Abstract
Objective
To assess the effect of therapeutic inhibition of interleukin 1β-converting enzyme (ICE) in an experimental model of severe acute pancreatitis (SAP).
Summary Background Data
Several lines of evidence suggest that cytokines activated by ICE play an instrumental role in the course of acute pancreatitis. Recent studies have shown that pharmacologic or genetic blockade of ICE significantly ameliorates the overall severity of and the death rate in SAP.
Methods
Severe acute pancreatitis was induced by infusion of 5% sodium taurocholate in Wistar rats. A new, highly selective, irreversible inhibitor of ICE was intraperitoneally applied at a dosage of 0.25 mg every 12 hours. Control animals in group 1 received treatment with saline; in group 2 rats, ICE inhibition was started 6 hours after the onset of pancreatitis; and in group 3 rats, ICE inhibition was started 12 hours after the onset of pancreatitis. After a 7-day observation period, surviving rats were killed and blood, plasma, pancreas, lung, and liver were used for subsequent analysis.
Results
Inhibition of ICE decreased the 7-day death rate from 87.5% to 38.9% irrespective whether treatment was started 6 hours or 12 hours after induction of SAP. Morphometric analysis revealed a significant reduction of acinar cell necrosis in both treated groups, whereas pancreatitis-associated pulmonary and hepatic damage was uniformly low and not influenced by ICE inhibition. Tissue myeloperoxidase concentrations were dramatically decreased in the pancreas and the lung after either regimen of ICE inhibitor treatment. In contrast to lung and liver, marked upregulation of interleukin 1β, tumor necrosis factor α, and ICE mRNA was observed in the pancreas, with levels of interleukin 1β and tumor necrosis factor α being reduced in ICE-inhibited animals. Compared with nontreated rats, plasma amylase levels were higher in both treatment groups, whereas lipase and hematocrit showed no difference. Changes of the differential white blood count including neutrophils, lymphocytes, and monocytes were attenuated in both groups after ICE inhibitor treatment.
Conclusions
Pharmacologic inhibition of ICE significantly improves the overall severity of and the death rate in SAP. A substantial reduction of neutrophil-mediated tissue injury in pancreas and lung seems to contribute to the beneficial effects of this approach. Moreover, ICE inhibition is still effective after a therapeutic window of 12 hours. Based on the current findings, future studies on the clinical application of ICE-inhibiting substances in acute pancreatitis seem to be promising.
Acute pancreatitis is characterized by wide clinical variation, ranging from a mild, self-limiting form to a severe disease complicated by sepsis and multiorgan system failure, with death rates of 10% and higher. 1 Despite major efforts in the search for improved therapy, no effective pharmacologic approach is available for acute pancreatitis, and treatment continues to be supportive. 2
During the past decade, increasing evidence has suggested that cytokines are of central importance in mediating local and systemic complications in acute pancreatitis. 3–6 Based on the clinical observation that the severity of this disease is reflected by an early, dramatic increase in proinflammatory cytokines, subsequent experimental studies using different approaches to block cytokine function substantiated insights into their pathophysiologic implications. Among the family of proinflammatory cytokines, tumor necrosis factor α (TNF-α) and interleukin 1β (IL-1β) are currently believed to be the most powerful ones, with potent properties to enhance local tissue destruction, produce distant organ complications, and increase the overall death rate of acute pancreatitis. 7–11 Inhibition of TNF-α and IL-1β has proved to be effective if given even after induction of symptoms in the experimental setting;12–14 however, clinical trials of anti-TNF-α or anti-IL-1β therapy have so far failed to achieve similar beneficial results. 15,16
Caspase 1, also termed interleukin 1β-converting enzyme (ICE), was the first described member of the still-growing family of cysteine proteases called caspases. 17,18 One of the major functions of ICE is the proteolytic cleavage of the 31-kd IL-1β precursor into its biologically active 17.5-kd form. Besides IL-1β, interleukin 18 (IL-18), a recently described proinflammatory cytokine with striking structural and functional similarities to IL-1β, is cleaved into its active form by caspase 1 as well. 19 Although little is known about the role of IL-18 in acute pancreatitis, first clinical observations suggest that IL-18 might be another cytokine with potent properties to promote local tissue destruction and remote organ failure in the course of this disease. 20
Based on several experimental studies, activation of caspase 1/ICE has been recognized as an important step in the pathophysiology of various inflammatory disorders. Irrespective of the specific disease, inhibiting the function of this enzyme has been shown to uniformly decrease overall severity and the death rate. 21–23 In acute pancreatitis, both pretreatment of rats with a selective, irreversible inhibitor of ICE 24,25 and the use of mice deficient in the ICE gene 24 revealed a dramatic improvement in intrapancreatic necrosis and a reduction of the overall death rate in two different models of severe acute pancreatitis (SAP). However, no study has ever assessed the therapeutic effects of this approach with inactivation of ICE after the onset of symptoms, thus reflecting the clinical situation. In the present study, we aimed to investigate this issue in an experimental model of SAP by administering a new, highly selective inhibitor of ICE at two time intervals after disease onset.
MATERIALS AND METHODS
Experimental Procedures
Animal experiments were performed in accordance with the national guidelines for the use and care of laboratory animals and approved by the local animal care and use committee. Sixty-eight male Wistar rats (300 ± 9 g) were acclimated for at least 1 week before use on a 12:12 hour light/dark cycle with free access to standard rodent chow and water. Anesthesia was performed with sevoflurane inhalation (Sevorane, Abbott, Wiesbaden, Germany) after rats had received 0.015 mg/kg buprenorphine subcutaneously (Temgesic, Grünethal, Aachen, Germany) and were placed in a supine position.
