Abstract
Objective
To analyze a large, single-institution experience with routine frozen section (FS) of the sentinel lymph node (SLN) in patients with primary cutaneous melanoma.
Summary Background Data
Controversy exists over the utility of intraoperative FS analysis of the SLN in patients with primary cutaneous melanoma.
Methods
All patients with clinically node-negative cutaneous melanoma undergoing SLN biopsy from 1991 to 1999 were identified from a prospective database. All SLNs were examined by FS. Step-sectioning and immunohistochemistry of permanent section were performed for SLNs negative by FS.
Results
At least one SLN was identified in 98% (360/368) of patients. There were 74 (20%) SLNs positive on permanent section; FS was positive in 59% of these. The accuracy, sensitivity, and specificity of FS were 92%, 59%, and 100%. Because isolated recurrence developed in six patients in the nodal basin in which the SLN was negative, the failure rate was 1.7%. The false-negative rate for SLN biopsy was 7.5%.
Conclusions
Because the prevalence of metastases within the SLN and sensitivity of FS analysis are low, routine use of FS for all patients undergoing SLN biopsy is not recommended.
The incidence of melanoma has steadily increased during the past three decades, at a rate exceeding that of any other cancer in the United States. The relative cancer survival rates have progressively improved since 1974 from 80% to nearly 90% at 5 years. 1 The presence or absence of regional nodal metastases remains the most significant predictor of outcome for melanoma: 5-year survival is consistently less than 50% for patients with node-positive disease. 2,3
In the past, elective lymph node dissection had been advocated for patients with intermediate-thickness melanoma at risk for clinically occult regional nodal metastases. Because only 15% to 20% of these patients have nodal metastases, 80% to 85% would not be expected to benefit from the operation but would be exposed to significant potential complications. Only one of four randomized prospective trials has shown a possible survival advantage for elective lymph node dissection, this identified in specific low risk patient subsets comprising those younger than 60 years of age and those with thin melanomas. 4–7 Lymphatic mapping and sentinel lymphadenectomy is a minimally invasive technique that has been shown to accurately stage the regional nodal basin with a lower associated rate of complications and costs. 8 This simple and reliable technique identifies patients with micrometastatic disease who may benefit from complete regional lymphadenectomy.
The impact of the pathologic status of the sentinel lymph node (SLN) on relapse-free and disease-specific survival is being defined. A multiinstitutional outcome analysis of patients with early-stage melanoma identified micrometastatic disease in the SLN as the most significant independent predictor of tumor-related death. 9 Recent data also suggest that for thick melanomas, SLN status is the most powerful predictor of survival. 10 Recurrence in a regional nodal basin previously subjected to negative SLN biopsy occurs infrequently (1–4%). 11,12 These low regional failure rates have been attributed partly to improved histopathologic diagnostic techniques such as nodal serial step-sectioning and immunohistochemistry.
Controversy exists over the utility of intraoperative frozen section (FS) analysis of the SLN. Proponents argue that it allows conversion to regional lymphadenectomy during the same surgical setting, thereby avoiding a second anesthetic. Frozen section may also be relevant in the setting of primary head and neck melanoma, particularly for patients with an intraparotid SLN, where it is important to avoid a secondary procedure in a previously operated field, potentially increasing the risk of injury to the facial nerve. The potential loss of SLN tissue containing small foci of micrometastases during FS processing and the low sensitivity of intraoperative FS analysis have been arguments against the use of this method of pathologic evaluation. 13,14 The purpose of this study was to conduct a critical review of a relatively large single-institution patient cohort with primary cutaneous melanoma undergoing routine intraoperative FS evaluation of the SLN to define sensitivity and specificity against the gold standard of permanent section analysis.
METHODS
Patients with histologically confirmed primary cutaneous melanoma and clinically negative lymph nodes who underwent lymphatic mapping and SLN biopsy with FS analysis of the SLN as part of their treatment were included in the study. Three hundred sixty-eight such patients were treated at Memorial Sloan-Kettering Cancer Center from 1991 to 1999. Patient, pathologic, treatment, recurrence, and death data on all patients admitted for treatment of their melanoma were prospectively entered into a melanoma database.
