Abstract
Objective
To analyze a single center’s 6-year experience with 258 consecutive patients undergoing major hepatic resection for primary or secondary malignancy of the liver, and to examine the predictive value of preoperative liver function assessment.
Summary Background Data
Despite the substantial improvements in diagnostic and surgical techniques that have made liver surgery a safer procedure, careful patient selection remains mandatory to achieve good results in patients with hepatic tumors.
Methods
In this prospective study, 258 patients undergoing hepatic resection were enrolled: 111 for metastases, 78 for hepatocellular carcinoma (HCC), 21 for cholangiocellular carcinoma, and 48 for other primary hepatic tumors. One hundred fifty-eight patients underwent segment-oriented liver resection, including hemihepatectomies, and 100 had subsegmental resections. Thirty-two clinical and biochemical parameters were analyzed, including liver function assessment by the galactose elimination capacity (GEC) test, a measure of hepatic functional reserve, to predict postoperative (60-day) rates of death and complications and long-term survival. All variables were determined within 5 days before surgery. Data were subjected to univariate and multivariate analysis for two patient subgroups (HCC and non-HCC). The cutoffs for GEC in both groups were predefined. Long-term survival (>60 days) was subjected to Kaplan-Meier analysis and the Cox proportional hazard model.
Results
In the entire group of 258 patients, a GEC less than 6 mg/min/kg was the only preoperative biochemical parameter that predicted postoperative complications and death by univariate and stepwise regression analysis. A GEC of more than 6 mg/min/kg was also significantly associated with longer survival. This predictive value could also be shown in the subgroup of 180 patients with tumors other than HCC. In the subgroup of 78 patients with HCC, a GEC less than 4 mg/min/kg predicted postoperative complications and death by univariate and stepwise regression analysis. Further, a GEC of more than 4 mg/min/kg was also associated with longer survival.
Conclusions
This prospective study establishes the preoperative determination of the hepatic reserve by GEC as a strong independent and valuable predictor for short- and long-term outcome in patients with primary and secondary hepatic tumors undergoing resection.
The incidence of primary hepatic tumors, in particular hepatocellular carcinoma (HCC), is increasing. 1 Hepatic resection or liver transplantation is the only potentially curative option for these patients. 2–4 In addition, liver metastasis, in particular from colorectal cancer, is a common clinical situation, and surgical resection of these metastasis improves survival. 5
Because of considerable improvements in perioperative intensive care and refinements in surgical technique, rates of death and complications after major liver resection have significantly decreased during the past 20 years. 6–9 Because many patients also have liver cirrhosis or other chronic liver disease, death and complications after liver resection may occur, liver failure being one of the most dreaded complications. Different scores 10,11 and quantitative liver function tests 12 have been inaugurated to identify patients at risk for postoperative liver failure and other complications.
We evaluated the predictive value of determination of galactose elimination capacity (GEC) along with 31 other clinical and biochemical parameters in a prospective study. Galactose elimination capacity has been shown to have a predictive value for fulminant hepatic failure, 13 primary biliary cirrhosis, 14 and chronic active hepatitis. 15 Further, it provides additional prognostic information in cirrhotic patients when compared with the Child-Pugh classification. 16 However, its value has never been investigated in the setting of liver resection. This analysis in 258 consecutive patients at a single institution shows that preoperative assessment of functional liver parenchyma by determination of GEC has a predictive value not only for postoperative death and complications but also long-term survival.
METHODS
Between January 1994 and January 2000, data from 307 consecutive patients with liver tumors were entered in a prospective statistical database collection. Patients with extrahepatic tumor dissemination or recurrence of the extrahepatic malignancy at the primary site were not considered. Data from 49 patients with unresectable bilateral tumors were also excluded. The remaining 258 patients underwent liver resections for neoplasms and were further evaluated. Long-term data after surgery were obtained periodically by visits in our outpatient clinic or from the patient’s physician records.
Thirty-two parameters were analyzed for each patient (Table 1). The GEC was determined by serial measurements of the serum concentration of galactose after a single intravenous bolus dose of 0.5 mg/kg galactose according to Tygstrup, 17 with some modifications, as previously described in detail. 18 The cutoffs of GEC for the two groups were defined before the statistical analysis. The GEC cutoff in patients without HCC was set at 6 mg/min/kg because this is the lower limit of the normal range. The GEC cutoff in patients with HCC was set at 4 mg/min/kg because this represents a 50% reduction of the hepatic contribution to the total GEC. 19 Complications or death occurring either within 60 days from the date of surgery or before hospital discharge were considered postoperative. Major complications were defined as reoperation or massive postoperative bleeding (>300 mL/hour), hemodialysis resulting from renal insufficiency, prolonged antibiotic therapy (>7 days), bile drainage, myocardial infarction, encephalopathy, pulmonary distress with prolonged mechanical ventilation (>24 hours), or sepsis. Complications were defined as minor if discharge or treatment was not delayed and they could be resolved with simple medication.
