Abstract
Objective
To investigate the existence of a pathway between intraluminal products and the muscularis leukocytic infiltrate.
Summary Background Data
Mild intestinal manipulation or lipopolysaccharide initiates an intense inflammatory response within the intestinal muscularis, resulting in paralytic ileus. A major potential morbidity factor in ileus is luminal bacterial overgrowth.
Methods
ACI rats were subjected to small bowel manipulation, after which fluorescent carboxylated or paramagnetic microspheres were administered into the gut lumen. Animals were killed between 0 and 24 hours; unoperated rats served as controls.
Results
Intestinal manipulation led to an early transient transference of microspheres from the intestinal lumen into mesenteric lymph that was not observed in unmanipulated controls. A time- dependent, significant increase in microsphere-laden phagocytes was observed within the intestinal muscularis. Immunohistochemistry and electron microscopy of the intestinal muscularis identified the phagocytes as extravasating ED1+ monocytes. Interruption of the lymphatics abolished the accumulation of microsphere-laden monocytes within the muscularis, although a significant monocytic recruitment could still be observed within the intestinal muscularis.
Conclusions
These data show that intestinal manipulation leads to a transient increase in mucosal permeability and that the extraintestinal endocytotic uptake of transferred particles by circulating monocytes precedes their infiltration into the gut wall. The transference of luminal bacterial products may follow a similar route and time course as the microspheres. The authors hypothesize that endogenous bacterial products act synergistically with the inflammatory response within the postsurgical intestinal muscularis, leading to an exacerbation of postoperative ileus.
Sepsis is a life-threatening complication in trauma patients and in patients undergoing major abdominal and extraabdominal surgery. 1–4 In the United States alone, the yearly incidence of Gram-negative bacteremia is approximately 250,000, with an associated death rate of 20% to 50%. 5–7 During the past two decades, the role of the intestine has been investigated as the primary source of bacteria and bacterial toxins in causing systemic sepsis in critically ill, postsurgical, and trauma patients. 3,4,8–11 It has been estimated that the gastrointestinal tract contains more bacterial organisms than the total composition of cells making up the human body. 12–14 The colon has the largest microbial population of the intestine, with microscopic counts in the feces being on the order of 1012 microorganisms per gram wet weight. 15
It is known that ileus is accompanied by intestinal bacterial overgrowth, producing even higher amounts of bacterial products within the gut lumen. 16,17 However, the relevance and prevalence of intestinal bacterial translocation and of transference of bacterial products in the development of systemic sepsis continue to be debated. 18–23
In healthy subjects, translocation and transference mechanisms occur continuously, with the amount of transferred particles being normally filtered by the existing immune defense system. 22,24,25 Lipopolysaccharide (LPS) is known to be cleared rapidly from the blood by binding to high-density lipoproteins (HDL) and by being phagocytized by blood cells, liver, spleen, lungs, and adrenals. 26–29 However, as soon as normal intestinal motility or barrier function is disrupted, the host becomes subjected to the intraluminal overgrowth of virulent bacteria. 16 Bacterial translocation and transference of bacterial products from the gastrointestinal tract involve complex interactions between host defense mechanisms, motility, mucosal permeability, and the ability of particles to cross the intestinal barrier. 12,30,31 It is now accepted that gut-derived LPS and other bacterial products contribute to death and complications in trauma patients and patients undergoing major surgery. 20,32–34
We have shown that LPS causes ileus by initiating an inflammatory response within the intestinal smooth muscle layer and a subsequent decrease in in vitro and in vivo smooth muscle contractility. 35 We have also shown that gentle manipulation of the small intestine leads to postoperative ileus through the promotion of a postoperative cellular inflammatory response within the manipulated intestinal smooth muscle layer. 36 Recently, we have generated evidence to suggest that these two insults act synergistically to cause ileus. 37
Because postoperative ileus is known to favor intraluminal bacterial overgrowth and alter the intestinal barrier function, 38,39 the central aim in this study was to test the hypothesis that a pathway opens to allow an interaction between luminal bacterial products and the surgically traumatized intestine. We investigated this by visualizing the escape of fluorescent microspheres from the gut lumen in response to intestinal manipulation.
METHODS
Animals
AxC 9935 Irish, inbred strain male rats (ACI) (200–250 g) were obtained from Harlan-Sprague-Dawley (Indianapolis, IN). The University of Pittsburgh Institutional Animal Care and Use Committee approved all experimental animal protocols. Animals were housed in a pathogen-free facility that is accredited by the American Association for Accreditation of Laboratory Animal Care and complies with the requirements of humane animal care as stipulated by the U.S. Department of Agriculture and the Department of Health and Human Services. They were maintained on a 12-hour light/dark cycle and given free access to commercially available rat chow and tap water.
Experimental Groups and Surgical Procedures
In the first set of experiments, two groups of animals were studied. The intestinal manipulation group consisted of animals that had their small bowel subjected to eventration and an easily standardized, mild surgical manipulation as described previously. 36 The control group consisted of corresponding unoperated and untreated animals. In brief, intestinal manipulation was performed on methoxyflurane-anesthetized animals through a midline incision into the peritoneal cavity. The manipulation model was constructed by having the small bowel eventrated to the left onto a moist gauze with additional light manipulation of the entire bowel with two moist cotton applicators, which lasted a total of 15 minutes. After manipulation, the laparotomy was closed using a double-layer running suture. The bowel manipulation procedures caused no deaths. In both controls and manipulated animals, fluorescent or paramagnetic microspheres were instilled into the stomach by gastric gavage, the small bowel by duodenal injection, or the colon by injection into the proximal colon. The instilled volume was 0.2 mL, containing approximately 107 to 108 microspheres.