Severe acute pancreatitis was induced by a standardized, pressure-controlled retrograde infusion of 0.1 mL/100 g body weight of a freshly prepared 5% sodium taurocholate solution (Sigma-Aldrich Chemie, Steinheim, Germany) into the biliopancreatic duct, as previously described. 26
After induction of acute pancreatitis, rats were randomly assigned to intraperitoneal treatment with either isotonic saline or a highly specific, cell-permeable, competitive, and irreversible inhibitor of ICE. 23
In the nontreated pancreatitis group (SAP-S group, n = 32), 6 hours after induction of acute pancreatitis rats received the first intraperitoneal injection of 1 mL isotonic saline. This was repeated after 12 hours and continued at 12-hour intervals until surviving rats were killed at the end of the observation period.
In the ICE inhibitor-treated pancreatitis group (SAP-ICE-I 6 hours group, n = 18), 6 hours after induction of pancreatitis rats were first given 0.25 mg Ac-Tyr-Val-Ala-Asp-2,6-dimethylbenzoyloxymethylketone (Bachem Biochemica, Heidelberg, Germany) dissolved in 1 mL sterile phosphate-buffered saline (pH 7.4) intraperitoneally. As in nontreated animals, this was repeated at 12 hours and thereafter continued at 12-hour intervals until the end of the experiment at 7 days.
In the ICE inhibitor-treated pancreatitis group (SAP-ICE-I 12 hours group, n = 18), 6 hours after induction of acute pancreatitis rats were intraperitoneally injected with 1 mL isotonic saline. At the 12-hour interval, rats received the first intraperitoneal injection of 0.25 mg ICE inhibitor, which was then continued every 12 hours until the end of the observation period.
After an observation period of 7 days after induction of pancreatitis, surviving rats were exsanguinated under anesthesia by aortic puncture and intraperitoneal ascites was quantified. Total blood count and differential white blood count was determined in whole blood and plasma was analyzed for amylase and lipase concentrations. Pancreas, lungs, and liver were rapidly excised and processed for light microscopy or were snap-frozen and stored at −80°C for subsequent extraction of total RNA as well as analysis of myeloperoxidase (MPO) levels. Expression of TNF-α, IL-1β, and ICE mRNA was assessed by reverse transcriptase–polymerase chain reaction (RT-PCR). Healthy animals without any previous manipulations were used to provide baseline values of the respective parameters.
Light Microscopy
Representative tissue samples of the pancreas and the liver were fixed in 4% phosphate-buffered formalin for 24 hours and embedded in paraffin. Both lungs were excised en bloc and inflated with 4% phosphate-buffered formalin at a pressure never exceeding 30 mm Hg before 24 hours of formalin fixation. Two-micrometer-thick sections were stained with hematoxylin and eosin for morphologic/morphometric analysis and were graded in a blinded fashion by two independent investigators.
Morphometric Assessment of Pancreatic Damage
Pancreatic damage in terms of necrosis was defined as previously decribed. 25,26 Signs of ductal transformation in exocrine pancreatic tissue were defined as pancreatic acini with an enlarged luminal diameter and a decreased number/absence of apical zymogen granules. At 100× magnification a minimum of 1,000 random acini per histologic section were counted. The extent of acinar cell necrosis and pancreatic tissue undergoing ductal transformation was related to the total area of pancreatic parenchyma assessed and is given as a percentage.
Morphologic Assessment of Pulmonary and Hepatic Damage
Pulmonary and hepatic damage was assessed as recently reported. 27 At 250× magnification at least 10 random high-power fields per histologic section were evaluated. Architectural damage and inflammatory cell infiltrate were graded from 0 to 3 (0 normal, 3 severe).
Plasma/Blood Measurements
Alpha-amylase and lipase activities were determined in heparinized plasma by a standard clinical method for automated analysis (DADE Behring, Liederbach, Germany). Total blood count and differential white blood count were determined in EDTA whole blood with a rat-specific setup on a CELLDyn counter (Abbott, Santa Clara, CA) used for routine clinical analysis.
Myeloperoxidase was measured according to a method described by Bradley et al. 28 Tissue concentrations were related to the protein content of the pancreatic samples. The protein concentration was determined by the method of Lowry et al. 29
Semiquantitative Reverse Transcriptase–Polymerase Chain Reaction
Total RNA was extracted from the pancreas, lung, and liver using the single-step method of Chomczynski and Sacchi. 30 For detection of IL-1β, TNF-α, and ICE by RT-PCR, 5 μg total RNA was digested with 20 U DNAse I (Boehringer Mannheim, Mannheim, Germany) to eliminate genomic DNA contamination. Thereafter, 2.5 μg digested total RNA underwent reverse transcription into cDNA in a total volume of 20 μL in 50 mmol/L Tris-HCl (pH 8.3), 75 mmol/L KCl, 4 mmol/L MgCl2, 10 mmol/L of each dNTP, 10 mmol/L DL-dithiothreitol, 40 U human placental RNAse inhibitor, oligo(dT) primers, and 200 U SUPERSCRIPT II reverse transcriptase (Gibco BRL Life Technologies, Gaithersburg, MD) at 42°C for 50 minutes. Two microliters of the reaction mixture was further subjected to PCR. Amplification of IL-1β (453 bp), TNF-α (465 bp), and ICE (295 bp) was carried out with rat-specific primer pairs (Biosource, Camarillo, CA) at a concentration of 20 pmol each in a total volume of 20 μL containing 20 mmol/L Tris-HCl (pH 8.4), 50 mmol/L KCl, 10 mmol/L of each dNTP, 1.5 mmol/L MgCl2, and 1 U Taq DNA polymerase (Gibco BRL Life Technologies) for 35 cycles according to the manufacturer’s conditions. To show the absence of genomic DNA, control experiments were performed by amplification of 2.5 μg digested total RNA without RT under the same conditions. Beta-actin (289 bp) was used as an internal standard in each experiment to confirm equal loading of the gel. Polymerase chain reaction products were electrophoresed on 1.5% agarose gels containing 0.1 μg/mL ethidium bromide and photographed under UV transillumination.