Technique of Lymphatic Mapping and Biopsy
Patients selected for SLN biopsy were those with untreated or previously biopsied melanoma 1 mm or more thick, or Clark level IV (any thickness). Patients scheduled for SLN biopsy underwent preoperative lymphoscintigraphy to facilitate identification of all nodal basins at risk, to identify aberrant in-transit SLNs, and to detail secondary-echelon nodes. Technetium-99m sulfur colloid (CIS-US, Bedford, MA) was the radioisotope used for lymphatic mapping. The radiocolloid was passed through a 0.2-μm filter before use. A total dose of 200 to 400 microcuries in 1 to 2 mL normal saline was injected intradermally at four equal locations around the primary melanoma site or excisional biopsy scar. The injection and lymphoscintigraphy were performed in the nuclear medicine suite on the morning of surgery, 1 to 4 hours before the operation. Dynamic scans of all nodal basins at risk were obtained beginning 5 to 10 minutes after injection using a large field-of-view gamma camera set at 20% window and fitted with a low-energy, high-resolution parallel hole collimator. Anterior and lateral static images were obtained at 5-minute intervals over a period of 20 minutes to 2 hours. The patient was taken to the operating room after lymphoscintigraphy.
The SLN biopsy was carried out 1 to 4 hours after injection of the radiocolloid, and the images obtained were used to guide the procedure. This was conducted under anesthesia appropriate to the anatomy at the discretion of the surgeon. After induction of general anesthesia or sedation, 0.5 to 1.0 mL 1% Isosulfan Blue dye (Lymphazurin, Hirsch Industries, Inc., Richmond, VA; 0.5 to 1.0 mL) was injected intradermally on the side of the primary melanoma or previous biopsy site closest to the draining nodal basin indicated by lymphoscintigraphy. The amount of dye injected was limited such that the blue cutaneous stain caused by the injection would be encompassed by the subsequent wide excision.
A hand-held gamma probe was used to localize the SLN, guided by the preoperative lymphoscintigrams. On probes where the settings could be adjusted manually, we used a detection threshold of 120 KeV with a window of 40 KeV to center on the 140-KeV photopeak of technetium-99m.
After identification of the hot spot, the skin over the site of maximal transcutaneous counts was incised and limited flaps were elevated. Incisions were fashioned in such a way that they could be incorporated into the formal incisions used for nodal dissection in case of a positive SLN. Both the blue-stained afferent lymphatic channel and radioactivity counts were used to guide dissection and identification of the SLN. The SLN was identified as the lymph node with an afferent blue lymphatic channel staining the node hilus, and/or hot node or nodes. The identified SLN was correlated with the preoperative lymphoscintigrams. After identification of the SLN, the afferent and efferent lymphatics were clipped and divided and the node was excised. Additional hot nodes were removed from the lymphatic basin until the background radioactivity was less than 10% of the hottest node removed, based on ex vivo counts.
The ex vivo radioactivity and color of all excised nodes, as well as the final nodal basin background radioactivity, were recorded. The SLN was submitted for FS analysis. While the FS was being done, the primary melanoma or biopsy scar was widely excised with a margin appropriate to its Breslow thickness. The SLN biopsy site was closed. When a positive SLN was identified by FS, immediate regional lymphadenectomy was performed encompassing the entire SLN biopsy cavity. All patients with positive SLN by permanent section or immunohistochemistry were subsequently treated with complete regional lymphadenectomy.
Pathologic Examination
Before undergoing surgery, each patient’s primary melanoma was reviewed to confirm the diagnosis and to measure tumor thickness and level of invasion. Those slides were used for comparison between primary tumor cells and suspicious nodal cells examined at the time of FS. At the time of FS, the most representative slide of the primary tumor was available to the pathologist for comparison with any potential tumor cells in the lymph node.
The excised SLN or SLNs were measured and bisected at the level of the hilum along the longitudinal axis of the node. The entire surface of a single 4-mm-thick hematoxylin-and-eosin-stained FS was evaluated microscopically. Additional FSs were examined if the initial FS revealed suspicious but not fully diagnostic tumor cells, or if one lymph node did not fit on a single slide. More than one FS was also obtained if multiple lymph nodes were submitted for examination.
