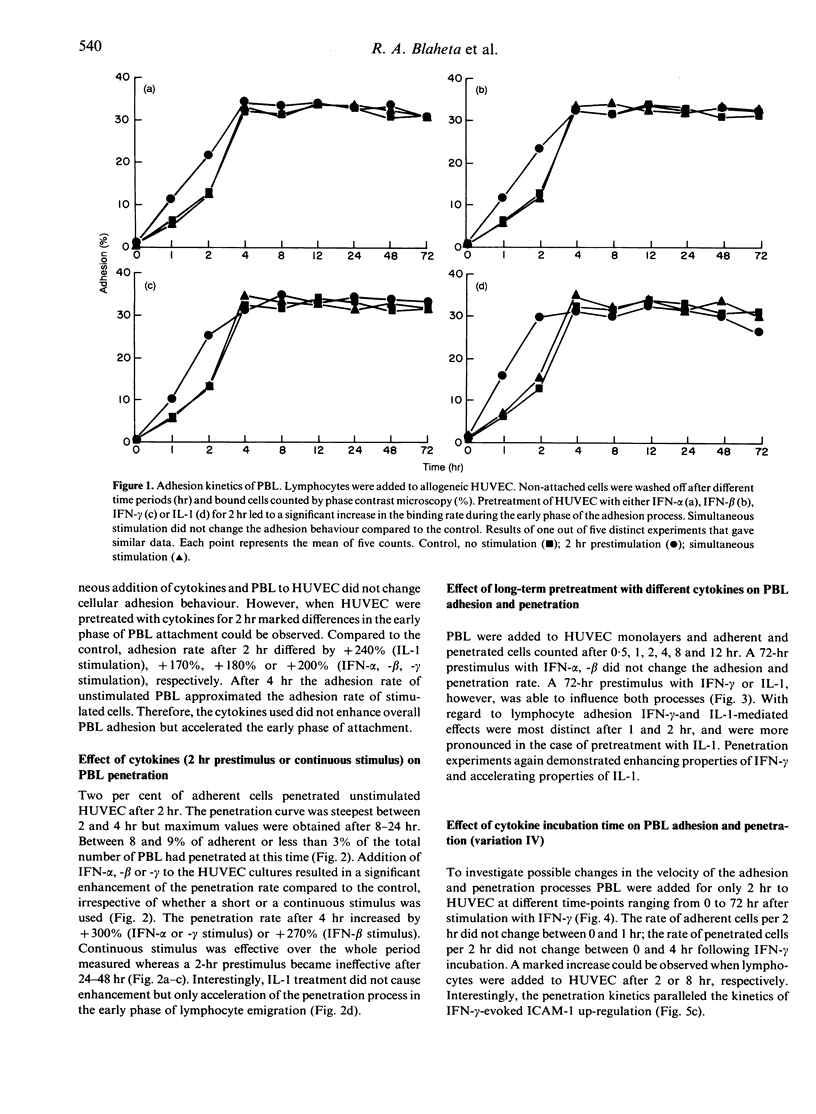
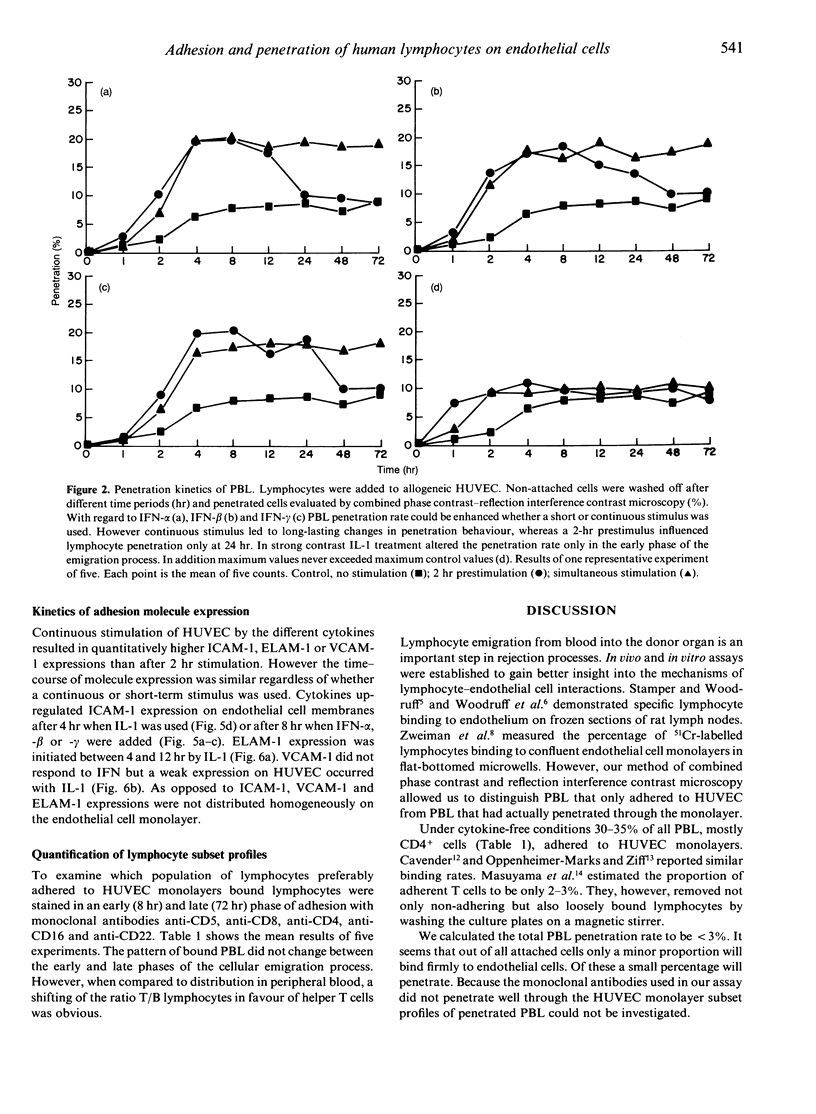
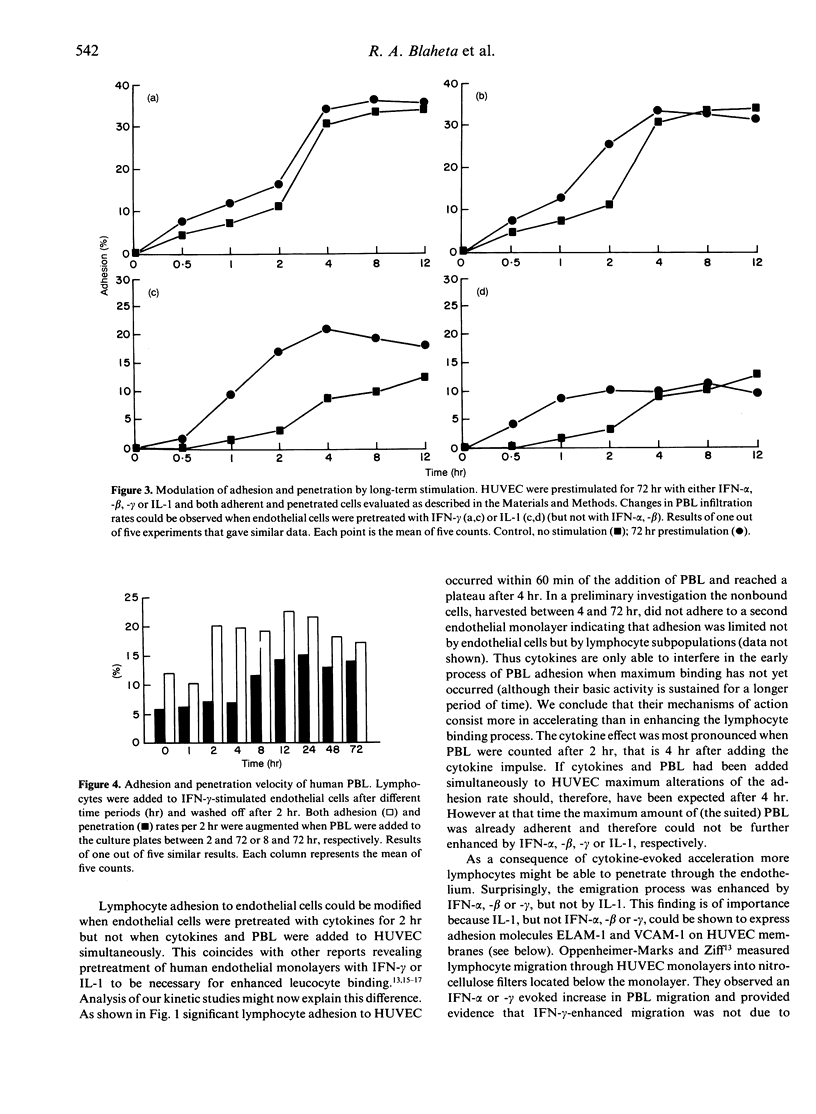
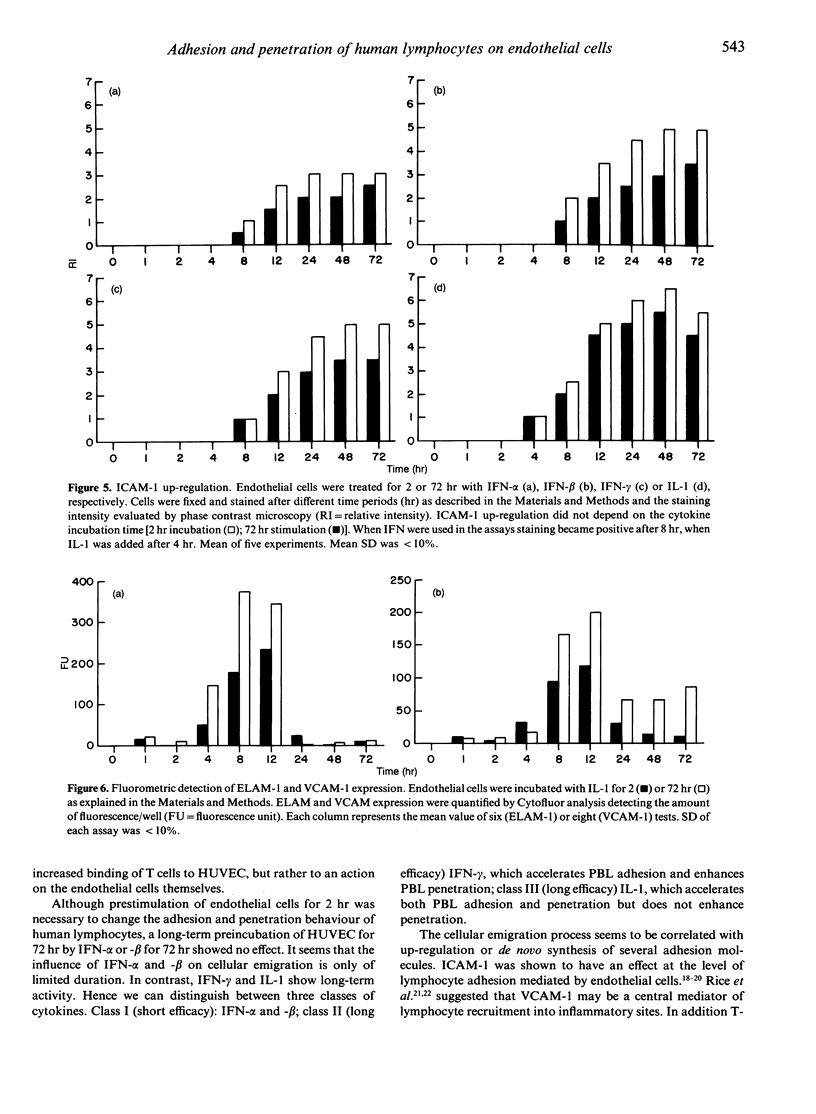
Lymphocyte infiltration through vascular endothelium is one important step in the course of graft rejection. To investigate this process more exactly we established a monolayer invasion assay which enabled us to discriminate between adherent and penetrated cells. Detailed studies of adhesion and penetration kinetics of peripheral blood lymphocytes (PBL) acting on allogeneic human umbilical vein endothelial cells (HUVEC) were carried out by combined phase contrast and reflection interference contrast microscopy. Between 30 and 35% of all PBL attached to HUVEC after 4 hr. Out of these less than 10% penetrated. When HUVEC were prestimulated for 2 hr by interferon (IFN)-alpha,-beta,-gamma or interleukin (IL)-1, PBL adhesion in the early phase of cellular attachment to endothelial cells was accelerated. Overall adhesion however did not increase. Long-term pretreatment of HUVEC for 72 hr with IFN-gamma or IL-1 also modified PBL-HUVEC interactions. However, a 72-hr pretreatment with IFN-alpha or -beta did not influence lymphocyte binding behaviour. PBL penetration was not only accelerated but also enhanced by IFN-alpha,-beta,-gamma, irrespective of whether HUVEC were prestimulated for 2 hr or PBL and cytokines were added simultaneously to HUVEC. On the other hand IL-1 was not able to enhance the amount of penetrated cells but only accelerated the infiltration process. Up-regulation or de novo expression of the adhesion molecules ICAM-1 (intercellular adhesion molecule), ELAM-1 (endothelial leucocyte adhesion