Statistical Analysis
All values are presented as mean ± standard error of the mean (SEM). Variables were tested for group differences with the Wilcoxon rank-sum test and percentages were compared by the Fisher exact test. In all instances, P < .05 at α < 0.05 were considered significant. Statistical calculations were done with the MedCalc (MedCalc Software, Mariakerke, Belgium) software package. 31
RESULTS
Ascites Formation and Death
No significant ascites formation was found in the surviving rats of any group at the 7-day interval. Most of the animals in the SAP-S group died in the first 3 days after induction of pancreatitis: at the 7-day interval, 28 of 32 rats had died, for a death rate of 87.5%. In contrast, ICE inhibition led to a significant reduction of the death rate, with only 7 deaths in 18 rats (38.9%), irrespective whether treatment was started 6 hours or 12 hours after disease onset (P < .001). Autopsy of the rats that died during the experiment revealed extended intra- and extrapancreatic necrosis, pancreatic hemorrhage, and moderate to massive hemorrhagic ascites in animals who died within 48 hours after induction of pancreatitis. At later time intervals, the upper abdomen had changed to an inflammatory mass harboring multiple abscess formations, which caused severe intestinal obstruction in some cases. In the lungs, exudative congestion was observed, whereas the liver and kidneys appeared grossly normal.
Plasma Amylase and Lipase
Compared with baseline values, all rats with pancreatitis showed increased lipase levels (P < .02), whereas amylase concentrations were found to be decreased (P < .02) in the SAP-S group (Table 1). In contrast to nontreated rats, amylase concentrations were significantly higher in both groups treated with the ICE inhibitor.
Table 1. AMYLASE AND LIPASE LEVELS
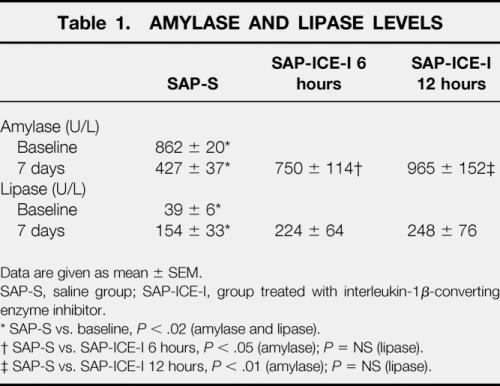
Data are given as mean ± SEM.
SAP-S, saline group; SAP-ICE-I, group treated with interleukin-1β-converting enzyme inhibitor.
* SAP-S vs. baseline, P < .02 (amylase and lipase).
† SAP-S vs. SAP-ICE-I 6 hours, P < .05 (amylase);P = NS (lipase).
‡ SAP-S vs. SAP-ICE-I 12 hours, P < .01 (amylase);P = NS (lipase).
Hematocrit and Differential White Blood Count
Systemic hematocrit was not different from baseline levels, nor did differ between nontreated and any of the treated groups (Table 2).
Table 2. HEMATOCRIT AND DIFFERENTIAL WHITE BLOOD COUNT
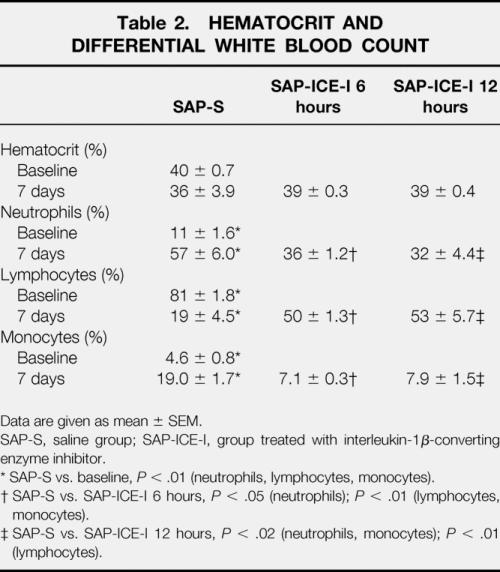
Data are given as mean ± SEM.
SAP-S, saline group; SAP-ICE-I, group treated with interleukin-1β-converting enzyme inhibitor.
* SAP-S vs. baseline, P < .01 (neutrophils, lymphocytes, monocytes).
† SAP-S vs. SAP-ICE-I 6 hours, P < .05 (neutrophils);P < .01 (lymphocytes, monocytes).
‡ SAP-S vs. SAP-ICE-I 12 hours, P < .02 (neutrophils, monocytes);P < .01 (lymphocytes).
Acute pancreatitis was associated with significant changes in the differential white blood count: an increased percentage of neutrophils (P < .01) and monocytes (P < .01) was paralleled by a decreased number of systemic lymphocytes (P < .01). Treatment with the ICE inhibitor led to a dramatic amelioration of the changes affecting all leukocyte subsets (P < .05 to .01), with similar results in both groups.
Histology
Light microscopy revealed an almost complete destruction of the pancreatic parenchyma by extended necrosis in rats of the SAP-S group; it was significantly reduced in both treated groups (P < .05 to .01). The extent of pancreatic parenchyma showing morphologic features of ductal transformation was not affected by ICE inhibition (Fig. 1).