When a diagnosis of metastatic melanoma was made on examination of the FS slide, only one routine hematoxylin-and-eosin-stained section was obtained from the formalin-fixed and paraffin-embedded FS remnant for confirmation of the diagnosis. If no or no definite melanoma was found on the FS, permanent sections were sectioned at three levels with 4-mm-thick hematoxylin-and-eosin-stained sections.
Immunohistochemistry was performed on formalin-fixed and paraffin-embedded sections using the avidin-biotin-peroxidase complex method (Vector, Burlingame, CA). The commercially obtained antibodies used in this study included S-100 protein (1:10,000; Dako, Carpinteria, CA) and HMB-45 (1:200; Dako). Endogenous peroxidase was suppressed by 20 minutes of incubation with 1% H2O2. Diaminobenzidine tetrahydrochloride (DAB, Biogenex, San Ramon, CA) was used as chromogen. Each assay was run with negative and positive internal controls.
A diagnosis of metastatic melanoma was made if pleomorphic tumor cells similar to the primary tumor were present in the lymph node. Immunohistochemical results were interpreted as positive for melanoma if HMB-45 immunopositive cells were present in the lymph node. For S-100 protein-positive cells, the diagnosis of metastatic melanoma required careful comparison of the morphology of the immunopositive cells with the primary tumor. If the cells were aggregated similar to the primary tumor or clearly pleomorphic, a diagnosis of melanoma was made. Individual isolated S-100 protein-positive cells with small cytologically bland nuclei and dendritic processes were interpreted as follicular dendritic cells and regarded as negative for melanoma. If melanocytes were detected in a nested pattern confined to the capsule of the lymph node, a diagnosis of nodal nevus was made.
Definitions, Study End points, and Statistical Analysis
Successful identification of the SLN and the sensitivity, specificity, accuracy, and positive and negative predictive values of FS analysis were determined. The failure rate of SLN biopsy was defined by the number of first recurrences in the regional nodal basin in which the SLN was negative divided by the total number of patients. The false-negative rate associated with SLN biopsy was defined as the number of such failures divided by the sum of the total number of SLN-positive patients and the number of failures.
Summary statistics were obtained using established methods. Associations between categorical variables were evaluated using the chi-square test. Clinical factors considered in the analysis included gender, age (60 years or younger vs. older than 60 years), primary melanoma site, Clark level, Breslow thickness, ulceration, and SLN status (positive and negative). Anatomic site was categorized into head and neck, extremity, trunk, and anogenital. Ulceration of the primary melanoma was defined as present if there was a history of bleeding, if the lesion was described as ulcerated, or if ulceration was noted in the pathology report. Statistical analysis was performed using JMP software (JMP, Cary, NC). For all statistical tests, P ≤ .05 was considered significant.
RESULTS
Clinical and Pathologic Factors
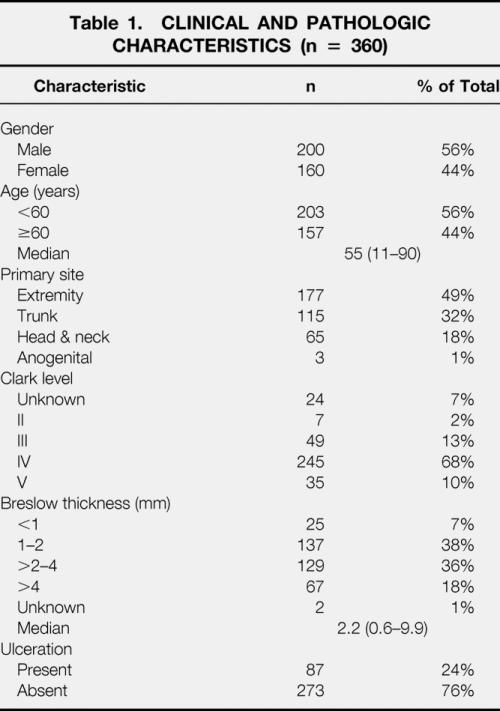
During the study period, 368 patients with clinically node-negative primary cutaneous melanoma underwent lymphatic mapping and SLN biopsy. The SLN was identified in 360 of 368 (98%) patients. The clinical and pathologic characteristics for the 360 patients are given in Table 1. There were 200 (56%) men and 160 (44%) women, ranging in age from 11 to 90 years (median 55 years) at primary presentation. The anatomic location of the primary melanoma included the extremities (49%), trunk (31%), head and neck (18%), and anogenital region (1%). Median tumor thickness was 2.2 mm. Fifty-five percent of primary lesions were more than 2 mm thick, 78% were Clark level IV/V, and 24% were ulcerated.