Two additional sets of animals were also studied. An ileocolostomy was performed on one set of animals to obtain two different small intestinal loops that emptied into the cecum. This model was used to investigate the route of different sizes and colors of microspheres. Another set of animals underwent lymphatic discontinuation at the subdiaphragmatic common lymph duct.
All animals recovered quickly from surgery and generally began to eat and drink within 12 hours. Animals were killed between 0 and 24 hours or 3 days after manipulation, and the intestine was used for histochemistry, immunohistochemistry, and electron microscopy.
Histochemistry and Immunohistochemistry Staining and Electron Microscopy
Whole mounts and cross-sections of the intestinal muscularis were studied for the presence of microspheres and resident and recruited leukocytes as well as the cellular localization of microspheres. Midjejunal segments were cut from the bowel and immersed in chilled Krebs Ringer buffer (KRB) in a Sylgard-bottom glass dish (Midland, MI) at 4°C, as described previously. 40 The length and width of each jejunal segment were measured with a caliper, and then it was gently pinned down along the mesenteric border. The bowel was opened along the mesentery and stretched to 150% of the length and 250% of the diameter. The opened segments of jejunum were fixed in 100% ethanol or 4% paraformaldehyde for 10 minutes. Each segment was washed twice in KRB, and mucosa and submucosa were stripped off under microscopic observation. The mucosa-free muscularis whole mounts were finally cut into 0.5 × 1-cm pieces and used for fluorescent microscopy and staining procedures. Cross-sections were made from paraformaldehyde-fixed intestinal tubes and used for the same histochemical and immunohistochemical staining procedures. Polymorphonuclear neutrophils were visualized by a myeloperoxidase (MPO) stain: freshly prepared whole mounts were immersed in a mixture of 10 mg Hanker-Yates reagent (Sigma, St. Louis, MO), 10 mL KRB, and 100 μL 3% hydrogen peroxide (Sigma) for 10 minutes. The reaction was stopped with cold KRB. Muscularis whole mounts were also used for immunohistochemical analysis. The muscularis whole mounts were incubated for 24 hours at 4°C in the primary antibody, followed by three 5-minute washes in 0.05 mol/L phosphate-buffered saline (PBS). We performed immunohistochemical stainings for ED1 (monocyte specific antibody [1:100]; Serotec, Indianapolis, IN) and ED2 (resident macrophage specific antibody [1:100]; Serotec). The specimens were then incubated in the appropriate secondary antibody (R-Phycoerythrin [RPE]-conjugated goat–antimouse secondary antibody [1:50]; DAKO Corp., Carpinteria, CA) at 4°C overnight and washed three times for 5 minutes in PBS. Whole mounts were cover slipped and inspected by light or fluorescent microscopy after staining. Leukocytes were counted in five randomly chosen areas in each specimen at a magnification of 200×. Nonspecific isotype-matched antibodies and primary antibody incubation without secondary antibody were used as negative controls.
Intestinal specimens from each animal, which had been previously fed with silica or paramagnetic microspheres, were fixed in 2.5% glutaraldehyde in PBS. Mucosa-free muscularis whole mounts as well as cross-sections from intestinal tubes were processed for standard electron microscopy.
Drugs and Solutions
A standard KRB was used as described previously. 41 This physiologic solution was gassed with 97% O2/3% CO2 to establish a pH of 7.4. KRB constituents and bethanechol were obtained from Sigma. Carboxylate-modified fluorescent latex microspheres were obtained in different sizes and fluorescent dyes from Sigma. Uniform silica microspheres with a mean diameter of 0.30 μm and Estapor superparamagnetic beads with a mean diameter of 0.46 μm were acquired from Bangs Laboratories (Fishers, IN). Antibodies were diluted in 0.05 mol/L PBS containing 0.2% bovine serum albumin (Sigma), 100 U/mL penicillin G, and 100 μg/mL streptomycin (Boehringer Mannheim, Indianapolis, IN).
Statistical Analysis
Data were compiled as mean ± standard error of the mean. Statistical analysis was performed using the unpaired and paired Student t test. Data were considered statistically significant at P < .05.
RESULTS
Recruitment of Fluorescent Microspheres into Intestinal Muscularis by Manipulation
Because of the synergistic potential for bacterial products to interact with the muscularis leukocytic infiltrate, we sought to determine whether luminal small particles could transfer out of the intestinal lumen after intestinal manipulation. To image and follow the pathway of transferring particles clearly, we intraluminally administrated fluorescent carboxylated latex microspheres of different sizes and colors. The fluorescent microspheres used ranged from 0.1 to 2.0 μm in diameter, which covers the size range of luminal contents from microbial products to intraluminally present bacteria. Whole mounts prepared after 24 hours from microsphere-injected control animals were devoid of fluorescent microspheres within the intestinal muscularis. However, when microspheres were injected into the duodenal lumen immediately after intestinal manipulation, we observed an increasing recruitment of bead-laden cells beginning at 6 hours after surgery and progressing through 24 hours after manipulation (Fig. 1). As shown in Figure 2, we were able to quantify the progressive time-dependent appearance of these bead-laden cells into the muscularis externa microscopically by recording the emitted fluorescence from whole mounts at specific time points (0, 6, 12, 18 and 24 hours) using a lux meter. We observed that the amount of bead-laden cells was dependent on the severity of manipulation. Three increasing degrees of manipulation were tested: eventration, running, and compression. Qualitatively, these data showed a progressive increase in bead-laden cells within the manipulated intestinal muscularis. The presence of fluorescent cells within the muscularis also depended on bead size: few microspheres larger than 0.8 μm were observed within whole mounts of the manipulated muscularis after 24 hours.