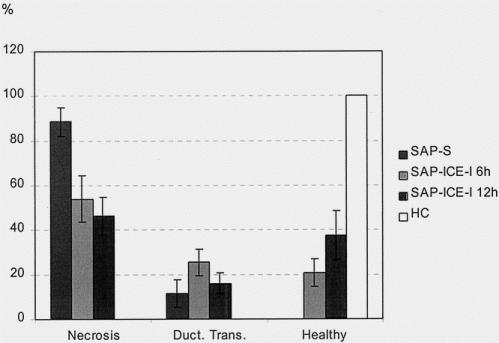
Figure 1. Morphometric assessment of the extent of acinar cell necrosis (% of total pancreatic parenchyma), pancreatic parenchyma undergoing ductal transformation (% of total pancreatic parenchyma), and remaining healthy pancreatic tissue in rats given saline (SAP-S) and interleukin-1β-converting enzyme inhibitor (SAP-ICE-I) (mean ± SEM). Healthy controls (HC) showed no morphologic changes. The extent of necrosis was significantly reduced in both treated groups (SAP-S vs. SAP-ICE-I 6 hours, P < .05; SAP-S vs. SAP-ICE-I 12 hours, P < .01), along with a significantly higher percentage of remaining viable pancreatic parenchyma in both treated groups (SAP-S vs. SAP-ICE-I 6 hours and SAP-ICE-I 12 hours, P < .02). Ductal transformation was not altered by ICE inhibition.
In terms of pulmonary damage, no significant morphologic changes were found between healthy controls and either treated or nontreated animals with pancreatitis (Fig. 2). Except for single, small abscess formations in both treated and nontreated rats, no morphologic abnormalities were found in the liver.
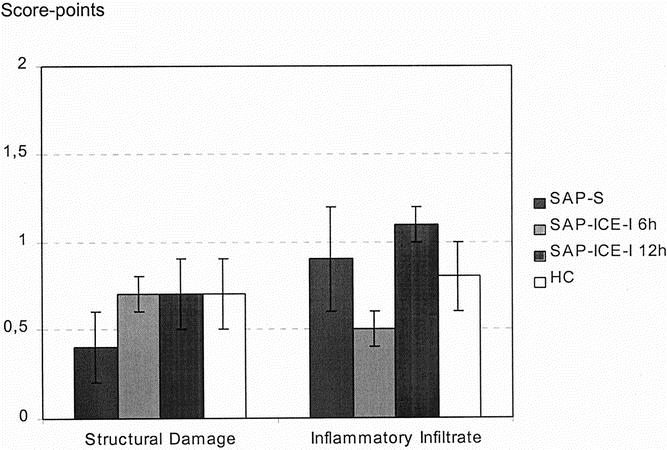
Figure 2. Morphologic scoring of pulmonary damage in rats given saline (SAP-S) and interleukin-1β-converting enzyme inhibitor (SAP-ICE-I) (mean ± SEM). In healthy controls (HC), moderate structural damage and inflammation were present that were not different from both treated and nontreated rats with pancreatitis.
Pancreatic, Pulmonary, and Hepatic Tissue Myeloperoxidase
In nontreated rats of the SAP-S group, MPO concentrations indicated excessive neutrophil infiltration in the pancreas and lungs. The highest levels of more than 2,000 U/g protein were found in the pancreas, followed by the lungs, with concentrations of approximately 800 U/g protein. Along with the absence of relevant morphologic changes, MPO levels remained within normal ranges in the liver (Fig. 3). Inhibition of ICE led to a dramatic decrease of neutrophil tissue infiltration in the pancreas (P < .01) and the lungs (P < .05 to .01), as shown by significantly lower MPO concentrations in both groups.
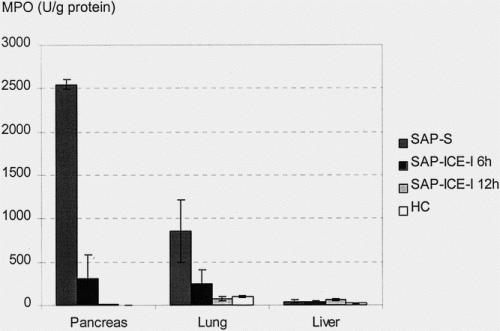
Figure 3. Tissue myeloperoxidase (MPO) concentrations in rats given saline (SAP-S) and interleukin-1β-converting enzyme inhibitor (SAP-ICE-I) (mean ± SEM). Treatment with the ICE inhibitor significantly reduced MPO levels as an indirect marker of neutrophil tissue infiltration in pancreas (SAP-S vs. SAP-ICE-I 6 hours, P < .05; SAP-S vs. SAP-ICE-I 12 hours, P < .01) and lung (SAP-S vs. SAP-ICE-I 6 hours, P < .05; SAP-S vs. SAP-ICE-I 12 hours, P < .01), whereas MPO concentrations remained within normal limits in the liver in all groups.
IL-1β, TNF-α, and ICE mRNA Expression
Semiquantitative RT-PCR revealed constitutive expression of ICE and weak expression of IL-1β mRNA in the pancreas of healthy animals, whereas TNF-α mRNA could not be detected (Fig. 4). In both lung and liver, marked constitutive expression of IL-1β and ICE but not of TNF-α mRNA was found. In nontreated rats with pancreatitis, a dramatic upregulation of IL-1β and ICE and to a lesser extent of TNF-α mRNA was evident in the pancreas. In contrast, IL-1β and ICE mRNA levels were only minimally increased in lung and liver, whereas TNF-α mRNA concentrations showed a clear elevation in both organs.