molecule) and VCAM-1 (vascular cell adhesion molecule) did not parallel PBL binding kinetics. Therefore an ICAM-, ELAM- and VCAM-independent modulation in the early phase of lymphocyte attachment to endothelium seems likely. The lymphocyte cytoskeleton may have a role in this process.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker J. N., Jones M. L., Mitra R. S., Crockett-Torabe E., Fantone J. C., Kunkel S. L., Warren J. S., Dixit V. M., Nickoloff B. J. Modulation of keratinocyte-derived interleukin-8 which is chemotactic for neutrophils and T lymphocytes. Am J Pathol. 1991 Oct;139(4):869–876. [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Wheeler M. E., Cotran R. S., Gimbrone M. A., Jr Interleukin 1 acts on cultured human vascular endothelium to increase the adhesion of polymorphonuclear leukocytes, monocytes, and related leukocyte cell lines. J Clin Invest. 1985 Nov;76(5):2003–2011. doi: 10.1172/JCI112200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P., Stengelin S., Gimbrone M. A., Jr, Seed B. Endothelial leukocyte adhesion molecule 1: an inducible receptor for neutrophils related to complement regulatory proteins and lectins. Science. 1989 Mar 3;243(4895):1160–1165. doi: 10.1126/science.2466335. [DOI] [PubMed] [Google Scholar]
- Boyd A. W., Novotny J. R., Wicks I. P., Salvaris E., Welch K., Wawryk S. O. The role of accessory molecules in lymphocyte activation. Transplant Proc. 1989 Feb;21(1 Pt 1):38–40. [PubMed] [Google Scholar]