Table 1. PARAMETERS
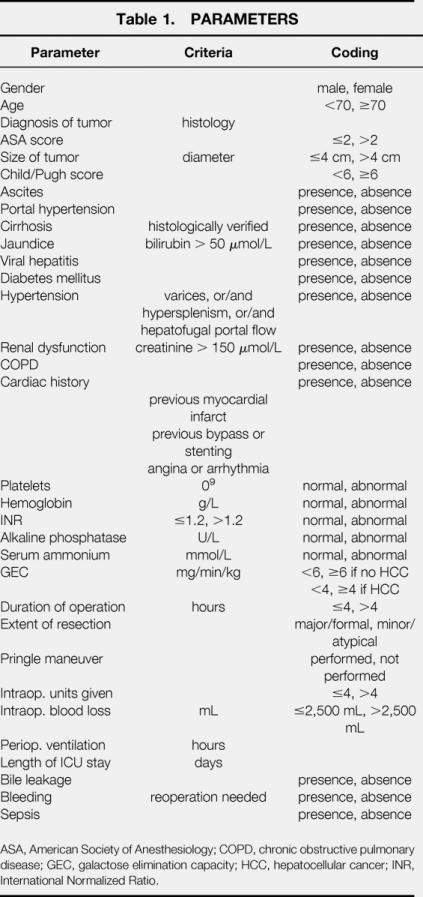
ASA, American Society of Anesthesiology; COPD, chronic obstructive pulmonary disease; GEC, galactose elimination capacity; HCC, hepatocellular cancer; INR, International Normalized Ratio.
All patients had combined epidural and general anesthesia with standardized macrocirculatory and respiratory monitoring. An epidural catheter was introduced at level T5–T7 before introduction of general anesthesia, and an epidural solution containing bupivacaine, fentanyl, and epinephrine was infused. General anesthesia was induced with sodium thiopentone, fentanyl, and pancuronium and maintained with 0% to 70% N2O in O2 and isoflurane as well as repeated intermittent bolus doses of fentanyl and pancuronium. Blood pressure was monitored continuously through a radial arterial line and maintained at a mean arterial pressure of 70 to 90 mm Hg by adjusting anesthetics or infusing colloids and crystalloids. Arterial blood oxygen saturation was measured continuously by pulse oximetry and maintained at more than 90%. The individual surgical approach was determined during surgery according to the size, location, and extent of the liver tumor as well as the results of the preoperative liver function tests. A clear resection margin of at least 1 cm was a standard requirement for resection and was not influenced or determined by other factors. The tumor tissue was completely resected macroscopically in all patients. Nonanatomic or atypical resections were defined as resections of a lesion without regard to segmental or lobar anatomy, including the classically defined wedge resection. Hemihepatectomies or segment-based resections (formal resections) followed the anatomic definitions into segments and lobes according to Couinaud 20 and were performed along the modified resection lines for extended liver resection proposed by Blumgart. 21 Patients routinely underwent intraoperative ultrasonography to determine tumor localization and extent and to exclude the presence of additional lesions in the residual liver. To minimize blood loss, parenchymal dissections were performed using the Cavitron Ultrasonic Surgical Aspirator (Valley Lab, Inc. Stamford, CT). Hemostasis was achieved by argon beam coagulation (Deltamed-Erbe, Winterthur, Switzerland) and by ligation of individual blood vessels and bile ducts. One or two silicon drains were positioned to detect postoperative bleeding or bile leakage.
Statistical Analysis
To determine the risk factors related to postoperative death, complications, and survival, all relevant data were entered into a statistical file. Using a statistical package program (SPSS, Chicago, IL), all variables were analyzed by the Fisher exact test, the chi-square test, and the Mann-Whitney test where appropriate. First, differences in the various factors were examined between patient groups with and without major postoperative complications, and then multivariate analysis using a logistic stepwise regression model was performed to detect comprehensive correlations and risk factors. Stepwise regression and selection were based on the maximal likelihood ratio test. Only variables with P < .1 after univariate analysis were retained for the multiple logistic model. Survival analysis was performed by the Kaplan-Meier method; the significance of the difference between different survival curves was assessed using the log-rank test. Only variables with P < .1 were included in the multivariate stepwise regression survival analysis using the Cox proportional hazard model. All data are reported as median and standard deviation and range.
In this study, 32 factors were considered, 27 for the complications analysis and 29 for the survival analysis. Testing all the factors in univariate analysis is the first step in an explorative data analysis (i.e., the extensive multiple testing is not controlling the global first error rate). Applying a correction such as Bonferroni or Holm-Bonferroni indicates that only a few of the factors are still significant prognostic parameters. The multivariate analysis is more informative because the univariate analysis can be confounded by other factors. The univariate analyses were used as indicators for the multivariate one. Factors with P < .05 were considered statistically significant.
RESULTS
A total of 258 patients undergoing liver resection for neoplasms were included in the study. Their median age was 59 years (range 17–85) and the median ASA score was 2.0 (range 1–4). Demographic characteristics of the study population are summarized in Table 2. Hepatic metastases of colon carcinoma (81/258 [31%]) or other hepatic metastases from other sites (30/258 [12%]) and primary HCC (78/258 [30%]) were the predominant indications for liver resection, followed by other primary hepatic tumors (48/258 [19%]) and hilar cholangiocarcinoma (21/258 [8%]). Among the patients with HCC, 89% had cirrhosis, compared with 6% in patients with metastatic liver disease (10/180). Six of these 10 patients with metastatic disease and cirrhosis had a preoperative GEC less than 6 mg/min/kg.
Table 2. DEMOGRAPHICS (n = 258)
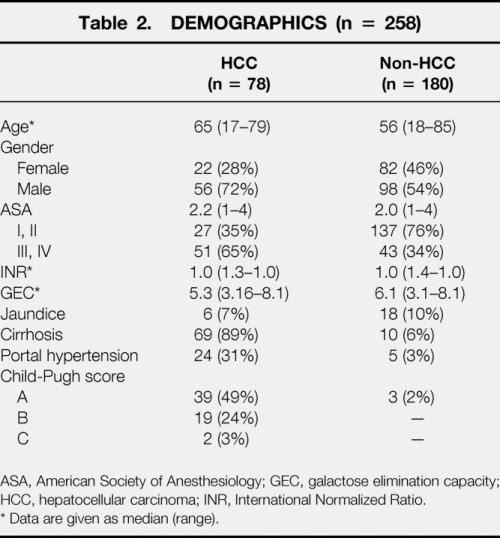
ASA, American Society of Anesthesiology; GEC, galactose elimination capacity; HCC, hepatocellular carcinoma; INR, International Normalized Ratio.