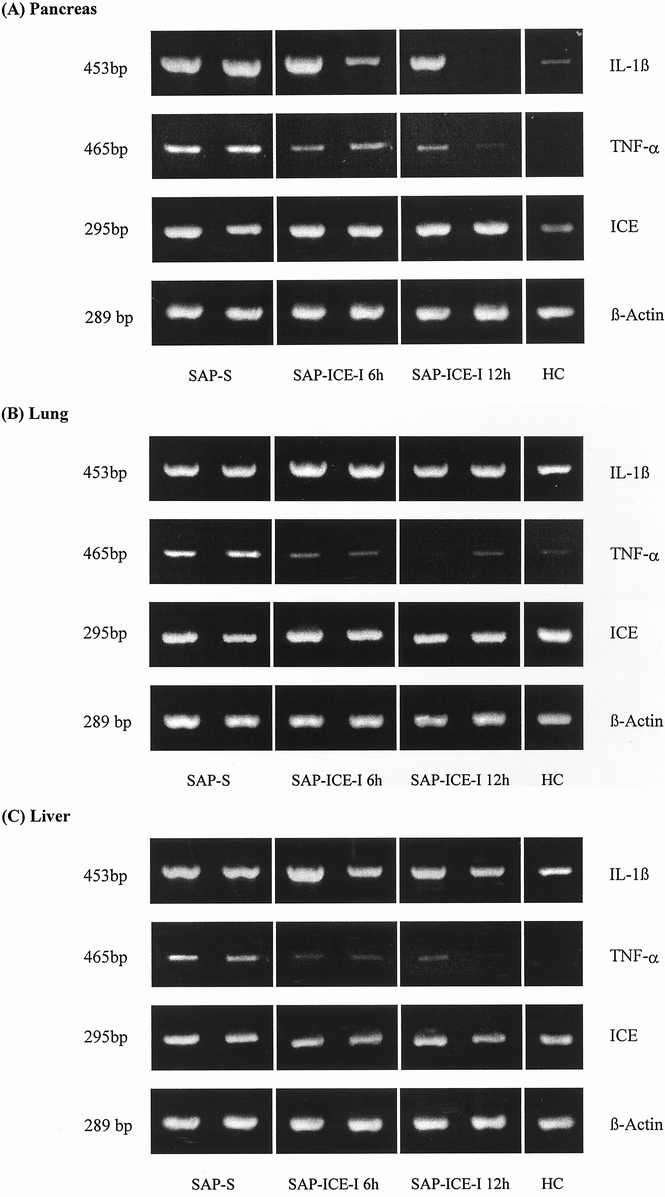
Figure 4. (A) Intrapancreatic, (B) intrapulmonary, and (C) intrahepatic mRNA expression of interleukin-1β (IL-1β), tumor necrosis factor-alpha (TNF-α), and interleukin-1β-converting enzyme (ICE) in nontreated rats (SAP-S) and rats treated with the ICE inhibitor (SAP-ICE-I) during taurocholate-induced severe acute pancreatitis. β-actin was used as internal standard. HC, healthy controls.
Compared with the SAP-S group, rats receiving either regimen of ICE inhibition showed intrapancreatic downregulation of IL-1β and TNF-α mRNA levels, whereas ICE mRNA concentrations remained unchanged. In lung and liver, ICE mRNA expression was not affected in any group treated with the ICE inhibitor, IL-1β mRNA was minimally reduced, and TNF-α mRNA was uniformly downregulated.
DISCUSSION
Although alternative pathways have recently been described, 32,33 caspase 1/ICE belongs to the family of cysteine proteases that generates the majority of the mature 17-kd unit of the biologically inactive IL-1β precursor. 17,18 The importance of this enzyme in the regulation of the IL-1β pathway was initially studied in vitro using monocytes from mice with a homozygous deletion of the ICE gene, 34 known to be a major source of IL-1β production. Beyond an almost complete absence of cellular export of mature IL-1β, a significant reduction of TNF-α, and IL-6 was observed, underscoring the importance of IL-1β in the cytokine cascade in general.
Only a few studies have investigated the effects of ICE inhibition in acute pancreatitis, but they uniformly showed a substantial improvement in overall severity and the death rate. 24,25 We previously showed in a model of lesser severity using retrograde intraductal infusion of 4% sodium taurocholate that prophylactic inhibition of ICE 25 resulted in a dramatic reduction of the extent of intrapancreatic necrosis between 3 hours and 3 days after induction of pancreatitis (Table 3). This was accompanied by significantly decreased pancreatic neutrophil tissue infiltration as well as downregulated IL-1β and TNF-α expression on both mRNA and protein levels. In addition, systemic IL-1β and TNF-α release, reflecting overall disease severity, was significantly attenuated in treated animals (Table 4).
Table 3. PERCENTAGE OF INTRAPANCREATIC NECROSIS
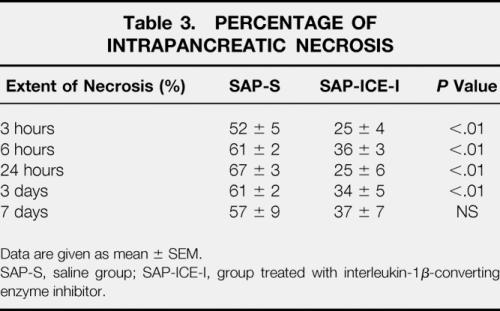
Data are given as mean ± SEM.
SAP-S, saline group; SAP-ICE-I, group treated with interleukin-1β-converting enzyme inhibitor.