Table 1. CLINICAL AND PATHOLOGIC CHARACTERISTICS (n = 360)
A median of two (range 1–3) SLNs per patient underwent biopsy, the majority (93%) from a single regional nodal basin, most commonly the axilla (n = 153 [43%]), followed by the groin (n = 119 [33%]) and neck (n = 63 [18%]). The median number identified for extremity, trunk, head and neck, and anogenital primary melanomas were 2.3, 2.6, 3.2, and 2.0 SLNs per patient. A significantly higher median number of SLNs were identified in the head and neck region than any other anatomic location (P = .01). Lymphoscintigraphy identified more than one SLN-containing nodal basin in 25 (7%) patients.
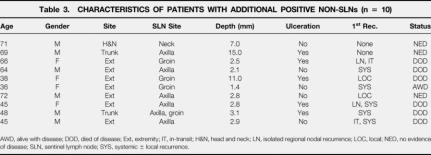
The SLN was positive by permanent section in 74 (20%) patients, three of which were positive only by immunohistochemistry. The incidence of micrometastases in SLN progressively increased with Breslow tumor thickness and Clark level of invasion (Table 2). Ulcerated primary cutaneous melanomas had a significantly higher incidence of nodal micrometastases than nonulcerated lesions (16 vs. 34%, P < .001). In a single patient (1/289 [0.3%]), a nonsentinel node was found to contain micrometastases when the SLN was found to be histologically negative. The SLN was the only positive lymph node in 86% (64/74) of patients. Additional positive non-SLNs were identified in 10 of 74 patients (14%). The characteristics of these 10 patients are given in Table 3.
Table 2. DISTRIBUTION OF PROGNOSTIC VARIABLES AND INCIDENCE OF HISTOLOGICALLY POSITIVE SLNs (n = 360)
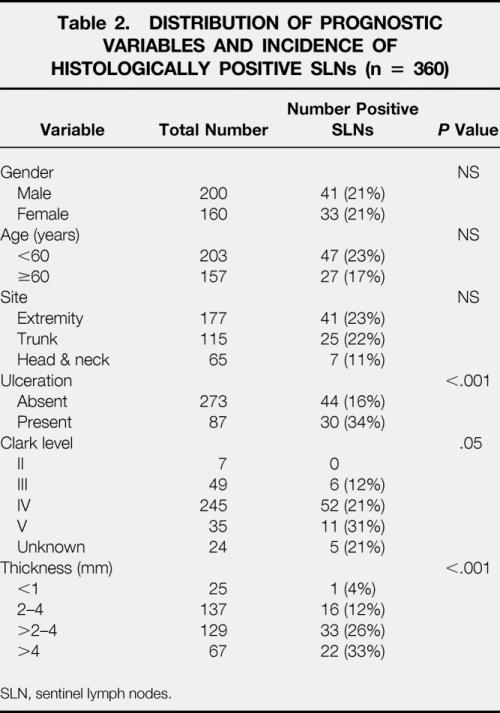
SLN, sentinel lymph nodes.
Table 3. CHARACTERISTICS OF PATIENTS WITH ADDITIONAL POSITIVE NON-SLNs (n = 10)
AWD, alive with disease; DOD, died of disease; Ext, extremity; IT, in-transit; H&N, head and neck; LN, isolated regional nodal recurrence; LOC, local; NED, no evidence of disease; SLN, sentinel lymph node; SYS, systemic ± local recurrence.
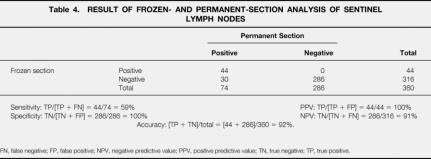
Frozen-section analysis was performed in all 360 patients. Of the 74 SLNs positive by permanent section, FS was positive in 44 instances (59%). There were no false-positive FS diagnoses. The accuracy of FS was 93%; the sensitivity and specificity were 59% and 100%, respectively (Table 4). The positive and negative predictive values of FS were 100% and 91%. The sensitivity for FS was highest for patients with thick melanomas and lowest for thin melanomas (<1 mm, 33%; 1–2 mm, 61%; 2–4 mm, 51%; >4 mm, 71%). The sensitivity of FS was identical (62%) for patients with ulcerated and nonulcerated primary cutaneous melanomas.