* Data are given as median (range).
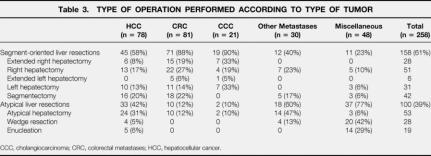
Most patients (158/258 [61%]) underwent segmental or sector-oriented hepatic resections. Fifty-one patients had classical and 28 had extended right hepatectomy. In 31 patients a formal left hepatectomy was performed, and 6 patients underwent extended left hepatectomy. Tissue-preserving nonanatomic liver resections, wedge resections, or left lobar subsegmentectomies were performed in 100 patients (39%). The surgical procedure with regard to the underlying diagnosis is detailed in Table 3. The surgical time averaged 4.1 ± 1.8 hours, and perioperative blood loss was 2.3 ± 1.9 L. Intermittent portal triad clamping was used in 39 of the 258 patients (15%) and continuous pedicular clamping (Pringle maneuver) in 21 of the 258 (8%).
Table 3. TYPE OF OPERATION PERFORMED ACCORDING TO TYPE OF TUMOR
CCC, cholangiocarcinoma; CRC, colorectal metastases; HCC, hepatocellular cancer.
Postoperative Death and Complications
Six patients (2%) died within 60 days after surgery; all postoperative deaths occurred after extended hepatic resections. Two patients were older than 70 years of age and died of acute myocardial infarction after uneventful surgery. Neither of these two patients had a history of cardiac disease. The other four hospital deaths occurred in patients younger than 60 years of age: one patient died of acute respiratory distress syndrome after multiorgan failure, acute liver failure developed in two patients after extended right hepatectomy, and one patient died of uncontrollable sepsis with consecutive multiorgan failure.
Univariate analysis of the 67 patients (26%) who had major postoperative complications identified 6 of 27 parameters to be significant risk factors for major complications for both groups of patients: concomitant cardiovascular risks; pathologic GEC; ASA score more than 2; duration of surgery more than 4 hours; intraoperative blood loss more than 2.5 L; and red blood cell transfusion more than 4 units (Table 4). In patients with HCC, five more factors were determined to be significantly predictors for complications: age older than 70 years; presence of cirrhosis with Child-Pugh classification B or C; portal hypertension; jaundice; and the extent of resection. Concomitant chronic obstructive pulmonary disease and insulin-dependent diabetes mellitus significantly increased the risk of complications in non-HCC patients.
Table 4. UNIVARIATE ANALYSIS OF PREDICTING FACTORS FOR POSTOPERATIVE COMPLICATIONS
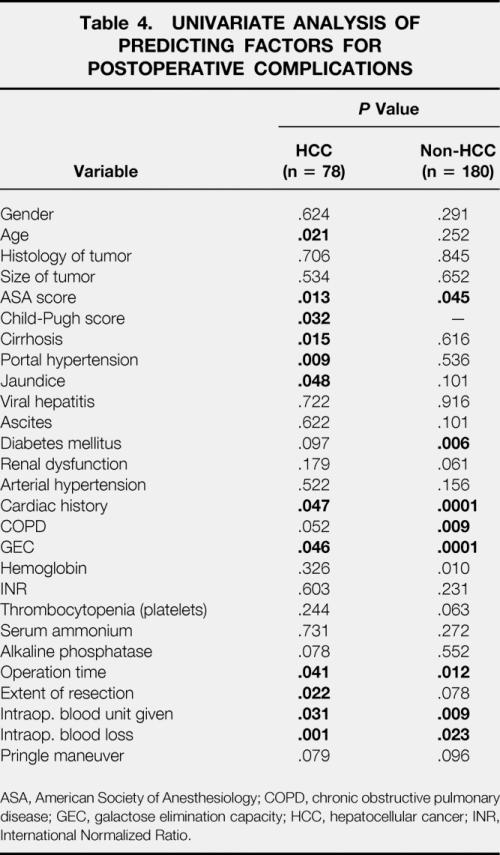
ASA, American Society of Anesthesiology; COPD, chronic obstructive pulmonary disease; GEC, galactose elimination capacity; HCC, hepatocellular cancer; INR, International Normalized Ratio.
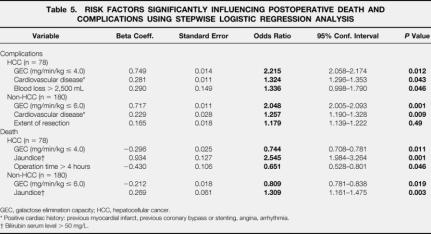
The multivariate analysis by a stepwise logistic regression model identified two independent significant variables for postoperative complications in both groups: pathologic GEC and concomitant cardiovascular disease. Intraoperative blood loss more than 2.5 L was a strong predictor for patients undergoing liver resection for HCC. An extended hepatic resection was predictive for non-HCC patients (Table 5). The multivariate analysis revealed pathologic liver function (low GEC), preoperative jaundice, and prolonged duration of surgery (>4 hours) were independent risk factors for death.
Table 5. RISK FACTORS SIGNIFICANTLY INFLUENCING POSTOPERATIVE DEATH AND COMPLICATIONS USING STEPWISE LOGISTIC REGRESSION ANALYSIS
GEC, galactose elimination capacity; HCC, hepatocellular cancer.
* Positive cardiac history: previous myocardial infarct, previous coronary bypass or stenting, angina, arrhythmia.
