Abstract
Objective
To determine whether reduction of circulating female sex hormones by ovariectomy causes suppression of macrophage (Mφ) function after trauma-hemorrhage and increases susceptibility to subsequent sepsis.
Summary Background Data
Studies indicate that immune functions are markedly depressed in males but not in proestrus females after trauma-hemorrhage. Although male sex steroids are immunosuppressive, it remains unknown whether female sex hormones are immunoprotective after trauma-hemorrhage.
Methods
Circulating female sex hormones were reduced by ovariectomy of 8-week-old female CBA/J mice. Two weeks afterward, ovariectomy and proestrus sham-ovariectomy mice were subjected to laparotomy (i.e., soft tissue trauma) and hemorrhagic shock (35 ± 5 mm Hg for 90 minutes, then resuscitated) or sham operation. Two hours afterward, splenic and peritoneal Mφ and Kupffer cells were isolated and cytokine production was assessed. In a second series of experiments, animals were subjected to sepsis by cecal ligation and puncture at 24 hours after trauma-hemorrhage or sham operation, and survival was assessed.
Results
Release of interleukin-1 and interleukin-6 by splenic and peritoneal Mφ from proestrus mice was maintained after trauma-hemorrhage, whereas release of interleukin-1 and interleukin-6 by Mφ from ovariectomized mice was depressed by approximately 50%. In contrast, trauma-hemorrhage resulted in a fourfold increase of Kupffer cell release of tumor necrosis factor-alpha in ovariectomized females and a fivefold increase in plasma concentrations of tumor necrosis factor-alpha. Release of tumor necrosis factor-alpha and plasma concentrations were unchanged in proestrus mice under such conditions. When proestrus and ovariectomized animals were subjected to sepsis by cecal ligation and puncture at 24 hours after trauma-hemorrhage or sham operation, ovariectomized mice had a significantly higher death rate than proestrus mice.
Conclusions
These findings suggest that female sex hormones play a critical role in maintaining immune responses after trauma-hemorrhage by suppressing the elaboration of tumor necrosis factor-alpha and prevent the increased lethality from subsequent sepsis. Thus, female sex hormones may be a useful adjunct in preventing trauma-induced immunodepression and increased susceptibility to subsequent sepsis.
Trauma-hemorrhage produces a pronounced depression of immune functions in males that persists for up to 10 days after resuscitation. 1,2 Alterations in the function of various macrophage (Mφ) populations (peritoneal, splenic, hepatic [Kupffer cells]) has been implicated in the immune depression and subsequent increased susceptibility to sepsis observed under such conditions. 3–5 Recent studies have shown that testosterone plays a significant role in producing this immunodepression and increased susceptibility to subsequent sepsis after trauma-hemorrhage. 6–8 In contrast, female mice in the proestrus state of the estrus cycle have maintained or enhanced immune responses under such conditions;9 this is associated with improved survival after the induction of subsequent sepsis. 10 Previous studies support the concept that female sex hormones are responsible for the maintained immune response observed in females after trauma-hemorrhage. 11,12 Administration of a single dose of 17β-estradiol to males after resuscitation reduced circulating concentrations of interleukin (IL)-6 and normalized splenocyte and splenic Mφ cytokine release. 12 Similarly, administration of the female sex hormone prolactin to males after trauma-hemorrhage has been shown to normalize immune function. 11 Thus, it appears that elevated levels of female sex hormones (e.g., estrogen, prolactin) in females during the proestrus stage of the estrus cycle 13 might be a causative factor in the sexual dimorphism of immune responses after hemorrhagic shock.
Several epidemiologic studies indicate gender differences in the susceptibility to sepsis or infections after trauma, with an increased death rate in male patients versus female patients. 14–16 Studies that included postmenopausal women, with decreased estrogen levels, showed a higher death rate in female patients versus male patients. 17,18 These studies suggest that female sex hormones provide a survival advantage after traumatic injury.
The aim of the present study was to determine whether reduction of female sex hormones by surgical ovariectomy might result in the development of immunosuppression and increased susceptibility to subsequent sepsis after trauma-hemorrhage.