- Boyd A. W., Wawryk S. O., Burns G. F., Fecondo J. V. Intercellular adhesion molecule 1 (ICAM-1) has a central role in cell-cell contact-mediated immune mechanisms. Proc Natl Acad Sci U S A. 1988 May;85(9):3095–3099. doi: 10.1073/pnas.85.9.3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavender D. E., Haskard D. O., Joseph B., Ziff M. Interleukin 1 increases the binding of human B and T lymphocytes to endothelial cell monolayers. J Immunol. 1986 Jan;136(1):203–207. [PubMed] [Google Scholar]
- Cavender D. E. Lymphocyte adhesion to endothelial cells in vitro: models for the study of normal lymphocyte recirculation and lymphocyte emigration into chronic inflammatory lesions. J Invest Dermatol. 1989 Aug;93(2 Suppl):88S–95S. doi: 10.1111/1523-1747.ep12581077. [DOI] [PubMed] [Google Scholar]
- Damle N. K., Eberhardt C., Van der Vieren M. Direct interaction with primed CD4+ CD45R0+ memory T lymphocytes induces expression of endothelial leukocyte adhesion molecule-1 and vascular cell adhesion molecule-1 on the surface of vascular endothelial cells. Eur J Immunol. 1991 Dec;21(12):2915–2923. doi: 10.1002/eji.1830211204. [DOI] [PubMed] [Google Scholar]
- Duijvestijn A., Hamann A. Mechanisms and regulation of lymphocyte migration. Immunol Today. 1989 Jan;10(1):23–28. doi: 10.1016/0167-5699(89)90061-3. [DOI] [PubMed] [Google Scholar]
- Dustin M. L., Garcia-Aguilar J., Hibbs M. L., Larson R. S., Stacker S. A., Staunton D. E., Wardlaw A. J., Springer T. A. Structure and regulation of the leukocyte adhesion receptor LFA-1 and its counterreceptors, ICAM-1 and ICAM-2. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 2):753–765. doi: 10.1101/sqb.1989.054.01.089. [DOI] [PubMed] [Google Scholar]
- Dustin M. L., Springer T. A. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature. 1989 Oct 19;341(6243):619–624. doi: 10.1038/341619a0. [DOI] [PubMed] [Google Scholar]