† Bilirubin serum level > 50 mg/L.
With the predefined cutoff of 4.0 mg/min/kg, the GEC had a sensitivity of 51.9% and a specificity of 100% in the HCC group. In the non-HCC group (with a cutoff of 6.0 mg/min/kg), GEC showed a sensitivity of 92.5% and a specificity of 64.5%. The positive and negative predictive values were 100% and 79.7%, respectively, for the HCC group (with a cutoff of 4.0) and 43.0% and 96.7%, respectively, for the non-HCC group (with a cutoff of 6.0).
Survival
The median follow-up period was 36 months (range 1–67). Overall 1-year, 3-year, and 5-year survival rates were 89%, 62%, and 39% (median 42 months) in patients with HCC, 96%, 70%, and 48% (median 48 months) for patients with resected colorectal metastases, and 78%, 35%, and 11% (median 18 months) in patients with hilar cholangiocarcinoma (Fig. 1).
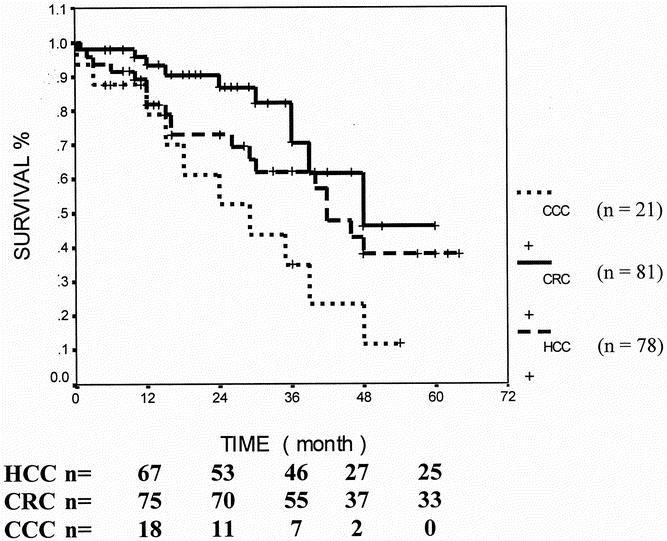
Figure 1. Comparison of survival rates (Kaplan-Meier) between different diagnoses in patients undergoing hepatic resection for malignancies. Median survival for cholangiocarcinoma (CCC) was significantly worse (18 months) than that for colorectal carcinoma (CRC, 48 months) and that for hepatocellular carcinoma (HCC, 41 months). Log-rank test, P = .0173.
Abnormal GEC, sepsis after surgery, and a high ASA score (>2) significantly predicted decreased survival in all patients undergoing liver surgery. In patients with colorectal metastases and other secondary liver malignancies, age older than 70 years and coexistent jaundice before surgery were additional predictors for poor long-term outcome. In patients with HCC, a duration of surgery of more than 4 hours and postoperative sepsis were significantly associated with shortened survival (Table 6). In addition, patients undergoing liver resection for HCC had significantly poorer survival if their preoperative GEC was decreased (<4 mg/min/kg, P = .003 by log-rank test) (Fig. 2). Patients with tumors other than HCC had a better survival if their preoperative GEC exceeded 6 mg/min/kg (Fig. 3;P < .03 by log-rank test). Twenty-eight non-HCC patients had a GEC less than 6 mg/min/kg, but none was below 5.6. Six of these 28 patients had liver cirrhosis.
Table 6. RESULTS OF UNIVARIATE ANALYSIS FOR SURVIVAL
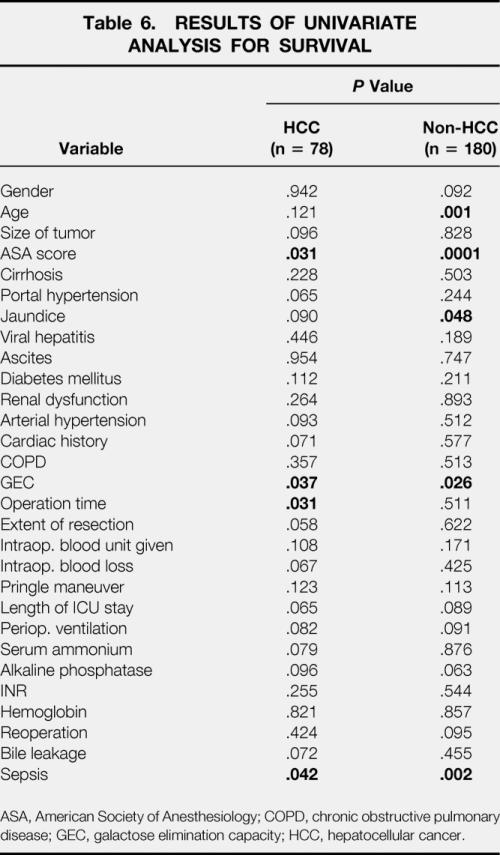
ASA, American Society of Anesthesiology; COPD, chronic obstructive pulmonary disease; GEC, galactose elimination capacity; HCC, hepatocellular cancer.
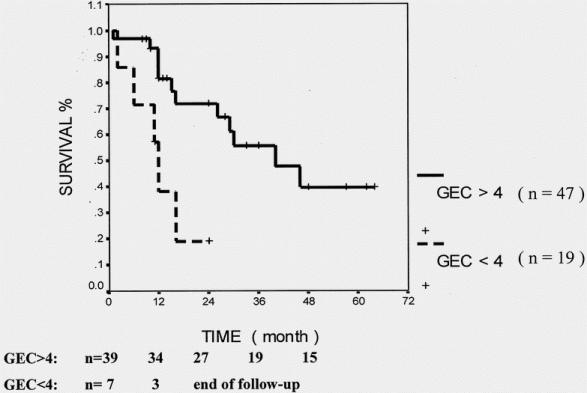
Figure 2. Cumulative survival for patients with hepatocellular carcinoma in terms of preoperative liver function (galactose elimination capacity [GEC], mg/min/kg). Log-rank test, P = .003.