METHODS
Animals
Inbred female CBA/J mice (Jackson Laboratories, Bar Harbor, ME), 8 to 9 weeks old (24–26 g), were used in this study. All procedures were carried out in accordance with the guidelines set forth in the Animal Welfare Act and the Guide for the Care and Use of Laboratory Animals by the National Institutes of Health. The Institutional Animal Care and Use Committee of Rhode Island Hospital and Brown University approved this project.
Experimental Groups
To determine the role of female sex hormones in the regulation of immune responses, ovariectomy or sham-ovariectomy was performed in female CBA/J mice 2 weeks before trauma-hemorrhage. Two weeks after ovariectomy or sham- ovariectomy, the animals were divided into four groups. Groups 1 and 2 consisted of sham-ovariectomy females in the proestrus state of the estrus cycle; this was determined by microscopic examination of vaginal cytology. Animals in groups 3 and 4 consisted of ovariectomized females. The animals in groups 1 and 3 served as sham-operated animals (neither hemorrhaged nor resuscitated). Animals in groups 2 and 4 were subjected to the trauma-hemorrhage procedure. Each group consisted of seven or eight animals.
In additional studies, ovariectomy and sham-ovariectomy females were subjected to sepsis at 24 hours after trauma-hemorrhage and resuscitation or sham operation. In all experimental groups, polymicrobial sepsis was induced by cecal ligation and puncture (CLP), and survival was assessed for up to 10 days after the induction of sepsis. Each group consisted of 20 animals.
Trauma-Hemorrhage Procedure
Mice in the trauma-hemorrhage groups were lightly anesthetized with methoxyflurane (Metofane, Pitman Moore, Mundelein, IL) and restrained in a supine position, and a 2.5-cm midline laparotomy (i.e., soft tissue trauma induced) was performed. It was then closed aseptically in two layers using 6-0 Ethilon sutures (Ethicon, Inc., Somerville, NJ). Both femoral arteries were then aseptically cannulated with polyethylene 10 tubing (Clay-Adams, Parsippany, NJ) using a minimal dissection technique, and the animals were allowed to awaken. Blood pressure was constantly monitored by attaching one of the catheters to a blood pressure analyzer (Micro-Med, Inc., Louisville, KY). Lidocaine was applied to the incision sites to provide analgesia during the study period. On awakening, the animals were bled rapidly through the other catheter to a mean arterial blood pressure of 35 ± 5 mm Hg (mean arterial blood pressure before hemorrhage was 95 ± 5 mm Hg); this was maintained for 90 minutes. At the end of that procedure, the animals were resuscitated with four times the shed blood volume in the form of lactated Ringer’s solution. The catheters were then removed, the vessels ligated, and the groin incisions closed. Sham-operated animals in groups 1 and 3 underwent the same surgical procedure, which included ligation of both femoral arteries, but neither hemorrhage nor fluid resuscitation was carried out. There were no deaths observed in this model of trauma-hemorrhage. The animals were killed by methoxyflurane overdose at 2 hours after trauma-hemorrhage and resuscitation to obtain the spleen, liver, peritoneal Mφ, uterus, and whole blood.
Cecal Ligation and Puncture Procedure
Polymicrobial sepsis was induced at 24 hours after trauma-hemorrhage and resuscitation using the CLP method described by Baker et al. 19 Briefly, mice were anesthetized with methoxyflurane and a 2-cm midline laparotomy was performed. The cecum was isolated and ligated just below the ileocecal valve. The cecum was then punctured twice with a 22-gauge needle, a small amount of bowel contents was extruded through the puncture holes, and the cecum was returned to the peritoneal cavity. The abdominal incision was closed in two layers using 6-0 Ethilon sutures. Normal saline solution (20 mL/kg) was administered subcutaneously at that time. Previous studies have shown that blood cultures taken from mice after CLP are positive for gram-positive (e.g., Streptococcus bovis) as well as gram-negative (e.g., Bacteroides fragilis, Escherichia coli, Klebsiella, Proteus mirabilis) bacteria as early as 1 hour after CLP. 19
Plasma Collection and Storage
Whole blood was obtained by cardiac puncture. Plasma was separated by centrifugation in pyrogen-free microcentrifuge tubes and samples were immediately frozen and stored (−80°C) until assayed.