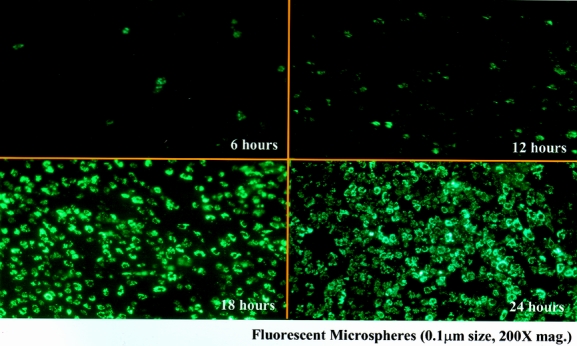
Figure 1. Jejunal muscularis layers at different time points after intestinal manipulation of the rat small intestine with the intraluminal administration of fluorescent microspheres 0.1 μm in diameter. (Upper left) Starting at 6 hours after manipulation, cells containing fluorescent microspheres begin to be observed within muscularis externa whole mounts. This recruitment time-dependently increased until at 24 hours after intestinal manipulation a virtual carpet of microsphere-laden cells was seen within the muscularis (200× original magnification).
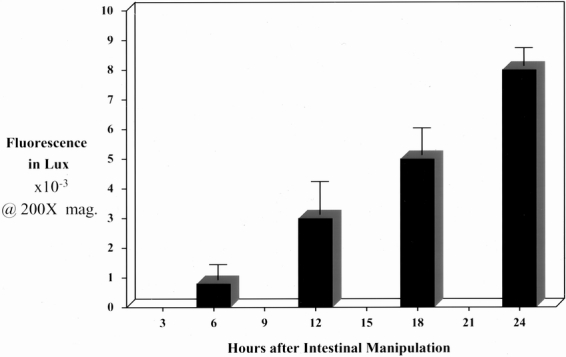
Figure 2. The histogram quantifies the time-dependent increase in fluorescent microsphere accumulation within manipulated jejunal muscularis whole mounts measured with a lux meter at a magnification of 200× at 6, 12, 18, and 24 hours after intestinal manipulation (n = 4).
We have previously timed the observed suppression in jejunal circular muscle contractility and found that it directly correlated with the temporal recruitment of leukocytes into the muscularis externa. 42 In a subsequent study, we showed that the late suppression in muscle function was directly caused by the leukocytic infiltrate. 43 Therefore, we designed an experiment to determine whether microsphere transference was also caused by the cellular inflammatory response within the intestinal wall.
First, yellow fluorescent beads (0.4 μm in diameter) were given immediately after the intestinal manipulation; 12 hours later, red fluorescent microspheres (0.1 μm) were administered by gavage. The use of two different colors of microspheres administered at different time points after intestinal manipulation resulted in the presence of only yellow fluorescent bead-laden monocytes 24 hours after the procedure, showing the transient nature of the mechanism by which the intraluminal particles exit the gut. The sequence of colored bead administration did not change our observation: a red–yellow bead temporal sequence resulted in only red bead-laden monocytes within the muscularis. Thus, the leukocytic inflammatory response is not responsible for the luminal transference.
Phagocytosis of Microspheres by Monocytes
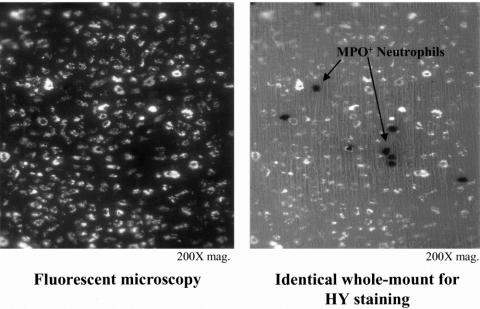
To determine the cellular identity of the bead-laden cells, we histochemically and immunohistochemically stained the bead-laden whole mounts for MPO and specific leukocyte populations. Images from MPO stainings of whole mounts from the manipulated intestine showed that the fluorescent beads were contained in a cell type other than infiltrating neutrophils (Fig. 3). As shown in Figure 4, intestinal manipulation also resulted in the recruitment of numerous ED1+ monocytes into the manipulated muscularis externa. Double-labeling the fluorescent cells with the monocyte-specific antibody ED1 showed a coalescence of these two signals. These infiltrating monocytes joined an already dense network of normally resident macrophages (see Fig. 4). 40 Further, we observed that after 3 days, the fluorescing cells transformed into a morphology that began to resemble stellate macrophages (Fig. 5).
Figure 3. Double-labeling of this manipulated whole mount after 24 hours shows that the microsphere-laden cells in panel A photographed under fluorescent lighting are not identified as the strongly myeloperoxidase neutrophils shown in panel B, photographed with partial transilluminating light (200× original magnification).