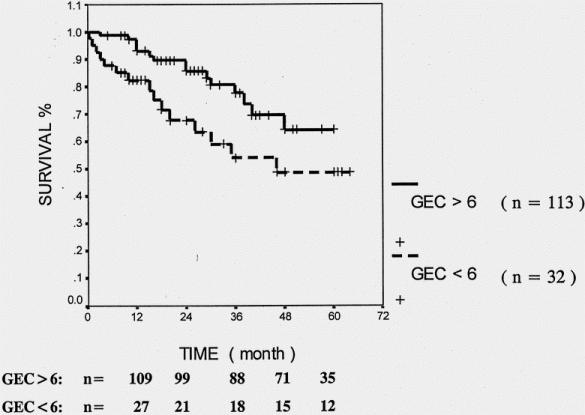
Figure 3. Cumulative survival for patients without hepatocellular carcinoma in terms of preoperative pathologic galactose elimination capacity (GEC) test. Log-rank test, P = .0282.
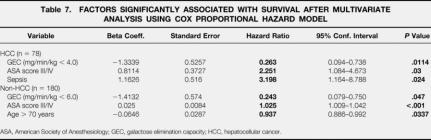
The multivariate Cox regression analysis assessing the significant prognostic factors of univariate analysis revealed a GEC less than 6 mg/min/kg and an ASA score greater than 2 as independent factors for shorter survival in patients regardless of tumor etiology. In non-HCC patients the same factors were identified, plus age older than 70 years. In patients with HCC, predictive factors were GEC less than 4 mg/min/kg, ASA score greater than 2, and postoperative sepsis (Table 7).
Table 7. FACTORS SIGNIFICANTLY ASSOCIATED WITH SURVIVAL AFTER MULTIVARIATE ANALYSIS USING COX PROPORTIONAL HAZARD MODEL
ASA, American Society of Anesthesiology; GEC, galactose elimination capacity; HCC, hepatocellular cancer.
DISCUSSION
In this prospective study, we analyzed a variety of parameters with respect to their ability to predict postoperative death and complications after hepatic resection for primary or secondary hepatic malignancy. We showed that GEC is consistently better than other parameters, including the Child-Pugh classification, to predict not only postoperative complications but also long-term survival. This was true for patients undergoing hepatic resection for metastasis of colorectal cancer as well as for those undergoing hepatic resection for HCC.
Our figures compare well with the literature. 5–7,22 In the short term, 20% to 50% of cirrhotic patients undergoing hepatic resection for HCC have postoperative complications, hepatic failure being the most frequent and most lethal. 23 The volume of liver that can be safely resected without inducing liver insufficiency is unknown. It is certainly less in patients with cirrhosis, because their liver has not only a diminished preoperative hepatic reserve but also a reduced capacity to regenerate. 24,25
Numerous factors have been suggested to influence the prognosis of patients undergoing hepatic resection, such as age;26 presence of cirrhosis;23 distribution, 27 size, 28 and number 29 of liver metastases; size of the HCC;22,30 Okuda stage of the HCC;31 tumor invasion of the portal vein;32 type of surgery;33 blood loss during surgery;34–36 and concomitant diseases, such as diabetes mellitus. 37 We took these parameters into account in our analysis (see Table 1); in addition, we performed preoperative determination of the GEC. This test is safe, inexpensive, and reproducible. In animals, it correlates closely with liver weight 38 and, more relevant for patients with chronic liver disease, with hepatocellular mass in animal models of chronic liver disease. 39 The rate-limiting step in galactose metabolism is phosphorylation of galactose by galactokinase, an enzyme located in the cytosol of hepatocytes;19,40 therefore, hepatic GEC is considered to depend primarily on the mass of functional hepatocytes. 19 In patients, it has been found to be of prognostic value in chronic active hepatitis, 15 cirrhosis, 16 and primary biliary cirrhosis. 14
Liver cirrhosis, even when compensated, may complicate hepatic resection as a result of coagulopathy or portal hypertension. Conventional biochemical liver tests have only limited value when it comes to estimating hepatocellular reserve; elevated serum bilirubin or decreased prothrombin time before surgery represent warning signs. Therefore, Hasegawa et al 41 considered a serum bilirubin level of more than 2.0 mg/dL (35 μmol/L) an absolute contraindication for hepatic resection. These two parameters are part of the Child-Pugh classification, originally described to identify patients for portocaval shunting. 10 Indeed, different studies found the Child-Pugh classification to be a good predictor of perioperative death in patients with cirrhosis undergoing abdominal resection, with 10%, 30%, and 76% to 82% death rates in patients with Child class A, B, and C, respectively. 42,43 In contrast, Japanese groups did not find the Child-Pugh classification to be helpful to identify long-term survivors. 44,45 One of these authors reported more recently a correlation between the Child-Pugh classification and long-term survival, 46 but the number of patients with a Child class C was small. This probably reflects the fact that surgeons today exclude Child class C patients from surgery; this is also reflected in our series, where only two patients with Child class C underwent surgery. Thus, the Child classification can be used to exclude patients from surgery, but in those who do undergo surgery, it fails to predict death and complications.