Table 4. RESULT OF FROZEN- AND PERMANENT-SECTION ANALYSIS OF SENTINEL LYMPH NODES
FN, false negative; FP, false positive; NPV, negative predictive value; PPV, positive predictive value; TN, true negative; TP, true positive.
Follow-Up and Recurrence
Median time from the primary operation to the date of last clinical follow-up was 32 months (range 7–109). During this period recurrent disease developed in 87 (24%) patients. The median time to first relapse was 15 months (range 2–67). The most common forms of relapse were distant (50/87 [57%]) and regional (in-transit with or without simultaneous local or regional nodal recurrence, 22/87 [25%]) metastases.
In six patients, an isolated first recurrence developed in the regional nodal basin in which the previous SLN biopsy was negative. Therefore, the failure rate of lymphatic mapping and SLN biopsy was 1.7% ([6/360] × 100%). Because the total number of patients with positive SLNs was 74, the false-negative rate for lymphatic mapping and SLN biopsy in this study was 7.5% ([6/80] × 100%). Immunohistochemistry was performed in 146 patients and found to be positive in 22 (15%), 19 of whom had proven micrometastases on hematoxylin-and-eosin-stained sections. In one of the three patients with a positive SLN by immunohistochemistry alone, subsequent systemic disease developed after completion lymphadenectomy.
Of the 50 patients with systemic first relapse, 37 had distant disease alone; 13 had systemic disease in the setting of simultaneous regional nodal or in-transit recurrence. Fifteen of 22 patients had isolated in-transit recurrence; 4 and 3 had simultaneous regional nodal and local recurrence, respectively. Six (7%) patients had isolated local and seven (8%) isolated regional nodal recurrences.
The majority (five of six) of patients with isolated local recurrences was salvaged with wide re-resection. All seven patients with isolated regional nodal recurrence were rendered disease-free with lymphadenectomy, but in five of these seven patients progression of melanoma developed; three of these have died of disease. Forty-one percent (9/22) of patients with regional recurrence were salvaged with further treatment and remain without evidence of disease after a median follow-up of 15 months. Only 10% (5/50) of patients in whom systemic relapse developed could be rendered free of disease (median postrecurrence survival, 8 months).
At the time of last follow-up, 317 (86%) patients are alive, 296 (80%) disease-free and 21 (6%) with disease. Forty-seven (13%) patients have died of disease. Median disease-specific survival was 24 months (range 6–63). Three-year relapse-free and disease-specific survival rates for the entire cohort were 73% and 86%, respectively.
DISCUSSION
The optimal treatment of the draining nodal basin for patients with melanoma remains controversial. Although the presence of nodal metastasis has been shown to be one of the strongest predictors of survival, only one of four prospective randomized trials has shown a survival benefit to elective lymphadenectomy, and this was within a subset of patients younger than 60 or with melanomas 1–2 inches thick. 2,3,7 Lymphatic mapping with SLN biopsy has become increasingly accepted in the management of patients with melanoma. This technique enables the surgeon to accurately stage patients without subjecting them all to the substantial complications of a formal nodal dissection.
When the SLN is found to contain metastatic cells, most surgeons advocate removing the remaining nodes within the regional nodal basin with therapeutic intent. 15,16 This approach of selective lymphadenectomy has gained popularity because several studies have reported regional nodal recurrence rates as low as 1% when the SLN was found to be negative. 11,12 This high level of accuracy has been thought to be due to the intensive pathologic evaluation of SLNs, which usually consists of step-sectioning and immunohistochemical analysis.
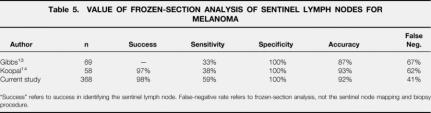
Because the pathologic result of the SLN may determine the need for additional surgery, many studies describe the use of FS as part of SLN evaluation. Frozen-section analysis of SLNs from patients with breast cancer has been reported to have a sensitivity as high as 91% and a specificity of 100%. 17,18 Proponents of this technique for patients with melanoma argue that it allows conversion to completion lymphadenectomy during the same anesthetic, thereby avoiding a second operation. The sensitivity of SLN analysis for patients with melanoma however, has been reported to be as low as 33% to 38%. 13,14 Because of this low sensitivity, as well as the low overall incidence of nodal metastasis for patients with melanoma, the use of FS in the evaluation of SLNs for patients with melanoma may not be warranted.