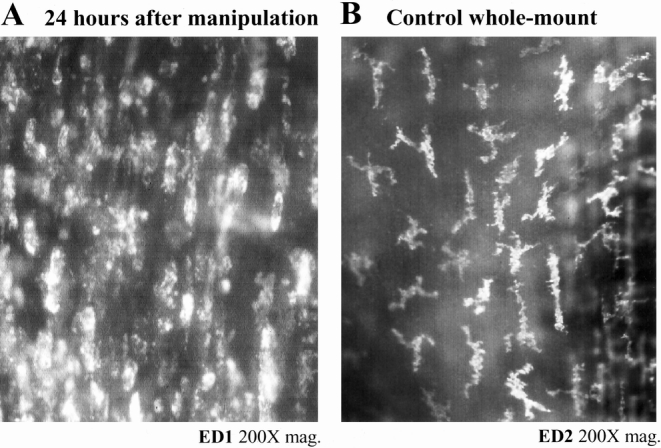
Figure 4. Manipulation induced recruitment of monocytes (panel A) to the already dense network of normally resident macrophages (panel B) within the jejunal muscularis. Twenty-four hours after intestinal manipulation, a large amount of ED1+ round monocytes can be stained lying within the intestinal jejunal muscularis (A). A dense network of ED2+ macrophages could be discriminated in muscularis externa whole mounts from controls using a highly selective monoclonal antibody (B).
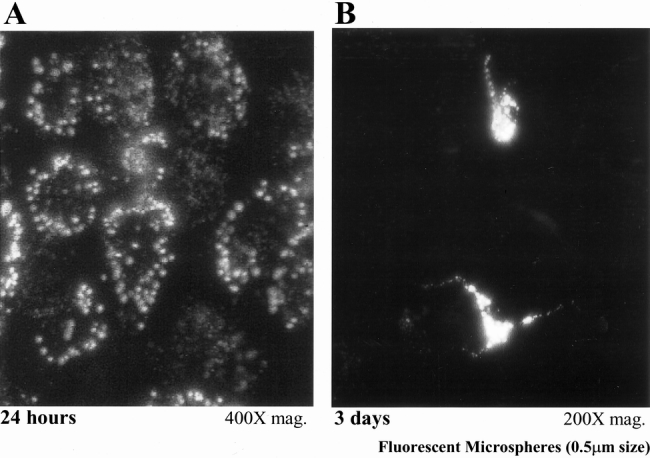
Figure 5. Morphologic transformation of newly recruited microsphere-laden monocytes to resident macrophages. Panel A shows a typical whole mount from an animal 24 hours after manipulation, illustrating the typical round aspect of these microsphere-containing phagocytes (400×). The morphologic appearance of the microsphere-laden monocytes changed over a 3-day period by transforming into more dendritic stellate resident macrophages (panel B, 400×).
The monocytic identity of the bead-laden cells was confirmed using electron microscopy on intestinal specimens obtained from animals 24 hours after intestinal manipulation, which had been fed 0.3-μm silica or 0.46-μm paramagnetic beads. As shown in Figure 6, all bead-containing cells had the morphologic appearance of recently extravasated monocytes. In most specimens, muscularis smooth muscle cells adjacent to the phagocytes showed signs of necrosis. The pattern of monocyte recruitment into the muscularis was also visualized by performing confocal microscopy on the inflamed microsphere-laden muscularis whole mounts. Examination of successive confocal images at various planes of the muscularis revealed the predominant recruitment of monocytes to the serosa and to a lesser extent to the circular muscle layer (Fig. 7).
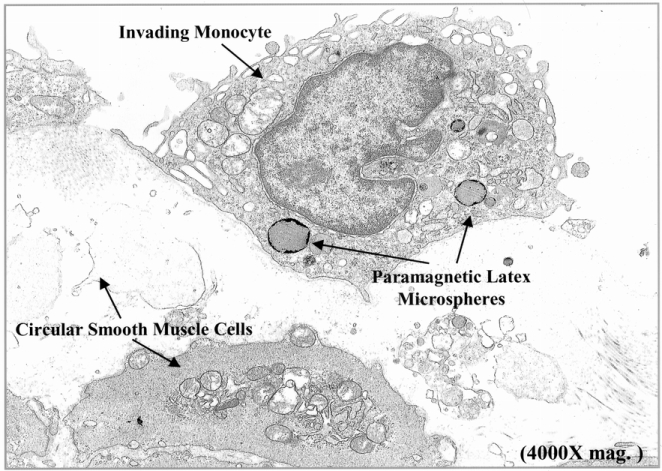
Figure 6. Electron microscopic confirmation of the microsphere-laden phagocytes as monocytes lying within the manipulated jejunal muscularis. Jejunal muscularis preparations fixed in glutaraldehyde for electron microscopy show the accumulation of 0.46-μm paramagnetic latex beads in phagocytes, which had structural cellular characteristics of monocytes. Each monocyte generally contained several microspheres and could be observed to be engulfing necrotic smooth muscle cells (4,000× original magnification).
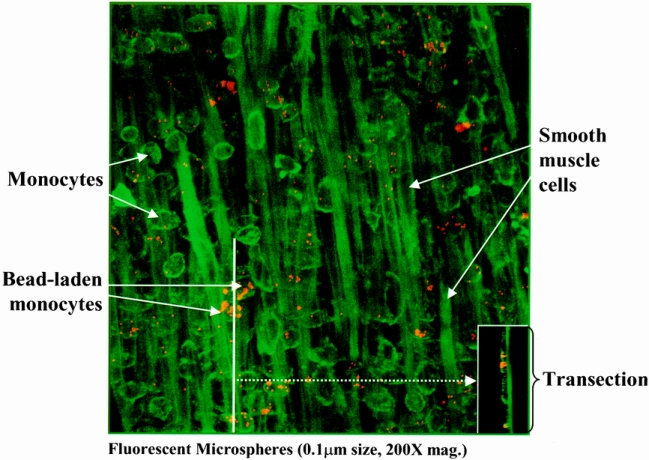
Figure 7. The postoperative distribution of microsphere-laden monocytes within the manipulated muscularis externa was investigated using confocal microscopy. The composite of the planar images shows numerous extravasated monocytes within jejunal muscularis 24 hours after intestinal manipulation. The transection composite (insert) shows the predominant localization of the fluorescently labeled monocytes near the serosa surface of the muscularis (panel, 200× magnification).