- Fuggle S. V., Sanderson J. B., Gray D. W., Richardson A., Morris P. J. Variation in expression of endothelial adhesion molecules in pretransplant and transplanted kidneys--correlation with intragraft events. Transplantation. 1993 Jan;55(1):117–123. doi: 10.1097/00007890-199301000-00022. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Jutila M. A., Berg E. L., Kishimoto T. K., Picker L. J., Bargatze R. F., Bishop D. K., Orosz C. G., Wu N. W., Butcher E. C. Inflammation-induced endothelial cell adhesion to lymphocytes, neutrophils, and monocytes. Role of homing receptors and other adhesion molecules. Transplantation. 1989 Nov;48(5):727–731. doi: 10.1097/00007890-198911000-00001. [DOI] [PubMed] [Google Scholar]
- Kishimoto T. K., Larson R. S., Corbi A. L., Dustin M. L., Staunton D. E., Springer T. A. The leukocyte integrins. Adv Immunol. 1989;46:149–182. doi: 10.1016/s0065-2776(08)60653-7. [DOI] [PubMed] [Google Scholar]
- Kishimoto T. K., O'Connor K., Lee A., Roberts T. M., Springer T. A. Cloning of the beta subunit of the leukocyte adhesion proteins: homology to an extracellular matrix receptor defines a novel supergene family. Cell. 1987 Feb 27;48(4):681–690. doi: 10.1016/0092-8674(87)90246-7. [DOI] [PubMed] [Google Scholar]
- Kupiec-Weglinski J. W., Tilney N. L. Lymphocyte migration patterns in organ allograft recipients. Immunol Rev. 1989 Apr;108:63–82. doi: 10.1111/j.1600-065x.1989.tb00013.x. [DOI] [PubMed] [Google Scholar]
- Masuyama J., Minato N., Kano S. Mechanisms of lymphocyte adhesion to human vascular endothelial cells in culture. T lymphocyte adhesion to endothelial cells through endothelial HLA-DR antigens induced by gamma interferon. J Clin Invest. 