Table 4. LEVELS OF TUMOR NECROSIS FACTOR ALPHA (TNF-α) AND INTERLEUKIN-1β (IL-1β)
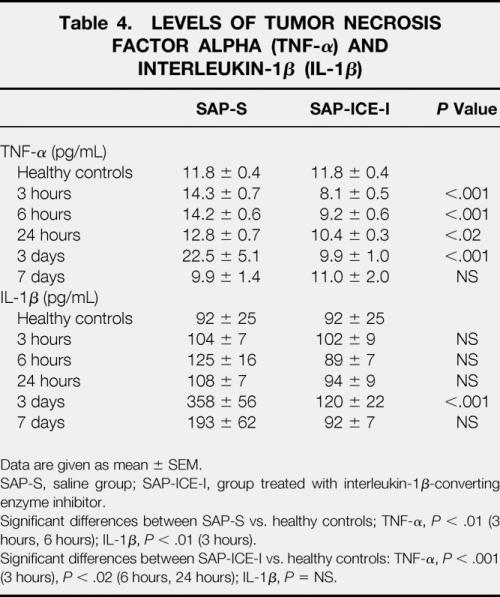
Data are given as mean ± SEM.
SAP-S, saline group; SAP-ICE-I, group treated with interleukin-1β-converting enzyme inhibitor.
Significant differences between SAP-S vs. healthy controls; TNF-α, P < .01 (3 hours, 6 hours); IL-1β, P < .01 (3 hours).
Significant differences between SAP-ICE-I vs. healthy controls: TNF-α, P < .001 (3 hours), P < .02 (6 hours, 24 hours); IL-1β, P = NS.
However, in all reports a prophylactic study design was used: either the inhibitor was given before induction of pancreatitis, or transgenic, ICE-deficient mice were used. It therefore remained unknown whether this regimen would prove effective if applied after the onset of symptoms. Because in the clinical setting patients are usually admitted to the hospital and treated beyond 48 hours after the onset of symptoms, any new potential therapeutic approach must be tested under conditions that meet these demands. To address this issue we used a severe, rapid-onset model of acute pancreatitis by retrograde infusion of 5% sodium taurocholate into the biliopancreatic duct. Thereafter, a cell-permeable and highly selective inhibitor of ICE 23,25 was administered up to 12 hours after induction of pancreatitis, a time point at which intrapancreatic damage has already peaked and systemic complications develop.
Our results confirm that ICE activation is detrimental with regard to the progression of intrapancreatic damage as well as the overall severity and death rate of severe acute pancreatitis. Even if treatment is started during full progression of the disease, death rates are significantly decreased. In terms of organ-specific damage, marked intrapancreatic necrosis developed in both treated and nontreated animals, but the overall extent of necrosis was significantly inhibited irrespective whether treatment was started 6 or 12 hours after disease onset. In contrast, pulmonary and hepatic cell damage was less expressed, and there were no significant differences between treated and nontreated animals. In terms of MPO concentrations as well as IL-1β, TNF-α, and ICE mRNA upregulation, similar observations were made: the changes of any single parameter were most expressed in the pancreas, followed by the lung, whereas no overt changes were found in the liver. In addition, almost every systemic biochemical parameter was significantly ameliorated in rats that received treatment with the inhibitor either 6 or 12 hours after induction of pancreatitis.
The prophylactic intraperitoneal administration of irreversible, highly specific inhibitors of ICE in lethal models of acute pancreatitis has been investigated in two studies so far. 24,25 The effects observed were similar to those obtained by the use of transgenic mice deficient in the gene and show the efficacy of the inhibitor in antagonizing ICE in vivo. Both prophylactic and delayed anticytokine therapy is known to differentially affect severity and survival in acute pancreatitis. Whereas TNF-α antagonism was even more effective if started in a delayed fashion, with symptoms of pancreatitis already being manifest, 12 IL-1 antagonism was slightly less effective in reducing pancreatitis-related severity and death rate under the same conditions. 13,14 This suggests that TNF-α seems to be of greater importance than IL-1β in promoting local tissue destruction and persisting organ failure, both of which are known as major determinants of survival in clinical acute pancreatitis. 35,36 The fact that prophylactic and therapeutic inhibition of ICE did not differ in terms of local and systemic effects as well as death rate implies that additional mechanisms must be involved. Beyond generating major amounts of active IL-1β, ICE is also known to process the inactive precursor of IL-18 into its bioactive form. 19 In a recent study, IL-18 antagonism was highly effective in protecting mice against gram-negative endotoxemia. 37 In instances of acute pancreatitis, we recently showed that serum IL-18 concentrations were significantly increased in patients with a severe course complicated by the development of pancreatic necrosis and remote organ failure. 20 By comparing the serum concentrations of both IL-1β5 and IL-18 in the same patient series, an interesting observation becomes evident: IL-1β was significantly increased only during the first days after onset of symptoms. In contrast, IL-18 peaked after the first week of the disease, which closely paralleled the onset of severe organ failure. The effectiveness of delayed ICE treatment could therefore be a result of inhibited generation of mature IL-18 rather than IL-1β. In addition, it is well established that IL-1β, IL-18, and TNF-α share a close interrelationship by inducing the synthesis of each other. 3,4 Because TNF-α mRNA expression was uniformly downregulated in all animals receiving treatment, a decrease in TNF-α-mediated severity may have additionally contributed in this context.
