Abstract
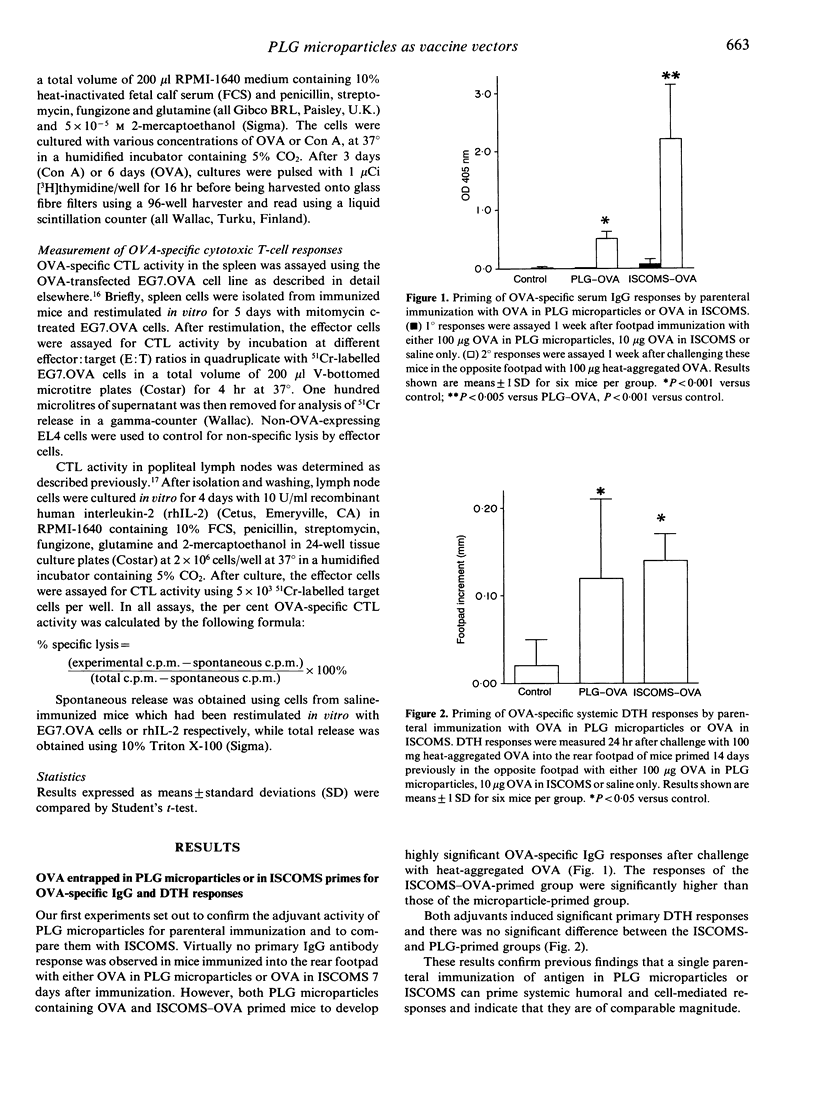
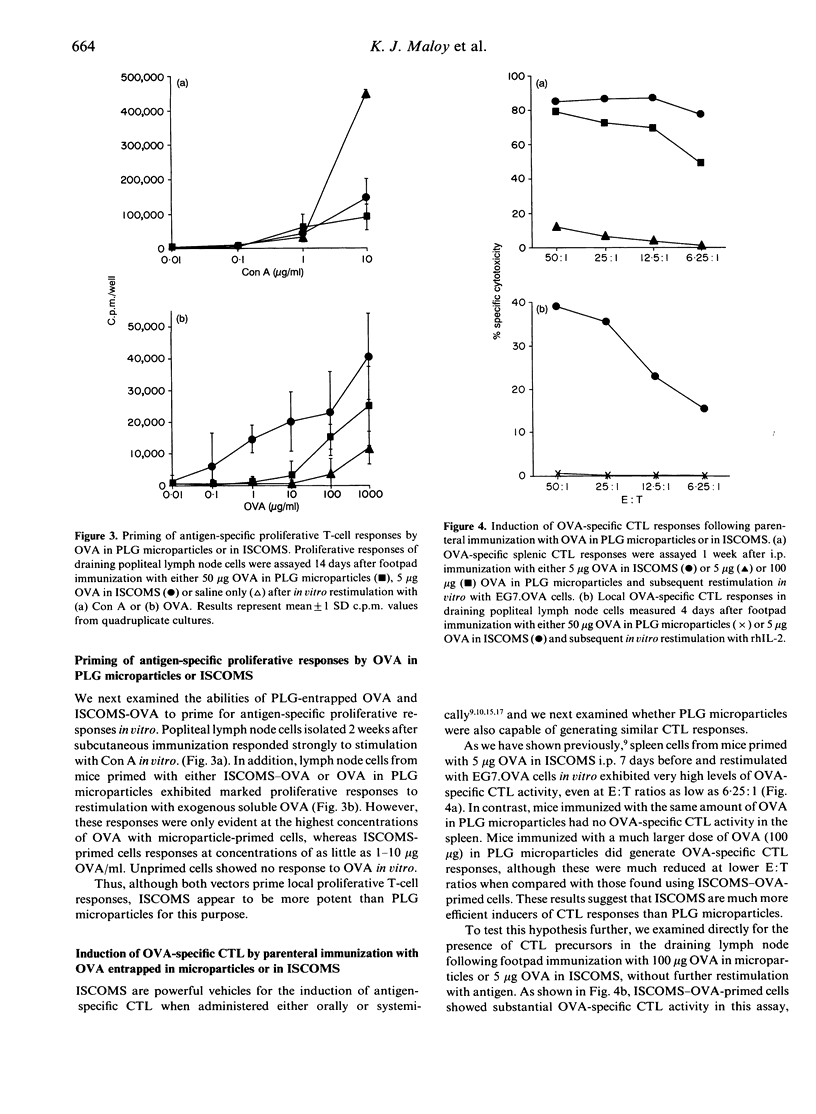
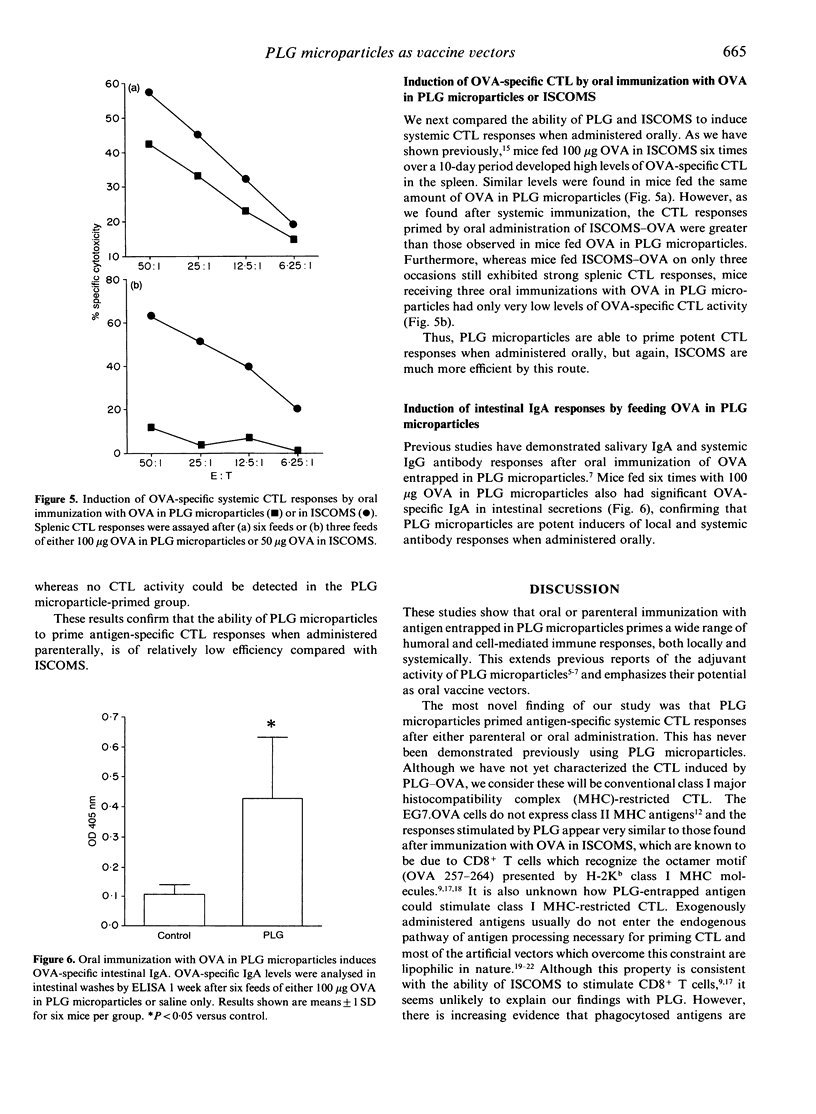
We have examined the range of mucosal and systemic immune responses induced by oral or parenteral immunization with ovalbumin (OVA) entrapped in poly(D,L-lactide-co-glycolide) (PLG) microparticles. A single subcutaneous immunization with OVA-PLG primed significant OVA-specific IgG and delayed-type hypersensitivity (DTH) responses. The DTH responses were of similar magnitude to those obtained using immunostimulating complexes (ISCOMS) as a potent control adjuvant, although ISCOMS stimulated higher serum IgG responses. Both vectors also primed OVA-specific in vitro proliferative responses in draining lymph node cells following a single immunization and strong OVA-specific CTL responses were found after intraperitoneal (i.p.) immunization. ISCOMS were more efficient in inducing cytotoxic T lymphocytes (CTL), requiring much less antigen and only ISCOMS could stimulate primary OVA-specific CTL responses in the draining lymph nodes. Multiple oral immunizations with OVA in PLG microparticles or in ISCOMS resulted in OVA-specific CTL responses and again ISCOMS seemed more potent as fewer feeds were necessary. Lastly, multiple feeds of OVA in PLG microparticles generated significant OVA-specific intestinal IgA responses. This is the first demonstration that PLG microparticles can stimulate CTL responses in vivo and our results highlight their ability to prime a variety of systemic and mucosal immune responses which may be useful in future oral vaccine development.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguado M. T., Lambert P. H. Controlled-release vaccines--biodegradable polylactide/polyglycolide (PL/PG) microspheres as antigen vehicles. Immunobiology. 1992 Feb;184(2-3):113–125. doi: 10.1016/S0171-2985(11)80470-5. [DOI] [PubMed] [Google Scholar]