Preparation of Splenic and Peritoneal Macrophage Cultures
Spleens were harvested 2 hours after sham operation or trauma-hemorrhage aseptically, and splenic Mφ cultures were established as previously described. 11 Resident peritoneal Mφ were harvested at the same time and monolayers were established as previously described. 20 These protocols provided peritoneal and splenic Mφ populations that were at least 95% positive for nonspecific esterase staining and exhibited typical Mφ morphology. Splenic and peritoneal Mφ monolayers (1 × 106 cells/mL) were stimulated with 10 μg lipopolysaccharide (from E. coli 055:B5, Difco Laboratories, Detroit, MI)/mL Click’s medium containing 10% heat-inactivated fetal bovine serum (Gibco BRL, Grand Island, NY) for 48 hours (at 37°C, 5% CO2, and 90% humidity) to assess the cells’ ability to release cytokines. The same lot of fetal bovine serum was used for all experiments to control for steroid content of the culture media. Culture supernatants were collected and stored at −80°C until assayed for IL-1, IL-6, and IL-10.
Preparation of Kupffer Cell Cultures
Kupffer cells were harvested as previously described. 21 In brief, retrograde perfusion of the liver was performed with 35 mL ice-cold Hank’s balanced salt solution (HBSS, Ca2+/Mg2+ free, 37°C, Gibco BRL) through the portal vein. This was immediately followed by perfusion with 10 mL 0.075% collagenase type IV (162 U/mg, Sigma Chemical Co., St. Louis, MO) in HBSS at 37°C. The liver was transferred to a petri dish containing warm 0.075% collagenase, minced finely, incubated at 37°C for 10 minutes, and passed through a sterile 150-mesh stainless-steel screen into a beaker containing 10 mL cold HBSS and 10% fetal bovine serum. The cell suspension was then layered over 16% metrizamide (Accurate Chemical & Scientific Corp., Westbury, NY) in HBSS and centrifuged at 3,000 g, 4°C, for 45 minutes to separate the Kupffer cells from the remaining parenchymal cells in the pellet. After removal of the nonparenchymal cells from the interface with a Pasteur pipette, the cells were washed twice by centrifugation (800 g, 15 minutes, 4°C) with HBSS, and resuspended in Click’s medium containing 10% fetal bovine serum. The cells were transferred to a 24-well plate that was precoated with 0.5 mL of 6-μg vitrogen 100 (Collagen Biomaterials, Collagen Corporation, Palo Alto, CA)/mL (plates were washed with phosphate-buffered saline three times before cell transfer) and incubated for 3 hours at 37°C (5% CO2, 90% humidity). Nonadherent cells were then removed by washing three times with Click’s medium. This protocol provides adherent cells that are more than 95% positive by nonspecific esterase staining and that exhibit typical Mφ morphology. 21 The Kupffer cells (1.5 × 106 Kupffer cells mL−1 per well) were incubated for 24 hours (37°C, 5% CO2) with 10 μg lipopolysaccharide/mL Click’s medium with 10% fetal bovine serum. Culture supernatants were collected and stored at −80°C until assayed for tumor necrosis factor (TNF)-α, IL-1β, IL-6, and IL-10.
Assessment of 17β-Estradiol and Cytokine Concentrations
17β-estradiol plasma concentrations were determined using a commercially available radioimmunoassay (ICN Biomedicals, Costa Mesa, CA) as described by the manufacturer.
Activity of IL-1 was determined by adding serial dilutions of plasma or supernatants to D10.G4.1 cells in the presence of Concanavalin A, as previously described. 22 In certain experiments IL-1β was determined by enzyme-linked immunosorbent assay according to the manufacturer’s recommendations (Genzyme Diagnostics, Cambridge, MA). Activity of IL-6 was determined by assessing the 72-hour proliferation of the IL-6-dependent murine hybridoma 7TD1 stimulated by serial dilutions of plasma or supernatant, as described previously. 23 Activity of TNF-α was determined by the 24-hour cytotoxicity induced in WEHI-164 clone 13 cells in the presence of serial dilutions of plasma or supernatant, as described elsewhere. 23 Concentrations of IL-10 were determined by enzyme-linked immunosorbent assay according to the manufacturer’s recommendations (Pharmingen, San Diego, CA).