From a pathophysiologic standpoint, our results support the theory that the inflamed gland acts as a pacemaker for the subsequent development of remote organ failure. Although the presence of necrosis is substantial in the overall severity determination, 35 infiltrating inflammatory cells seem to be of even greater importance in promoting further tissue destruction and are known to be the major source of continuous release of cytokines 38,39 and other toxic agents. 26,40,41 This is evident if the organ-specific changes are compared. Cellular damage and neutrophil tissue infiltration, as well as cytokine and ICE mRNA levels, were most expressed in the pancreas, whereas liver and even lungs revealed only moderate or no changes of any parameter assessed. Besides a dramatic reduction of pancreatic necrosis, neutrophil tissue infiltration was significantly reduced in both pancreas and lungs after ICE inhibition. Thus, an important source for the release of deleterious mediators was suppressed, with subsequent significant benefits on systemic severity and survival. However, the bile reflux model of acute pancreatitis has been reported to be associated with only moderate pulmonary damage in some reports. 40 Because experimental studies in CDE diet or cerulein-induced acute pancreatitis in mice 27 are in contrast to the present observations, model- or species-dependent influences must be taken into account.
In summary, activation of caspase 1/ICE plays a central role in the determination of local and systemic severity and survival in SAP. As a consequence of downregulated local ICE-dependent cytokine synthesis, a substantial decrease of neutrophil-mediated tissue injury in pancreas and lung seems to contribute to the beneficial effects of this approach. Moreover, ICE inhibition is still effective after a therapeutic window of 12 hours. Based on the current findings, future studies on the clinical application of ICE-inhibiting substances in SAP seem to be promising.
Acknowledgments
The authors thank Ms. Silke Dauser, Mr. Michael and Mrs. Elke Marzinzig, and Mrs. Erika Schmidt for their support and excellent technical assistance.
Footnotes
Supported by the Deutsche Forschungsgemeinschaft, SFB/A6 (B.R., P.M.).
The first two authors contributed equally to this paper.
Correspondence: Prof. Dr. med. H.G. Beger, Department of General Surgery, University of Ulm, Steinhoevelstr. 9, 89075 Ulm, Germany.
E-mail: hans.beger@medizin.uni-ulm.de
Accepted for publication May 22, 2001.
References
- 1.Beger HG, Rau B, Mayer J, et al. Natural course of acute pancreatitis. World J Surg 1997; 21: 130–135. [DOI] [PubMed] [Google Scholar]
- 2.Dervenis C, Johnson CD, Bassi C, et al. Diagnosis, objective assessment of severity, and management of acute pancreatitis: Santorini consensus conference. Int J Pancreatol 1999; 25: 195–210. [DOI] [PubMed] [Google Scholar]
- 3.Norman J. The role of cytokines in the pathogenesis of acute pancreatitis. Am J Surg 1998; 175: 76–83. [DOI] [PubMed] [Google Scholar]
- 4.Ogawa M. Acute pancreatitis and cytokines: “Second attack” by septic complications leads to organ failure. Pancreas 1998; 16: 312–315. [PubMed] [Google Scholar]
- 5.Mayer J, Rau B, Gansauge F, et al. Inflammatory mediators in human acute pancreatitis: clinical and pathophysiological implications. Gut 2000; 47: 546–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Beaux AC, Ros JA, Maingay JP, et al. Proinflammatory cytokine release by peripheral blood mononuclear cells from patients with acute pancreatitis. Br J Surg 1996; 83: 1071–1075. [DOI] [PubMed] [Google Scholar]
- 7.Grewal HP, Mohey el Din AB, Gaber L, et al. Amelioration of the physiologic and biochemical changes of acute pancreatitis using an anti TNF-α polyclonal antibody. Am J Surg 1994; 167: 214–219. [DOI] [PubMed] [Google Scholar]
- 8.Hughes CB, Grewal HP, Gaber LW, et al. Anti-TNF alpha therapy improves survival and ameliorates the pathophysiologic sequelae in acute pancreatitis in the rat. Am J Surg 1996; 17: 274–280. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka N, Murata A, Uda K, et al. Interleukin-1 receptor antagonist modifies the changes in vital organs by acute necrotizing pancreatitis in a rat experimental model. Crit Care Med 1995; 23: 901–908. [DOI] [PubMed] [Google Scholar]
- 10.Norman JG, Fink G, Franz M, et al. Active interleukin-1 receptor is required for maximal progression of acute pancreatitis. Ann Surg 1996; 223: 163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denham W, Yang J, Fink G, et al. Gene targeting demonstrates additive detrimental effects of interleukin-1 and tumor necrosis factor during pancreatitis. Gastroenterology 1997; 113: 1741–1746. [DOI] [PubMed] [Google Scholar]
- 12.Norman JG, Fink GW, Messina J, et al. Timing of tumor necrosis factor antagonism is critical in determining outcome in murine lethal acute pancreatitis. Surgery 1996; 120: 515–521. [DOI] [PubMed] [Google Scholar]
- 13.Norman J, Franz M, Messina J, et al. Interleukin-1 receptor antagonist decreases severity of experimental acute pancreatitis. Surgery 1995; 117: 648–655. [DOI] [PubMed] [Google Scholar]
- 14.Norman JG, Franz MG, Fink GS, et al. Decreased mortality of severe acute pancreatitis after proximal cytokine blockade. Ann Surg 1995; 221: 625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cain BS, Meldrum DR, Harken AH, et al. The physiologic basis for anticytokine clinical trials in the treatment of sepsis. J Am Coll Surg 1998; 186: 337–350. [DOI] [PubMed] [Google Scholar]