- Audibert F. M., Lise L. D. Adjuvants: current status, clinical perspectives and future prospects. Immunol Today. 1993 Jun;14(6):281–284. doi: 10.1016/0167-5699(93)90046-N. [DOI] [PubMed] [Google Scholar]
- Bloom B. R. Vaccines for the Third World. Nature. 1989 Nov 9;342(6246):115–120. doi: 10.1038/342115a0. [DOI] [PubMed] [Google Scholar]
- Collins D. S., Findlay K., Harding C. V. Processing of exogenous liposome-encapsulated antigens in vivo generates class I MHC-restricted T cell responses. J Immunol. 1992 Jun 1;148(11):3336–3341. [PubMed] [Google Scholar]
- Debrick J. E., Campbell P. A., Staerz U. D. Macrophages as accessory cells for class I MHC-restricted immune responses. J Immunol. 1991 Nov 1;147(9):2846–2851. [PubMed] [Google Scholar]
- Deres K., Schild H., Wiesmüller K. H., Jung G., Rammensee H. G. In vivo priming of virus-specific cytotoxic T lymphocytes with synthetic lipopeptide vaccine. Nature. 1989 Nov 30;342(6249):561–564. doi: 10.1038/342561a0. [DOI] [PubMed] [Google Scholar]
- Eldridge J. H., Staas J. K., Meulbroek J. A., Tice T. R., Gilley R. M. Biodegradable and biocompatible poly(DL-lactide-co-glycolide) microspheres as an adjuvant for staphylococcal enterotoxin B toxoid which enhances the level of toxin-neutralizing antibodies. Infect Immun. 1991 Sep;59(9):2978–2986. doi: 10.1128/iai.59.9.2978-2986.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hora M. S., Rana R. K., Nunberg J. H., Tice T. R., Gilley R. M., Hudson M. E. Release of human serum albumin from poly(lactide-co-glycolide) microspheres. Pharm Res. 1990 Nov;7(11):1190–1194. doi: 10.1023/a:1015948829632. [DOI] [PubMed] [Google Scholar]
- Jeffery H., Davis S. S., O'Hagan D. T. The preparation and characterization of poly(lactide-co-glycolide) microparticles. II. The entrapment of a model protein using a (water-in-oil)-in-water emulsion solvent evaporation technique. Pharm Res. 1993 Mar;10(3):362–368. doi: 10.1023/a:1018980020506. [DOI] [PubMed] [Google Scholar]