Statistical Analysis
Data are presented as mean ± standard error. One-way analysis of variance followed by the Student-Newman-Keuls test as a post hoc test for multiple comparisons was used to determine the significance of the differences between experimental means. A t test was used to determine the significance of differences in 17β-estradiol plasma concentrations and uterine wet weight between proestrus and ovariectomized mice. To determine the significance of the differences between death rates in the survival study, a z test was used. P < .05 was considered significant.
RESULTS
Biologic Effect of Ovariectomy
At 2 weeks after ovariectomy, plasma concentrations of 17β-estradiol were significantly lower in ovariectomized females compared with sham-ovariectomy females in the proestrus state of the estrus cycle (22.5 ± 2.9 vs. 10.0 ± 1.9 pg/mL; mean ± SEM of seven or eight mice per group). Uterine wet weight was also significantly lower in ovariectomized females compared with sham-ovariectomy females (63.9 ± 12.6 vs. 19.5 ± 3.4 mg).
Macrophage Interleukin-1 Release
Splenic Mφ IL-1 release was significantly increased in proestrus sham-ovariectomy females after trauma-hemorrhage (P < .05 vs. sham-operated proestrus females;Fig. 1). In ovariectomized females, splenic Mφ IL-1 release was significantly depressed after trauma-hemorrhage (P < .05 vs. sham-operated ovariectomized females). Similar changes in peritoneal Mφ IL-1 release were observed. In contrast to splenic and peritoneal Mφ, hepatic Mφ (Kupffer cell) IL-1 release was not significantly increased in proestrus females after trauma-hemorrhage. In ovariectomized females, however, Kupffer cell IL-1 release was significantly increased (P < .05) approximately 2.5-fold in animals that underwent trauma-hemorrhage compared with ovariectomy shams.
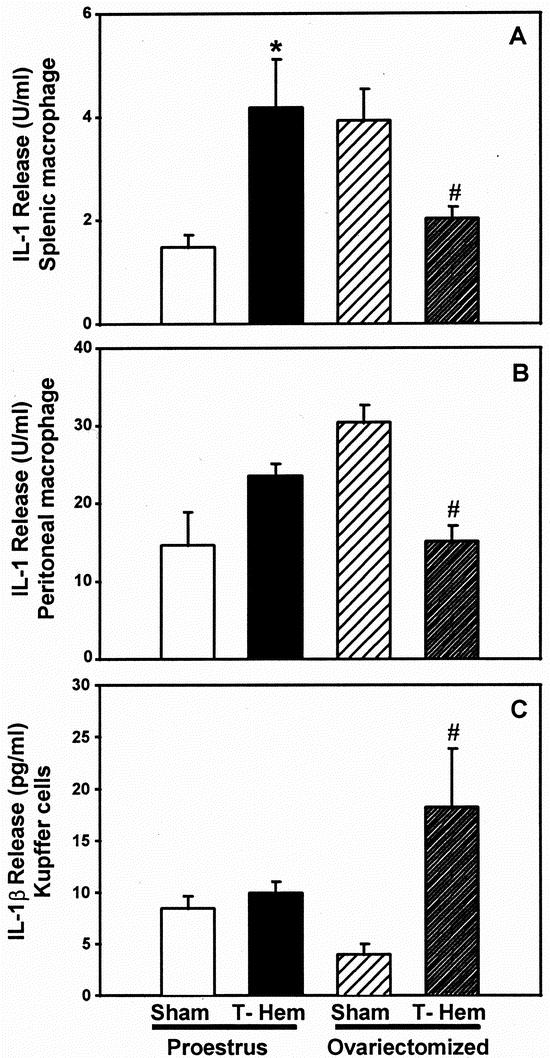
Figure 1. Release of interleukin-1 (IL-1) by splenic macrophages (A) or peritoneal macrophages (B) and Kupffer cells (C) at 2 hours after sham operation or trauma-hemorrhage. Concentrations of IL-1 in supernatants from splenic and peritoneal macrophages were measured by a specific bioassay (D10.G4.1), whereas Kupffer cell IL-1 release was measured by enzyme-linked immunosorbent assay specific for murine IL-1β. Values are means ± SEM of seven or eight animals per group; analysis of variance, *P < .05 vs. proestrus sham; #P < .05 vs. ovariectomy sham.