1986 May;77(5):1596–1605. doi: 10.1172/JCI112475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morzycki W., Sadowska J., Issekutz A. C. Interleukin-1 and tumour necrosis factor alpha induced polymorphonuclear leukocyte-endothelial cell adhesion and transendothelial migration in vitro: the effect of apical versus basal monolayer stimulation. Immunol Lett. 1990 Sep;25(4):331–340. doi: 10.1016/0165-2478(90)90204-4. [DOI] [PubMed] [Google Scholar]
- Oppenheimer-Marks N., Ziff M. Migration of lymphocytes through endothelial cell monolayers: augmentation by interferon-gamma. Cell Immunol. 1988 Jul;114(2):307–323. doi: 10.1016/0008-8749(88)90324-3. [DOI] [PubMed] [Google Scholar]
- Osborn L., Hession C., Tizard R., Vassallo C., Luhowskyj S., Chi-Rosso G., Lobb R. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell. 1989 Dec 22;59(6):1203–1211. doi: 10.1016/0092-8674(89)90775-7. [DOI] [PubMed] [Google Scholar]
- Rice G. E., Bevilacqua M. P. An inducible endothelial cell surface glycoprotein mediates melanoma adhesion. Science. 1989 Dec 8;246(4935):1303–1306. doi: 10.1126/science.2588007. [DOI] [PubMed] [Google Scholar]
- Rice G. E., Munro J. M., Bevilacqua M. P. Inducible cell adhesion molecule 110 (INCAM-110) is an endothelial receptor for lymphocytes. A CD11/CD18-independent adhesion mechanism. J Exp Med. 1990 Apr 1;171(4):1369–1374. doi: 10.1084/jem.171.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothlein R., Dustin M. L., Marlin S. D., Springer T. A. A human intercellular adhesion molecule (ICAM-1) distinct from LFA-1. J Immunol. 1986 Aug 15;137(4):1270–1274. [PubMed] [Google Scholar]