Pathway of Microsphere Transference
The pathway for microsphere transference was limited to particle sizes of less than 1 μm; therefore, we hypothesized that there would be a specific anatomic pathway through which the beads would reach the intestinal muscularis. To determine whether the beads directly traversed the intestinal wall, we constructed a two-loop model in which the proximal half of the small intestine was anastomosed to the cecum as an ileocolostomy and the anatomically distal small bowel was closed at its oral end and drained normally through the ileal cecal valve. Two weeks later, the entire intestine of these two-loop animals was manipulated and each loop was selectively injected with a different color of fluorescent beads (red or yellow). The instillation of the two differently colored microspheres into the respective loops resulted in the appearance of both colored beads within the same monocyte, which appeared equally recruited into the muscularis externa of each small intestinal loop (Fig. 8). To further show that the beads were not transferring directly through the intestinal wall, we injected fluorescent microspheres directly into the colon of small bowel-manipulated animals, which additionally had the ileocecal junction ligated. This was done to prevent reflux of the microspheres into the small intestine. Despite ileocecal ligation, we observed the massive postoperative recruitment of bead-laden phagocytes within the manipulated jejunal muscularis externa. We also observed microsphere-laden cells within the mesentery of the manipulated animals.
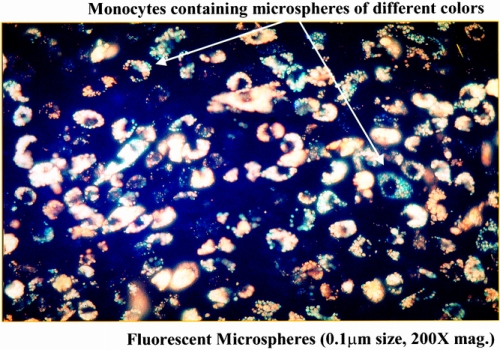
Figure 8. Coalescence within monocytes of two different-colored microspheres coming from two separate and independent loops of the manipulated small intestine. A two-loop small intestinal model was created to investigate the luminal transference pathway of the microspheres. The selective injection of yellow and red microspheres into individual intestinal loops and their combined appearance within extravasated monocytes within a single muscularis whole mount indicated that the microspheres do not transmurally pass through the gut wall to the muscularis, but that a more indirect extraintestinal route is taken.
We hypothesized from the above data that the microspheres must be transferring out of the lumen through either lymphatics or the mesenteric circulation. We investigated these two alternatives by examining the presence of microspheres in the mesenteric lymph vessels. Early after intestinal manipulation and microsphere administration, we observed the existence of numerous beads within small mesenteric lymph vessels and lymph nodules (Fig. 9). The existence of beads in the lymph vessels could be shown only at early time points, within 3 to 4 hours after intestinal observation, which again underlined a transient permeability of the gut wall for the transference of microspheres. These data further led us to examine the effect of lymph vessel ligation or discontinuation on bead transference. Interruption of the subdiaphragmatic common lymph duct abolished the presence of fluorescence-laden cells within the manipulated muscularis externa, even though these specimens contained phagocytic infiltrates, as shown in Figure 10 by the presence of numerous MPO-positive cells. This observation showed the mesenteric lymphatics as the preferential pathway of transluminal bead transference after intestinal manipulation and suggests that the circulating monocytes ingested the beads and subsequently extravasated into the muscularis externa.
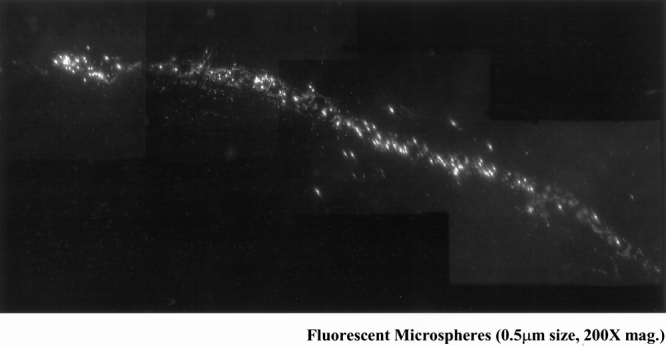
Figure 9. The early appearance of fluorescent microspheres within the mesenteric lymph vessels could be observed within 4 hours after intestinal manipulation and bead administration. This figure shows a four-picture composite of microspheres draining through a mesenteric lymph vessel 4 hours after intestinal manipulation (200× original magnification).
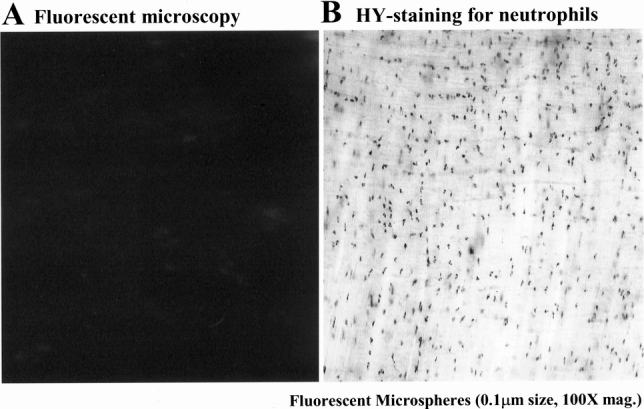
Figure 10. Subdiaphragmatic common lymph duct diversion prevented the appearance of fluorescent microspheres within phagocytes, which had extravasated into the manipulated jejunal muscularis. (Panel A) The muscularis whole mount from an animal in which the common subdiaphragmatic lymph duct was diverted by cutting after intestinal manipulation and microsphere injection showed the presence of no fluorescent beads in the muscularis (100× original magnification). However, these animals continued to have a strong leukocytic infiltrate, as shown by the presence of numerous myeloperoxidase-positive neutrophils within the manipulated muscularis (panel B, 200× original magnification).