Different studies have evaluated a variety of quantitative liver function tests to predict postoperative death and complications. In an early study on a limited number of patients (38 patients, mostly with alcoholic liver disease) undergoing a variety of visceral surgical procedures, the aminopyrine breath test, a measure of microsomal function, was found to be superior to the Child-Pugh classification in predicting complications and survival. 47 Indocyanine green retention in combination with radiologic estimation of the liver volume was found to be of value to predict posthepatectomy liver failure. 48,49 Others have reported the indocyanine green clearance test to be a good preoperative predictor of death and complications in patients undergoing liver resection for HCC. 35,50–52
Quantitative liver function tests, whether indocyanine green retention or GEC, are superior presumably because they are less determined by extrahepatic factors (as is the case with serum bilirubin, serum albumin, or prothrombin time), and that allows a better estimate of hepatic functional reserve than conventional liver tests, whether alone or combined into a score.
Postoperative liver failure in patients with hepatic metastasis and presumably normal functional reserve is much rarer than in patients with HCC, who practically all have underlying structural disease. 53 In noncirrhotic patients having resection for metastases, preoperative serum alkaline phosphatase has been reported to predict postoperative liver insufficiency. 54 In our univariate analysis, we found abnormal alkaline phosphatase to predict short-term survival but not postoperative complications; this prognostic value was not confirmed in multivariate analysis. In contrast, GEC was informative regarding postoperative complications and survival in patients undergoing liver resection for tumors other than HCC, in particular those with metastatic disease. This finding was surprising because liver function is thought to be maintained in patients with metastasis to the liver. In some patients the GEC was certainly diminished as a result of underlying liver disease, in particular cirrhosis in 10 patients. This cannot explain the discriminant capacity of GEC alone, however.
This raises the question of whether liver function is affected by the presence of hepatic metastasis. In a recent large series on hepatic resections for metastatic disease, mostly tumor burden and time to recurrence determined survival. 11 In line with this concept, antipyrine clearance was maintained regardless of tumor burden. 55,56 However, in the latter study a decrease in conjugation reactions was found, suggesting that liver metastases could affect certain aspects of liver function. 56 Earlier studies found sulfobromophthalein clearance to be impaired in patients with hepatic metastases, 57 suggesting that unrecognized cholestasis could impair liver function. This contention is supported by the fact that even in the absence of metastasis, signs of cholestasis are often found in liver biopsy samples from patients with proven hepatic metastases. 58 Finally, nuclear medicine techniques 59 and duplex sonography 60 can show alterations of hepatic perfusion in the presence of metastases. Whether the diminution of GEC in some patients with metastatic disease is due to cholestasis or alterations of hepatic perfusion cannot be answered from the present investigation.
In conclusion, we found the GEC to be an important preoperative piece of information in assessing the risk of hepatic resection in patients with primary and secondary hepatic focal lesions. This simple, safe, and inexpensive test provides predictive information regarding both the short-term outcome and long-term survival. This analysis validates the use of performing a GEC before hepatic resection to stratify patients and exclude those at high risk.
Acknowledgments
The authors thank the nurses of the Hepatology Outpatient Clinic for their constant commitment to perform quantitative liver function tests, sometimes on very short notice.
Footnotes
Supported by a grant from the Cloëtta Foundation (C.A.R).
Presented at the 50th Annual Meeting of the American Association for the Study of Liver Diseases, November 1999, Dallas, Texas.
Correspondence: Markus W. Büchler, MD, Department of General Surgery, University of Heidelberg, Heidelberg, Germany.
E-mail: markus_buechler@med.uni-heidelberg.de
Accepted for publication May 22, 2001.
References
- 1.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med 1999; 340: 745–750. [DOI] [PubMed] [Google Scholar]
- 2.Michel J, Suc B, Montpeyroux F, et al. Liver resection or transplantation for hepatocellular carcinoma? Retrospective analysis of 215 patients with cirrhosis. J Hepatol 1997; 26: 1274–1280. [DOI] [PubMed] [Google Scholar]
- 3.Marsh JW, Dvorchik I, Iwatsuki S. Liver transplantation in the treatment of hepatocellular carcinoma. J Hepatobiliary Pancreat Surg 1998; 5: 24–28. [DOI] [PubMed] [Google Scholar]
- 4.Bismuth H, Chiche L, Adam R, et al. Liver resection versus transplantation for hepatocellular carcinoma in cirrhotic patients. Ann Surg 1993; 218: 145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fong Y, Salo J. Surgical therapy of hepatic colorectal metastasis. Semin Oncol 1999; 26: 514–523. [PubMed] [Google Scholar]
- 6.Matsumata T, Taketomi A, Kawahara N, et al. Morbidity and mortality after hepatic resection in the modern era. Hepato-Gastroenterology 1995; 42: 456–460. [PubMed] [Google Scholar]