- Shimizu Y., Newman W., Gopal T. V., Horgan K. J., Graber N., Beall L. D., van Seventer G. A., Shaw S. Four molecular pathways of T cell adhesion to endothelial cells: roles of LFA-1, VCAM-1, and ELAM-1 and changes in pathway hierarchy under different activation conditions. J Cell Biol. 1991 Jun;113(5):1203–1212. doi: 10.1083/jcb.113.5.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sica A., Matsushima K., Van Damme J., Wang J. M., Polentarutti N., Dejana E., Colotta F., Mantovani A. IL-1 transcriptionally activates the neutrophil chemotactic factor/IL-8 gene in endothelial cells. Immunology. 1990 Apr;69(4):548–553. [PMC free article] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Stamper H. B., Jr, Woodruff J. J. Lymphocyte homing into lymph nodes: in vitro demonstration of the selective affinity of recirculating lymphocytes for high-endothelial venules. J Exp Med. 1976 Sep 1;144(3):828–833. doi: 10.1084/jem.144.3.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff J. J., Katz M., Lucas L. E., Stamper H. B., Jr An in vitro model of lymphocyte homing. II. Membrane and cytoplasmic events involved in lymphocyte adherence to specialized high-endothelial venules of lymph nodes. J Immunol. 1977 Nov;119(5):1603–1610. [PubMed] [Google Scholar]
- Yu C. L., Haskard D. O., Cavender D., Johnson A. R., Ziff M. Human gamma interferon increases the binding of T lymphocytes to endothelial cells. Clin Exp Immunol. 1985 Dec;62(3):554–560. [PMC free article] [PubMed] [Google Scholar]
- Zweiman B., Moskovitz A. R., Lisak R. P. Quantitative assessment of the adherence of normal human blood mononuclear cells to vascular endothelial cell monolayers. Cell Immunol. 1982 Mar 15;68(1):165–172. doi: 10.1016/0008-8749(82)90099-5. [DOI] [PubMed] [Google Scholar]