DISCUSSION
The clinical prevalence and relevance of the frank escape of bacteria (translocation) and the leakage of bacterial products (transference) from the gastrointestinal tract remain controversial. 18–23 The central aim in this study was to test the hypothesis that a potential pathway exists that allows an interaction between luminal bacterial products and the surgically traumatized intestine. This hypothesis was formulated based on three observations previously made in the laboratory: intestinal manipulation suppresses in vitro postoperative jejunal smooth muscle contractility, resulting in a significant delay in in vivo intestinal transit;36 LPS also significantly decreases intestinal muscle contractility and delays transit;35 and these two insults appear to act synergistically in the induction of intestinal ileus. 37
We have observed that these isolated insults are accompanied by an early molecular inflammatory response, followed by a significant leukocytic extravasation into the muscularis. This leukocytic invasion consists predominantly of neutrophils and monocytes. 36,44 These invading phagocytes join the already dense network of resident macrophages. For our study, we hypothesized that the muscularis cellular infiltrate would be a likely primary site of LPS interaction within the manipulated bowel, because phagocytes abundantly express the Toll/CD14 LPS binding complex. 29,45–47 This observation would be important because it has been postulated by others that bacteria and bacterial products are not directly responsible for the septic response, but that immunologic mediators are major causative factors of systemic inflammatory response syndrome (SIRS) and multiple organ dysfunction (MODS). 2,10,48
The visualization of potential bacterial products from the gut lumen and their hypothetical interaction with leukocytes was simulated using fluorescent carboxylated latex microspheres of different sizes and colors. We showed that intestinal manipulation results in a massive accumulation and discrete cellular localization of intraluminally administered microspheres within the muscularis. The amount of microsphere-laden cells depended on the severity of the intestinal insult, with eventration, running, and compression resulting in a progressive increase in the recruitment of bead-laden cells. The mechanism responsible for this response could be our documented degree-dependent increase in leukocyte extravasation but could also be caused by a degree-dependent increase in luminal transference. Future studies will be needed to address this issue. We also observed that the accumulation of fluorescently labeled cells was time-dependent, occurring only within the first 12 hours after manipulation. This showed that the transference of microspheres out of the intestine is a transient response. Therefore, transference was not caused by the ensuing leukocytic infiltration. This is in contrast to the onset and progression of muscular dysfunction, which we have shown previously temporally correlates with and is directly attributable to this cellular infiltrate. 36 However, both the degree of trauma and the time dependency of the fluorescent accumulation correlated with our previous observations of the time- and trauma-dependent recruitment of MPO+ and ED1+ leukocytes into the muscularis. 36
The sequestration of fluorescence was observed to occur within newly extravasated monocytes using electron microscopy and double-labeling ED1-specific immunohistochemistry. Neutrophils and resident macrophages appeared to be devoid of the fluorescent microspheres. In addition, free fluorescent microspheres were not observed within the muscularis. Thus, the direct transmural transference of microspheres through the gut wall seemed unlikely. This hypothesis was confirmed by the cross-coalescence of red and yellow microspheres in the double intestinal loop model and by the selective instillation of microspheres into the unmanipulated colon.
The primary pathway for endogenous bacterial and endotoxin leakage from the gut remains controversial. 19,21,31,49,50 In light of our results and the considerable size of the latex particles, we speculated that the escaped microspheres are picked up by the mesenteric lymphatic vessels within a short time after intestinal trauma. 21,49,51 Indeed, our results show that the lymph vessels constitute a preferential pathway for the transferring intraluminally administered microspheres, because subdiaphragmatic lymph duct interruption abolished the presence of bead-laden cells within the manipulated muscularis externa, even though we observed a substantial infiltration of MPO+ neutrophils and ED1+ monocytes within the whole mounts. Further, these results support the extraintestinal phagocytosis of the transferred particles.
In summary, we have clearly established the existence of a transiently opening portal within the intestinal barrier through which large particles can transfer to the lymphatic vessels and thus gain access to the systemic circulation, where they can interact with circulating monocytes. The bead-laden leukocytes then extravasate into the manipulated intestinal muscularis in response to the local upregulation of ICAM-1 on the endothelium of the muscularis vasculature. Together with our earlier demonstration of a synergistic interaction between the traumatized intestine and exogenous endotoxin, we speculate that endogenous luminal particles and bacterial products will activate or prime circulating leukocytes that eventually extravasate into the manipulated muscularis, resulting in the potentiation of ileus.
Footnotes
Supported by National Institutes of Health grants RO1-GM-58241 and P50-GM-53789 and a grant from the Deutsche Forschungsgemeinschaft Schw. 745 1/1 (N.T.S.).
Correspondence: Anthony J. Bauer, PhD, Department of Medicine/Gastroenterology, 572 Scaife Hall, 3550 Terrace Street, University of Pittsburgh Medical School, Pittsburgh, PA 15261.
E-mail: tbauer@pitt.edu
Accepted for publication May 3, 2001.