- 7.Lai EC, Fan ST, Lo CM, et al. Hepatic resection for hepatocellular carcinoma. An audit of 343 patients. Ann Surg 1995; 221: 291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shuto T, Hirohashi K, Kubo S, et al. Changes and results of surgical strategies for hepatocellular carcinoma: results of a 15-year study on 452 consecutive patients. Surg Today 1998; 28: 1124–1129. [DOI] [PubMed] [Google Scholar]
- 9.Torzilli G, Makuuchi M, Inoue K, et al. No-mortality liver resection for hepatocellular carcinoma in cirrhotic and noncirrhotic patients: is there a way? A prospective analysis of our approach. Arch Surg 1999; 134: 984–992. [DOI] [PubMed] [Google Scholar]
- 10.Child CG, Turcotte JG. Surgery in portal hypertension. In: Child CG, ed. Major problems in clinical surgery. The liver and portal hypertension. Philadelphia: WB Saunders; 1964: 1–85. [PubMed]
- 11.Iwatsuki S, Dvorchik I, Madariaga JR, et al. Hepatic resection for metastatic colorectal adenocarcinoma: a proposal of a prognostic scoring system. J Am Coll Surg 1999; 189: 291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zimmermann H, Reichen J. Hepatectomy: preoperative analysis of hepatic function and postoperative liver failure. Dig Surg 1998; 15: 1–11. [DOI] [PubMed] [Google Scholar]
- 13.Ranek L, Andreasen PB, Tygstrup N. Galactose elimination capacity as a prognostic index in patients with fulminant liver failure. Gut 1976; 17: 959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reichen J, Widmer T, Cotting J. Accurate prediction of death by serial determination of galactose elimination capacity in primary biliary cirrhosis: a comparison with the Mayo model. Hepatology 1991; 14: 504–510. [PubMed] [Google Scholar]
- 15.Aebli N, Reichen J. [The prognostic value of the serial determination of galactose elimination capacity in chronic active hepatitis]. Schweiz Med Wochenschr 1991; 121: 970–976. [PubMed] [Google Scholar]
- 16.Merkel C, Gatta A, Zoli M, et al. Prognostic value of galactose elimination capacity, aminopyrine breath test, and ICG clearance in patients with cirrhosis. Comparison with the Pugh score. Dig Dis Sci 1991; 36: 1197–1203. [DOI] [PubMed] [Google Scholar]
- 17.Tygstrup N. Determination of the hepatic elimination capacity (Lm) of galactose by single injection. Scand J Clin Lab Invest Suppl 1966; 18: 118–125. [PubMed] [Google Scholar]
- 18.Heri M, Bircher J. [Galactose elimination capacity, a reliable test for quantitative comprehension of the liver function]. Schweiz Med Wochenschr 1971; 101: 735–736. [PubMed] [Google Scholar]
- 19.Bircher J. Quantitative assessment of deranged hepatic function: a missed opportunity? Semin Liver Dis 1983; 3: 275–284. [DOI] [PubMed] [Google Scholar]
- 20.Couinaud C. [Bases anatomiques des hépatectomies gauches et droites réglées]. J Chir 1954; 70: 933–966. [PubMed] [Google Scholar]
- 21.Blumgart LH. Liver resection: Liver and biliary tumors. In: Blumgart LH, ed. Surgery of the liver and biliary tract. Edinburgh: Churchill Livingstone; 1988: 1251–1280.
- 22.Fong Y, Sun RL, Jarnagin W, et al. An analysis of 412 cases of hepatocellular carcinoma at a Western center. Ann Surg 1999; 229: 790–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagasue N, Kohno H, Chang YC, et al. Liver resection for hepatocellular carcinoma. Results of 229 consecutive patients during 11 years. Ann Surg 1993; 217: 375–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagasue N, Yukaya H, Ogawa Y, et al. Human liver regeneration after major hepatic resection. A study of normal liver and livers with chronic hepatitis and cirrhosis. Ann Surg 1987; 206: 30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hashimoto M, Watanabe G. Functional capacity of the cirrhotic liver after partial hepatectomy in the rat. Surgery 1999; 126: 541–547. [PubMed] [Google Scholar]
- 26.Lui WY, Chau GY, Wu CW, et al. Surgical resection of hepatocellular carcinoma in elderly cirrhotic patients. Hepato-Gastroenterology 1999; 46: 640–645. [PubMed] [Google Scholar]
- 27.Bakalakos EA, Kim JA, Young DC, et al. Determinants of survival following hepatic resection for metastatic colorectal cancer. World J Surg 1998; 22: 399–405. [DOI] [PubMed] [Google Scholar]
- 28.Shirabe K, Takenaka K, Gion T, et al. Analysis of prognostic risk factors in hepatic resection for metastatic colorectal carcinoma with special reference to the surgical margin. Br J Surg 1997; 84: 1077–1080. [PubMed] [Google Scholar]
- 29.Taylor M, Forster J, Langer B, et al. A study of prognostic factors for hepatic resection for colorectal metastases. Am J Surg 1997; 173: 467–471. [DOI] [PubMed] [Google Scholar]
- 30.Lau H, Fan ST, Ng IO, et al. Long-term prognosis after hepatectomy for hepatocellular carcinoma: a survival analysis of 204 consecutive patients. Cancer 1998; 83: 2302–2311. [PubMed] [Google Scholar]
- 31.Yanaga K, Kanematsu T, Takenaka K, et al. Hepatic resection for hepatocellular carcinoma in elderly patients. Am J Surg 1988; 155: 238–241. [DOI] [PubMed] [Google Scholar]
- 32.Izumi R, Shimizu K, Ii T, et al. Prognostic factors of hepatocellular carcinoma in patients undergoing hepatic resection. Gastroenterology 1994; 106: 720–727. [DOI] [PubMed] [Google Scholar]
- 33.Miyagawa S, Makuuchi M, Kawasaki S, et al. Criteria for safe hepatic resection. Am J Surg 1995; 169: 589–594. [DOI] [PubMed] [Google Scholar]
- 34.Fujimoto J, Okamoto E, Yamanaka N, et al. Adverse effect of perioperative blood transfusions on survival after hepatic resection for hepatocellular carcinoma. Hepato-Gastroenterology 1997; 44: 1390–1396. [PubMed] [Google Scholar]