- Lipford G. B., Hoffman M., Wagner H., Heeg K. Primary in vivo responses to ovalbumin. Probing the predictive value of the Kb binding motif. J Immunol. 1993 Feb 15;150(4):1212–1222. [PubMed] [Google Scholar]
- Moore M. W., Carbone F. R., Bevan M. J. Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell. 1988 Sep 9;54(6):777–785. doi: 10.1016/s0092-8674(88)91043-4. [DOI] [PubMed] [Google Scholar]
- Mowat A. M., Donachie A. M. ISCOMS--a novel strategy for mucosal immunization? Immunol Today. 1991 Nov;12(11):383–385. doi: 10.1016/0167-5699(91)90133-E. [DOI] [PubMed] [Google Scholar]
- Mowat A. M., Donachie A. M., Reid G., Jarrett O. Immune-stimulating complexes containing Quil A and protein antigen prime class I MHC-restricted T lymphocytes in vivo and are immunogenic by the oral route. Immunology. 1991 Mar;72(3):317–322. [PMC free article] [PubMed] [Google Scholar]
- Mowat A. M., Maloy K. J., Donachie A. M. Immune-stimulating complexes as adjuvants for inducing local and systemic immunity after oral immunization with protein antigens. Immunology. 1993 Dec;80(4):527–534. [PMC free article] [PubMed] [Google Scholar]
- Nardelli B., Tam J. P. Cellular immune responses induced by in vivo priming with a lipid-conjugated multimeric antigen peptide. Immunology. 1993 Jul;79(3):355–361. [PMC free article] [PubMed] [Google Scholar]
- O'Hagan D. T., Jeffery H., Davis S. S. Long-term antibody responses in mice following subcutaneous immunization with ovalbumin entrapped in biodegradable microparticles. Vaccine. 1993;11(9):965–969. doi: 10.1016/0264-410x(93)90387-d. [DOI] [PubMed] [Google Scholar]
- O'Hagan D. T., McGee J. P., Holmgren J., Mowat A. M., Donachie A. M., Mills K. H., Gaisford W., Rahman D., Challacombe S. J. Biodegradable microparticles for oral immunization. Vaccine. 1993;11(2):149–154. doi: 10.1016/0264-410x(93)90011-l. [DOI] [PubMed] [Google Scholar]