References
- 1.Bates DW, Sands K, Miller E, et al. Predicting bacteremia in patients with sepsis syndrome. J Infect Dis 1997; 176: 1538–1551. [DOI] [PubMed] [Google Scholar]
- 2.Livingston DH, Mosenthal AC, Deitch EA. Sepsis and multiple organ dysfunction syndrome: a clinical-mechanistic overview. New Horizons 1995; 3: 257–266. [PubMed] [Google Scholar]
- 3.Pape H-C, Dwenger A, Regel G, et al. Increased gut permeability after multiple trauma. Br J Surg 1994; 81: 850–852. [DOI] [PubMed] [Google Scholar]
- 4.Saadia R. Trauma and bacterial translocation. Br J Surg 1995; 82: 1243–1244. [DOI] [PubMed] [Google Scholar]
- 5.O’Boyle C, MacFie J, Mitchell CJ. The microbiology of bacterial translocation in humans. Gut 1998; 42: 29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rangel-Frausto MS. The epidemiology of bacterial sepsis. Infect Dis Clin North Am 1999; 13: 299–310. [DOI] [PubMed] [Google Scholar]
- 7.Wells CL. Colonization and translocation of intestinal bacterial flora. Transplant Proc 1996; 28: 2653–2656. [PubMed] [Google Scholar]
- 8.Baker JW, Deitch EA, Li M, et al. Hemorrhagic shock induces bacterial translocation from the gut. J Trauma 1998; 28: 896–906. [DOI] [PubMed] [Google Scholar]
- 9.Marshall JC, Christou NV, Maekins JL. The gastrointestinal tract; the undrained abscess of multiple organ failure. Ann Surg 1993; 218: 111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nieuwenhuijzen GAP, Deitch EA, Goris JA. Infection, the gut and the development of the multiple organ dysfunction syndrome. Eur J Surg 1996; 162: 259–273. [PubMed] [Google Scholar]
- 11.Peitzman AB, Udekwu AO, Ochoa J, et al. Bacterial translocation in trauma patients. J Trauma 1991; 31: 1083–1087. [PubMed] [Google Scholar]
- 12.Berg RD. Bacterial translocation from the gastrointestinal tract. Trends Microbiol 1995; 3: 149–154. [DOI] [PubMed] [Google Scholar]
- 13.Berg RD. The indigenous gastrointestinal microflora. Trends Microbiol 1996; 4: 430–435. [DOI] [PubMed] [Google Scholar]
- 14.Unno N, Fink MP. Intestinal epithelial hyperpermeability. Mechanisms and relevance to disease. Gastroenterol Clin North Am 1998; 27: 289–307. [DOI] [PubMed] [Google Scholar]
- 15.Croucher SC, Houston AP, Bayliss CE, et al. Bacterial populations associated with different regions of the human colon wall. Appl Environ Microbiol 1983; 45: 1025–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deitch EA. Simple intestinal obstruction causes bacterial translocation in man. Arch Surg 1989; 124: 699–701. [DOI] [PubMed] [Google Scholar]
- 17.Merrett ND, Jorgenson J, Schwartz P, et al. Bacteremia associated with operative decompression of a small bowel obstruction. J Am Coll Surg 1994; 179: 33–37. [PubMed] [Google Scholar]
- 18.Deitch EA, Xu DZ, Qi L, et al. Bacterial translocation from the gut impairs systemic immunity. Surgery 1991; 109: 269–276. [PubMed] [Google Scholar]
- 19.Lemaire LCJM, van Lanschot JJB, Soutenbeek CP, et al. Thoracic duct in patients with multiple organ failure: no major route of bacterial translocation. Ann Surg 1999; 229: 128–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacFie J, O’Boyle C, Mitchell CJ, et al. Gut origin of sepsis: a prospective study investigating associations between bacterial translocation, gastric microflora, and septic morbidity. Gut 1999; 45: 223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mainous MR, Tso P, Berg RD, et al. Studies of the route, magnitude, and time course of bacterial translocation in a model of systemic inflammation. Arch Surg 1991; 126: 33–37. [DOI] [PubMed] [Google Scholar]
- 22.Sedman PC, Macfie J, Sagar P, et al. The prevalence of gut translocation in humans. Gastroenterology 1994; 107: 643–649. [DOI] [PubMed] [Google Scholar]
- 23.Van Leeuwen PA, Boermeester MA, Houdijk AP. Clinical significance of translocation. Gut 1994; 35: S28–S34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eckmann L, Stenson WF, Savidge TC, et al. Bacterial entry induces epithelial cells in the host secretory response to infection by invasive bacteria. Bacterial entry induces epithelial prostaglandin H synthase-2 expression and prostaglandin E2 and F2-alpha production. J Clin Invest 1997; 100: 296–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gautreaux MD, Deitch EA, Berg RD. T lymphocytes in host defense against bacterial translocation from the gastrointestinal tract. Infect Immun 1994; 62: 2874–2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corradin SB, Mauel J, Gallay P, et al. Enhancement of murine macrophage binding of and response to bacterial lipopolysaccharide (LPS) by LPS-binding protein. J Leukoc Biol 1992; 52: 363–368. [DOI] [PubMed] [Google Scholar]
- 27.Gallay P, Carrel S, Glauser MP, et al. Purification and characterization of murine lipopolysaccharide-binding protein. Infect Immun 1993; 61: 378–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gallay P, Barras C, Tobias PS, et al. Lipopolysaccharide (LPS)-binding protein in human serum determines the tumor necrosis factor response of monocytes to LPS. J Infect Dis 1994; 170: 1319–1322. [DOI] [PubMed] [Google Scholar]