Macrophage Interleukin-6 Release
Splenic Mφ IL-6 release was significantly increased (P < .05) in proestrus females after trauma-hemorrhage compared with their corresponding shams, whereas in ovariectomized females IL-6 production was significantly depressed (P < .05) under such conditions (Fig. 2). Peritoneal Mφ IL-6 release after trauma-hemorrhage was unchanged in proestrus females compared with their corresponding shams. In ovariectomized females, however, peritoneal Mφ IL-6 release was significantly depressed in hemorrhaged animals versus sham-operated animals (P < .05). Kupffer cell IL-6 release significantly increased after trauma-hemorrhage in proestrus and ovariectomized females (P < .05). No differences were observed in post-trauma-hemorrhage Kupffer cell IL-6 release between proestrus and ovariectomized females.
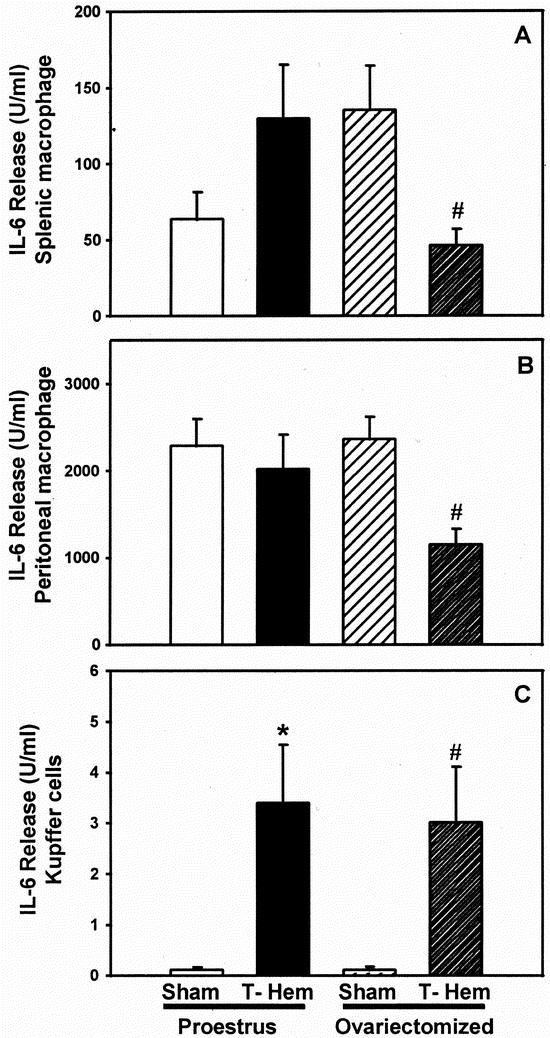
Figure 2. Release of interleukin-6 (IL-6) by splenic macrophages (A) or peritoneal macrophages (B) and Kupffer cells (C) at 2 hours after sham operation or trauma-hemorrhage. Values are means ± SEM of seven or eight animals per group; analysis of variance, *P < .05 vs. proestrus sham; #P < .05 vs. ovariectomy sham.
Macrophage Interleukin-10 Release
Production of IL-10 by splenic Mφ was significantly reduced in proestrus females at 2 hours after trauma-hemorrhage (Fig. 3;P < .05). In contrast, IL-10 release by splenic Mφ from ovariectomized females was not different from that in sham animals. Similar changes were observed in peritoneal Mφ, with the exception that ovariectomy significantly reduced IL-10 release independent of trauma-hemorrhage. After trauma-hemorrhage, Kupffer cell IL-10 release was significantly increased in proestrus and ovariectomized females to a similar extent (P < .05).