- 35.Nonami T, Nakao A, Kurokawa T, et al. Blood loss and ICG clearance as best prognostic markers of posthepatectomy liver failure. Hepato-Gastroenterology 1999; 46: 1669–1672. [PubMed] [Google Scholar]
- 36.Pol B, Campan P, Hardwigsen J, et al. Morbidity of major hepatic resections: a 100-case prospective study. Eur J Surg 1999; 165: 446–453. [DOI] [PubMed] [Google Scholar]
- 37.Shirabe K, Shimada M, Gion T, et al. Postoperative liver failure after major hepatic resection for hepatocellular carcinoma in the modern era with special reference to remnant liver volume. J Am Coll Surg 1999; 188: 304–309. [DOI] [PubMed] [Google Scholar]
- 38.Lauterburg BH, Sautter V, Preisig R, et al. Hepatic functional deterioration after portacaval shunt in the rat. Effects on sulfobromophthalein transport-maximum, indocyanine green clearance and galactose elimination capacity. Gastroenterology 1976; 71: 221–227. [PubMed] [Google Scholar]
- 39.Reichen J, Egger B, Ohara N, et al. Determinants of hepatic function in liver cirrhosis in the rat. Multivariate analysis. J Clin Invest 1988; 82: 2069–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keiding S, Johansen S, Tonnesen K. Kinetics of ethanol inhibition of galactose elimination in perfused pig liver. Scand J Clin Lab Invest 1977; 37: 487–494. [DOI] [PubMed] [Google Scholar]
- 41.Hasegawa H, Yamazaki S, Makuuchi M, et al. Hépatectomies pour hépatocarcinome sur foie cirrhotique: schémas décisionnels et principes de réanimation périopératoire. Expérience de 204 cas. J Chirurgie 1987; 124: 425–431. [PubMed] [Google Scholar]
- 42.Mansour A, Watson W, Shayani V, et al. Abdominal operations in patients with cirrhosis: still a major surgical challenge. Surgery 1997; 122: 730–736. [DOI] [PubMed] [Google Scholar]
- 43.Garrison RN, Cryer HM, Howard DA, et al. Clarification of risk factors for abdominal operations in patients with hepatic cirrhosis. Ann Surg 1984; 199: 648–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nagao T, Inoue S, Goto S, et al. Hepatic resection for hepatocellular carcinoma. Clinical features and long-term prognosis. Ann Surg 1987; 205: 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nagasue N, Yukaya H, Ogawa Y, et al. Clinical experience with 118 hepatic resections for hepatocellular carcinoma. Surgery 1986; 99: 694–701. [PubMed] [Google Scholar]
- 46.Nagasue N, Kohno H, Tachibana M, et al. Prognostic factors after hepatic resection for hepatocellular carcinoma associated with Child-Turcotte class B and C cirrhosis. Ann Surg 1999; 229: 84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gill RA, Goodman MW, Golfus GR, et al. Aminopyrine breath test predicts surgical risk for patients with liver disease. Ann Surg 1983; 198: 701–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Okamoto E, Kyo A, Yamanaka N, et al. Prediction of the safe limits of hepatectomy by combined volumetric and functional measurements in patients with impaired hepatic function. Surgery 1984; 95: 586–592. [PubMed] [Google Scholar]
- 49.Yamanaka N, Okamoto E, Oriyama T, et al. A prediction scoring system to select the surgical treatment of liver cancer. Further refinement based on 10 years of use. Ann Surg 1994; 219: 342–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fan ST, Lai EC, Lo CM, et al. Hospital mortality of major hepatectomy for hepatocellular carcinoma associated with cirrhosis. Arch Surg 1995; 130: 198–203. [DOI] [PubMed] [Google Scholar]
- 51.Kitano S, Kim YI. ICG clearance in assessing cirrhotic patients with hepatocellular carcinoma for major hepatic resection. HPB Surg 1997; 10: 182–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lau H, Man K, Fan ST, et al. Evaluation of preoperative hepatic function in patients with hepatocellular carcinoma undergoing hepatectomy. Br J Surg 1997; 84: 1255–1259. [PubMed] [Google Scholar]
- 53.Grando-Lemaire V, Guettier C, Chevret S, et al. Hepatocellular carcinoma without cirrhosis in the West: epidemiologic factors and histopathology of the non-tumorous liver. Groupe d’Etude et de Traitement du Carcinome Hepatocellulaire. J Hepatol 1999; 31: 508–513. [DOI] [PubMed] [Google Scholar]
- 54.Didolkar MS, Fitzpatrick JL, Elias EG, et al. Risk factors before hepatectomy, hepatic function after hepatectomy and computed tomographic changes as indicators of mortality from hepatic failure. Surg Gynecol Obstet 1989; 169: 17–26. [PubMed] [Google Scholar]
- 55.Grieco A, Barone C, Coletta P, et al. Antipyrine metabolism in patients with liver metastases from colorectal cancer. Cancer 1992; 70: 1477–1482. [DOI] [PubMed] [Google Scholar]
- 56.Robertz-Vaupel GM, Lindecken KD, Edeki T, et al. Disposition of antipyrine in patients with extensive metastatic liver disease. Eur J Clin Pharmacol 1992; 42: 465–469. [DOI] [PubMed] [Google Scholar]
- 57.Schaefer J, Schiff L. Liver function tests in metastatic tumor of the liver: study of 100 cases. Gastroenterology 1965; 49: 360–363. [PubMed] [Google Scholar]
- 58.Gerber MA, Thung SN, Bodenheimer HC, Jr., et al. Characteristic histologic triad in liver adjacent to metastatic neoplasm. Liver 1986; 6: 85–88. [DOI] [PubMed] [Google Scholar]
- 59.Kemeny MM, Sugarbaker PH, Smith TJ, et al. A prospective analysis of laboratory tests and imaging studies to detect hepatic lesions. Ann Surg 1982; 195: 163–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leen E, Goldberg JA, Anderson JR, et al. Hepatic perfusion changes in patients with liver metastases: comparison with those patients with cirrhosis. Gut 1993; 34: 554–557. [DOI] [PMC free article] [PubMed] [Google Scholar]