- 29.Ulevitch RJ, Mathison JC, Schumann RR, et al. A new model of macrophage stimulation by bacterial lipopolysaccharide. J Trauma 1990; 30: S189–192. [PubMed] [Google Scholar]
- 30.Schatz G, Dobberstein B. Common principles of protein translocation across membranes. Science 1996; 271: 1519–1526. [DOI] [PubMed] [Google Scholar]
- 31.Wells CL, Maddaus MA, Simmons RL. Proposed mechanisms for the translocation of intestinal bacteria. Rev Infect Dis 1988; 10: 958–979. [DOI] [PubMed] [Google Scholar]
- 32.Burd RS, Cody CS, Raymond CS, et al. Antiendotoxin monoclonal antibodies protect by enhancing bacterial and endotoxin clearance. Arch Surg 1993; 128: 145–151. [DOI] [PubMed] [Google Scholar]
- 33.Fink MP. Adoptive immunotherapy of gram-negative sepsis: use of monoclonal antibodies to lipopolysaccharide. Crit Care Med 1993; 21: S32–39. [DOI] [PubMed] [Google Scholar]
- 34.Wells CL, Barton RG, Wavatne CS, et al. Intestinal bacterial flora, intestinal pathology, and lipopolysaccharide-induced translocation of intestinal bacteria. Circ Shock 1992; 37: 117–123. [PubMed] [Google Scholar]
- 35.Eskandari MK, Kalff JC, Billiar TR, et al. Lipopolysaccharide activates the muscularis macrophage network and suppresses circular smooth muscle activity. Am J Physiol 1997; 273: G727–G734. [DOI] [PubMed] [Google Scholar]
- 36.Kalff JC, Schraut WH, Simmons RL, Bauer AJ. Surgical manipulation of the gut elicits an intestinal muscularis inflammatory response resulting in paralytic ileus. Ann Surg 1998; 228: 625–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwarz NT, Simmons RL, Bauer AJ. Minor intraabdominal injury followed by low dose LPS administration act synergistically to induce ileus. Neurogastroenterol Motil 2000; 11 (2): 288. [Google Scholar]
- 38.Deitch EA, Specian RD, Berg RD. Endotoxin-induced bacterial translocation and mucosal permeability: role of xanthine oxidase, complement activation, and macrophage products. Crit Care Med 1991; 19: 785–791. [DOI] [PubMed] [Google Scholar]
- 39.Fink MP, Antonsson JB, Wang HL, et al. Increased intestinal permeability in endotoxic pigs. Mesenteric hypoperfusion as an etiologic factor. Arch Surg 1991; 126: 211–218. [DOI] [PubMed] [Google Scholar]
- 40.Kalff JC, Schwarz NT, Walgenbach KJ, et al. Leukocytes of the intestinal muscularis externa: their phenotype and isolation. J Leukocyte Biol 1998; 63: 683–691. [DOI] [PubMed] [Google Scholar]
- 41.Eskandari MK, Kalff JC, Billiar TR, et al. LPS-induced muscularis macrophage nitric oxide suppresses rat jejunal circular muscle activity. Am J Physiol 1999; 277: G478–G486. [DOI] [PubMed] [Google Scholar]
- 42.Kalff JC, Buchholz BM, Eskandari MK, et al. Biphasic response to gut manipulation and temporal correlation of cellular infiltrates and muscle dysfunction in rat. Surgery 1999; 126: 498–509. [PubMed] [Google Scholar]
- 43.Kalff JC, Beer-Stolz D, Carlos TM, et al. Surgically induced leukocytic infiltrates within the rat intestinal muscularis mediate postoperative ileus. Gastroenterology 1999; 117: 378–387. [DOI] [PubMed] [Google Scholar]
- 44.Eskandari MK, Kalff JC, Billiar TR, et al. Lipopolysaccharide activates the intestinal muscularis macrophage network and suppresses circular smooth muscle activity. Am J Physiol 1997; 273: G727–G734. [DOI] [PubMed] [Google Scholar]
- 45.Tobias PS, Ulevitch RJ. Lipopolysaccharide binding protein and CD14 in LPS-dependent macrophage activation. Immunobiology 1993; 187: 227–232. [DOI] [PubMed] [Google Scholar]
- 46.Ulevitch RJ, Mathison JC, Mintz DN, et al. Understanding how macrophages recognize bacterial lipopolysaccharide. In: van Furth R, ed. Mononuclear phagocytes. Netherlands: Kluwer Academic Publishers; 1992: 468–471.
- 47.Wright SD, Tobias PS, Ulevitch RJ, et al. Lipopolysaccharide (LPS) binding protein opsonizes LPS-bearing particles for recognition by a novel receptor on macrophages. J Exp Med 1989; 170: 1231–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bone RC. Toward a theory regarding the pathogenesis of the systemic inflammatory response syndrome: what we do and do not know about cytokine regulation. Crit Care Med 1996; 24: 163–172. [DOI] [PubMed] [Google Scholar]
- 49.Magnotti LJ, Upperman JS, Xu D-Z, et al. Gut-derived mesenteric lymph but not portal blood increases endothelial cell permeability and promotes lung injury after hemorrhagic shock. Ann Surg 1998; 228: 518–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tokyay R, Zeigler ST, Loick HM, et al. Mesenteric lymphadenectomy prevents postburn systemic spread of translocated bacteria. Arch Surg 1992; 127: 384–388. [DOI] [PubMed] [Google Scholar]
- 51.Gautreaux MD, Deitch EA, Berg RD. Bacterial translocation from the gastrointestinal tract to various segments of the mesenteric lymph node complex. Infect Immun 1994; 62: 2132–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]