- O'Hagan D. T., Rahman D., McGee J. P., Jeffery H., Davies M. C., Williams P., Davis S. S., Challacombe S. J. Biodegradable microparticles as controlled release antigen delivery systems. Immunology. 1991 Jun;73(2):239–242. [PMC free article] [PubMed] [Google Scholar]
- Pfeifer J. D., Wick M. J., Roberts R. L., Findlay K., Normark S. J., Harding C. V. Phagocytic processing of bacterial antigens for class I MHC presentation to T cells. Nature. 1993 Jan 28;361(6410):359–362. doi: 10.1038/361359a0. [DOI] [PubMed] [Google Scholar]
- Reddy R., Zhou F., Nair S., Huang L., Rouse B. T. In vivo cytotoxic T lymphocyte induction with soluble proteins administered in liposomes. J Immunol. 1992 Mar 1;148(5):1585–1589. [PubMed] [Google Scholar]
- Reid G. Soluble proteins incorporate into ISCOMs after covalent attachment of fatty acid. Vaccine. 1992;10(9):597–602. doi: 10.1016/0264-410x(92)90439-q. [DOI] [PubMed] [Google Scholar]
- Rock K. L., Rothstein L., Gamble S., Fleischacker C. Characterization of antigen-presenting cells that present exogenous antigens in association with class I MHC molecules. J Immunol. 1993 Jan 15;150(2):438–446. [PubMed] [Google Scholar]
- Takahashi H., Takeshita T., Morein B., Putney S., Germain R. N., Berzofsky J. A. Induction of CD8+ cytotoxic T cells by immunization with purified HIV-1 envelope protein in ISCOMs. Nature. 1990 Apr 26;344(6269):873–875. doi: 10.1038/344873a0. [DOI] [PubMed] [Google Scholar]
- van Binnendijk R. S., van Baalen C. A., Poelen M. C., de Vries P., Boes J., Cerundolo V., Osterhaus A. D., UytdeHaag F. G. Measles virus transmembrane fusion protein synthesized de novo or presented in immunostimulating complexes is endogenously processed for HLA class I- and class II-restricted cytotoxic T cell recognition. J Exp Med. 1992 Jul 1;176(1):119–128. doi: 10.1084/jem.176.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]