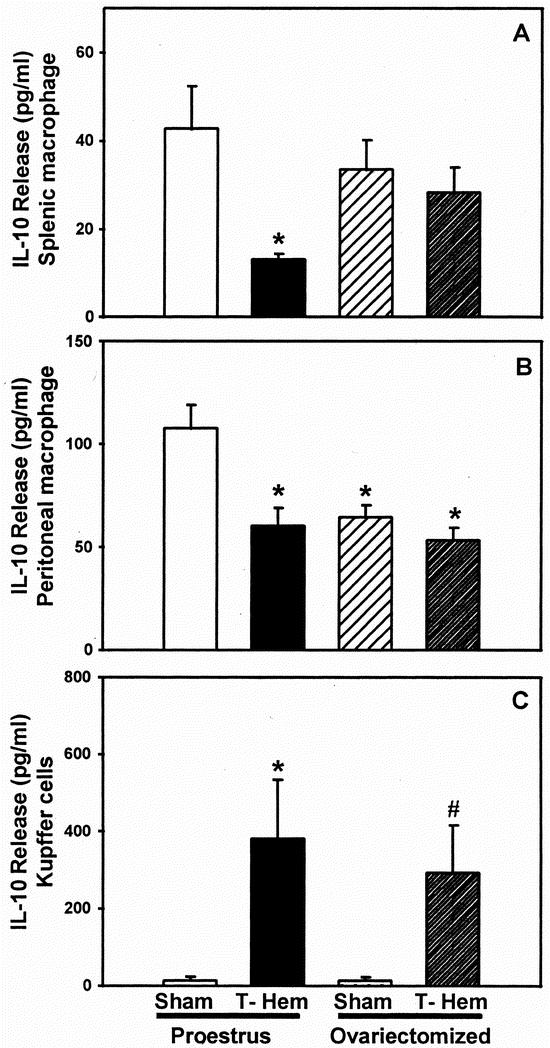
Figure 3. Release of interleukin-10 (IL-10) by splenic macrophages (A) or peritoneal macrophages (B) and Kupffer cells (C) at 2 hours after sham operation or trauma-hemorrhage. Values are means ± SEM of seven or eight animals per group; analysis of variance, *P < .05 vs. proestrus sham; #P < .05 vs. ovariectomy sham.
Kupffer Cell Tumor Necrosis Factor
In proestrus females, a slight but insignificant increase in Kupffer cell TNF-α release was observed after trauma-hemorrhage, whereas TNF-α release by Kupffer cells from ovariectomized females subjected to trauma-hemorrhage was increased approximately sevenfold compared with ovariectomy shams (Fig. 4;P < .05).
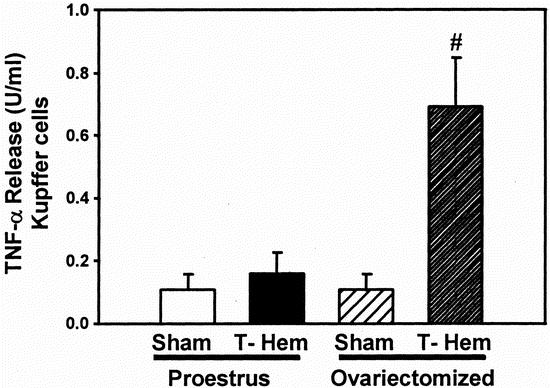
Figure 4. Release of tumor necrosis factor (TNF-α) by Kupffer cells at 2 hours after sham operation or trauma-hemorrhage (T-Hem). Kupffer cells were cultured in the presence of 10 μg/mL lipopolysaccharide for 24 hours. Values are means ± SEM of seven or eight animals per group; analysis of variance, #P < .05 versus ovariectomy sham.
Plasma Concentrations of Interleukin-6 and Tumor Necrosis Factor
At 2 hours after trauma-hemorrhage, a significant increase in plasma concentrations of IL-6 in proestrus and ovariectomized females was observed (Fig. 5;P < .05). There was no significant difference in plasma concentrations of IL-6 between proestrus females and ovariectomized females after trauma-hemorrhage. Trauma-hemorrhage did not increase plasma TNF-α concentrations in proestrus females, but in ovariectomized females TNF-α concentrations were significantly increased (P < .05) approximately sixfold after trauma-hemorrhage.