This study represents the largest single-institution experience published to date that evaluates the role of FS analysis of the SLN in the setting of malignant melanoma. The demographic, tumor, and outcome data in this study are similar to those of other large melanoma studies. The median age of patients in this study was 55 years, the most common site of the primary tumor was the extremity (49%), and the majority of melanomas were nonulcerated (76%) and of intermediate thickness (74%). Likewise, the results of this study with regard to the feasibility of SLN biopsy are also supportive of the results of other reported series. The SLN was identified in 98% of patients, with an overall incidence of nodal metastasis of 20%. The SLN was the only positive node in most patients (86%), a finding also consistent with other published data.
In this study, FS analysis was positive for metastasis in 44 of the 74 patients where nodal metastasis was present (sensitivity, 59%). Therefore, FS analysis provided information that changed the surgical approach in 44 of 360 (12%) patients whose SLNs were subjected to FS analysis. Because most patients with melanoma have node-negative disease, and because of the relatively low sensitivity of FS in detecting nodal metastasis, we do not advocate the routine use of FS (Table 5).
Table 5. VALUE OF FROZEN-SECTION ANALYSIS OF SENTINEL LYMPH NODES FOR MELANOMA
“Success” refers to success in identifying the sentinel lymph node. False-negative rate refers to frozen-section analysis, not the sentinel node mapping and biopsy procedure.
We do believe, however, that FS analysis may be beneficial in selected circumstances. In particular, it should be obtained in instances of head and neck melanoma when the SLN node is found within the parotid gland. The rationale for obtaining FS in this setting is to avoid the potential complications of facial nerve injury during a secondary procedure through a previously elevated cheek flap. Moreover, we believe that if a positive SLN in the neck can be identified by FS, immediate completion lymph node dissection is often safer than delayed completion dissection to reduce the likelihood of spinal accessory nerve injury.
Although the overall sensitivity of FS was relatively low (59%), the use of FS analysis may also be considered for patients with a higher likelihood of having nodal metastases, such as patients with deep or ulcerated lesions. In this study, the incidence of nodal metastasis for lesions that were more than 4 mm deep and ulcerated was 43%. The sensitivity of FS was identical (62%) for patients with ulcerated and nonulcerated melanomas. The sensitivity of FS was highest (71%) for thick (>4 mm) lesions. In this setting, even with a sensitivity of 71%, the high incidence of nodal metastases may warrant performing FS because many of these patients will ultimately require completion lymphadenectomy.
Regardless of the pathologic technique used for SLN analysis, this study emphasizes the utility of lymphatic mapping and SLN biopsy in patients with melanoma. The SLN was identified in 98% of patients; when positive, it represented the only positive node in 86% of patients. When the SLN was negative (n = 289), recurrence developed in only six (1.7%) patients within the basin from which the SLN was removed.
Lymphatic mapping and sentinel lymphadenectomy can be used to accurately stage the regional nodal basin for patients with primary cutaneous malignant melanoma. Only 4% of positive SLNs were positive only by immunohistochemistry analysis. Recurrence in a regional nodal basin previously subjected to negative SLN biopsy occurs rarely. Given the low prevalence (20%) of metastases within the SLN and the low sensitivity (59%) of FS analysis, routine use of FS in all patients undergoing SLN biopsy is not recommended. Frozen section may be appropriate for patients with head and neck melanoma to avoid a secondary procedure that may imperil the facial or spinal accessory nerve.
Footnotes
Correspondence: Daniel G. Coit, MD, Chief, Gastric, Mixed Tumor Service, Department of Surgery, Memorial Sloan-Kettering Cancer Center, 1275 York Ave., New York, NY 10021
E-mail: coitd@mskcc.org
Accepted for publication May 3, 2001.