- 16.Baumgartner JD, Calandra T. Treatment of sepsis: past and future avenues. Drugs 1999; 57: 127–132. [DOI] [PubMed] [Google Scholar]
- 17.Kostura MJ, Tocci MJ, Limjuco G, et al. Identification of a monocyte-specific pre-interleukin 1β convertase activity. Proc Natl Acad Sci USA 1989; 86: 5227–5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson KP, Black JAF, Thomson JA, et al. Structure and mechanism of interleukin-1β-converting enzyme. Nature 1994; 370: 270–274. [DOI] [PubMed] [Google Scholar]
- 19.Dinarello CA. Interleukin-1 beta, interleukin-18, and the interleukin-1 beta-converting enzyme. Ann NY Acad Sci 1998; 856: 1–11. [DOI] [PubMed] [Google Scholar]
- 20.Rau B, Baumgart K, Paszkowski AS, et al. Clinical relevance of caspase-1 activated cytokines in acute pancreatitis: High correlation of serum interleukin-18 with pancreatic necrosis and systemic complications. Crit Care Med 2001; 29: 1556–1562. [DOI] [PubMed] [Google Scholar]
- 21.Li P, Allen H, Banerjee S, et al. Mice deficient in IL-1β-converting enzyme are defective in production of mature IL-1β and resistant to endotoxic shock. Cell 1995; 80: 401–411. [DOI] [PubMed] [Google Scholar]
- 22.Ku G, Faust T, Lauffer LL, et al. Interleukin-1β-converting enzyme inhibition blocks progression of type II collagen-induced arthritis in mice. Cytokine 1996; 8: 377–386. [DOI] [PubMed] [Google Scholar]
- 23.Roquet N, Pages J-C, Molina T, et al. ICE inhibitor YVADcmk is a potent therapeutic agent against in vivo liver apoptosis. Curr Biol 1996; 6: 1192–1195. [DOI] [PubMed] [Google Scholar]
- 24.Norman J, Yang J, Fink G, et al. Severity and mortality of experimental pancreatitis are dependent on interleukin-1-converting enzyme. J Interferon Cytokine Res 1997; 17: 113–118. [DOI] [PubMed] [Google Scholar]
- 25.Rau B, Paszkowski A, Lillich S, et al. Differential effects of interleukin-1β-converting enzyme (ICE) on acinar cell death by necrosis and apoptosis in severe acute experimental pancreatitis. Lab Invest 2001; 81: 1001–1013. [DOI] [PubMed] [Google Scholar]
- 26.Rau B, Poch B, Gansauge F, et al. Pathophysiological role of oxygen free radicals in acute pancreatitis: initiating event or mediator of tissue damage? Ann Surg 2000; 231: 352–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mayer J, Laine VJO, Rau B, et al. Systemic lymphocyte activation modulates the severity of diet-induced acute pancreatitis in mice. Pancreas 1999; 19: 62–68. [DOI] [PubMed] [Google Scholar]
- 28.Bradley PP, Priebat DA, Christensen RD, et al. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol 1982; 78: 206–209. [DOI] [PubMed] [Google Scholar]
- 29.Lowry OH, Rosebrough NJ, Farr AL, et al. Protein measurement with the folin phenol reagent. J Biol Chem 1951; 193: 265–275. [PubMed] [Google Scholar]
- 30.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinum thiocyanate-phenol-chloroform extraction. Anal Biochem 1987; 162: 156–159. [DOI] [PubMed] [Google Scholar]
- 31.Schoonjans F, Zalata A, Depuydt CE, et al. MedCalc: a new computer program for medical statistics. Comp Meth Prog Biomed 1995; 48: 257–262. [DOI] [PubMed] [Google Scholar]
- 32.Miwa K, Asano M, Horai R, et al. Caspase-1 independent IL-1 beta release and inflammation induced by the apoptosis inducer Fas ligand. Nat Med 1998; 4: 1287–1292. [DOI] [PubMed] [Google Scholar]
- 33.Schonbeck U, Mach F, Libby P. Generation of biologically active IL-1 beta by matrix metalloproteinases: a novel caspase-1 independent pathway of IL-1 beta processing. J Immunol 1998; 161: 3340–3346. [PubMed] [Google Scholar]
- 34.Kuida K, Lippke J, Ku G, et al. Altered cytokine export and apoptosis in mice deficient in interleukin-1-converting enzyme. Science 1995; 267: 2000–2003. [DOI] [PubMed] [Google Scholar]
- 35.Beger HG, Bittner R, Block S, et al. Bacterial contamination of pancreatic necrosis. Gastroenterology 1986; 49: 433–438. [DOI] [PubMed] [Google Scholar]
- 36.Isemann R, Rau B, Beger HG. Bacterial infection and extent of necrosis are determinants of organ failure in patients with acute necrotizing pancreatitis. Br J Surg 1999; 86: 1020–1024. [DOI] [PubMed] [Google Scholar]
- 37.Netea MG, Fantuzzi G, Kullberg BJ, et al. Neutralization of IL-18 reduces neutrophil tissue accumulation and protects mice against lethal Escherichia coli and Salmonella typhimurium endotoxemia. J Immunol 2000; 164: 2644–2649. [DOI] [PubMed] [Google Scholar]
- 38.Fink G, Norman JG. Intrapancreatic interleukin-1β gene expression by specific leukocyte populations during acute pancreatitis. J Surg Res 1996; 63: 369–373. [DOI] [PubMed] [Google Scholar]
- 39.McKay CJ, Gallagher G, Brooks B, et al. Increased monocyte cytokine production in association with systemic complications in acute pancreatitis. Br J Surg 1996; 83: 919–923. [DOI] [PubMed] [Google Scholar]
- 40.Poch B, Gansauge F, Rau B, et al. The role of polymorphonuclear leukocytes and oxygen-derived free radicals in experimental acute pancreatitis: mediators of local destruction and activators of inflammation. FEBS Lett 1999; 461: 268–272. [DOI] [PubMed] [Google Scholar]
- 41.Yang J, Denham W, Tracey KJ, et al. The physiologic consequences of macrophage pacification during severe acute pancreatitis. Shock 1998; 10: 169–175. [DOI] [PubMed] [Google Scholar]