References
- 1.Greenlee RT, Bolden S. Cancer statistics 2000. CA Cancer J Clin 2000; 50: 7–33. [DOI] [PubMed] [Google Scholar]
- 2.American Joint Commission on Cancer Staging Manual, 5th ed. Philadelphia: Lippincott-Raven; 1997.
- 3.Morton DL, Wanek L, Nizze JA, et al. Improved long-term survival after lymphadenectomy of melanoma metastatic to regional nodes. Analysis of prognostic factors in 1134 patients from the John Wayne Cancer Clinic. Ann Surg 1991; 214: 491–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sim FH, Taylor WF, Ivins JC, et al. A prospective randomized study of the efficacy of routine elective lymphadenectomy in malignant melanoma. Cancer 1978; 41: 948–956. [DOI] [PubMed] [Google Scholar]
- 5.Veronesi U, Adamus J, Bandiera DC, et al. Inefficacy of immediate node dissection in stage I melanoma of the limbs. N Engl J Med 1977; 297: 627–630. [DOI] [PubMed] [Google Scholar]
- 6.Veronesi U, Adamus J, Bandiera DC, et al. Delayed regional lymph node dissection in stage I melanoma of the skin of the lower extremities. Cancer 1982; 49: 2420–2430. [DOI] [PubMed] [Google Scholar]
- 7.Balch CM, Soong SJ, Bartolucci AA, et al. Efficacy of an elective regional lymph node dissection of 1- to 4-mm-thick melanomas for patients 60 years of age and younger. Ann Surg 1996; 224: 255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morton DL, Thompson JF, Essner R, et al. Validation of the accuracy of intra-operative lymphatic mapping and sentinel lymphadenectomy for early-stage melanoma. Ann Surg 1999; 230: 253–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gershenwald JE, Thompson W, Mansfield PF, et al. Multi-institutional melanoma lymphatic mapping experience: the prognostic value of sentinel lymph node status in 612 stage I or II melanoma patients. J Clin Oncol 1999; 17: 976–983. [DOI] [PubMed] [Google Scholar]
- 10.Gershenwald JE, Mansfield PF, Lee JE, et al. Role of lymphatic mapping and sentinel lymph node biopsy in patients with thick (≥4 mm) primary melanoma. Ann Surg Oncol 2000; 7: 160–165. [DOI] [PubMed] [Google Scholar]
- 11.Gershenwald JE, Colome MI, Lee JE, et al. Patterns of recurrence following a negative sentinel lymph node biopsy in 243 patients with stage I or II melanoma. J Clin Oncol 1998; 16: 2253–2260. [DOI] [PubMed] [Google Scholar]
- 12.Statius MG, Borgstein PJ, Pijpers R, et al. Ann Surg Oncol 2000; 7: 461–468. [DOI] [PubMed] [Google Scholar]
- 13.Gibbs JF, Huang PP, Zhang PJ, et al. Accuracy of pathologic techniques for the diagnosis of metastatic melanoma in sentinel lymph nodes. Ann Surg Oncol 1999; 6: 699–704. [DOI] [PubMed] [Google Scholar]
- 14.Koopal SA, Tiebosch ATMG, Piers DA, et al. Frozen section analysis of sentinel lymph nodes in melanoma patients. Cancer 2000; 89: 1720–1725. [PubMed] [Google Scholar]
- 15.Morton DL, Chan AD. Current status of intraoperative lymphatic mapping and sentinel lymphadenectomy for melanoma: is it standard of care? J Am Coll Surg 1999; 189: 214–223. [DOI] [PubMed] [Google Scholar]
- 16.Reintgen DS, Brobeil A. Lymphatic mapping and selective lymphadenectomy as an alternative to elective lymph node dissection in patients with malignant melanoma. Hematol Oncol Clin North Am 1998; 12: 807–821. [DOI] [PubMed] [Google Scholar]
- 17.Van Diest PJ, Torrenga H, Bergstein PJ, et al. Reliability of intraoperative frozen section and imprint cytological investigation of sentinel lymph nodes in breast cancer. Histopathology 1999; 35: 14–18. [DOI] [PubMed] [Google Scholar]
- 18.Zurrida S, Galimberti V, Orvieto E, et al. Radioguided sentinel node biopsy to avoid axillary dissection in breast cancer. Ann Surg Oncol 2000; 7: 28–31. [DOI] [PubMed] [Google Scholar]