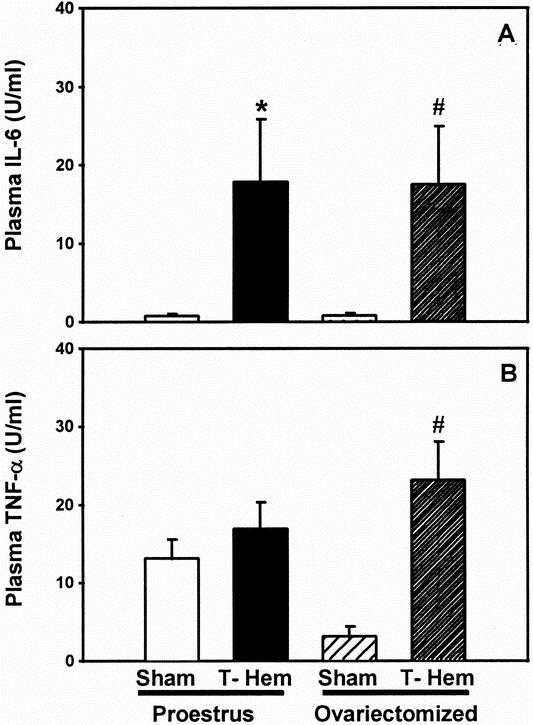
Figure 5. Plasma concentrations of (A) interleukin-6 (IL-6) and (B) tumor necrosis factor (TNF-α) at 2 hours after sham operation or trauma-hemorrhage. Values are means ± SEM of seven or eight animals per group; analysis of variance, *P < .05 vs. proestrus sham; #P < .05 vs. ovariectomy sham.
Survival From Subsequent Sepsis
The results of the trauma-hemorrhage and subsequent sepsis experiment are shown in Figure 6. No difference was observed in the death rates of proestrus females that underwent trauma-hemorrhage or sham operation 24 hours before the induction of sepsis. However, in ovariectomized females, the death rate of sham-operated animals subjected to sepsis was significantly higher than that of proestrus shams (P < .05). Further, from postoperative day 6, the death rate of ovariectomized females that underwent trauma-hemorrhage before sepsis induction was significantly higher than that of the sham-operated ovariectomized females (P < .05).
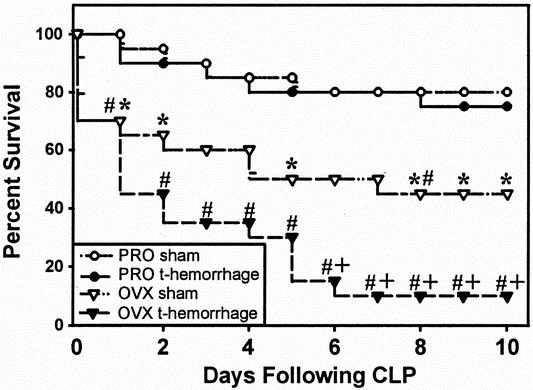
Figure 6. Ten-day survival of sham or trauma-hemorrhage mice after the induction of sepsis by cecal ligation and puncture (CLP). z test; *P < .05 vs. proestrus sham; #P < .05 vs. proestrus trauma-hemorrhage; +P < .05 vs. ovariectomy sham; n = 20 per group. PRO, proestrus; OVX, ovariectomy.
DISCUSSION
The differences in systemic sex hormone levels between proestrus and ovariectomized females are associated with profound alterations in the immunologic response to trauma-hemorrhage. Consistent with previous findings, 9 our results indicate that females in the proestrus state had increased splenic and peritoneal Mφ proinflammatory cytokine production after trauma-hemorrhage. However, in ovariectomized females, trauma-hemorrhage resulted in depressed splenic and peritoneal Mφ proinflammatory cytokine production under such conditions. Because ovariectomy led to a depression of Mφ IL-1 and IL-6 production after trauma-hemorrhage, it appears that physiologic levels of female sex steroids are involved in the regulation of Mφ proinflammatory cytokine production. The depression of splenic and peritoneal Mφ function seen in ovariectomized females after trauma-hemorrhage is comparable to the depression observed in males under such conditions, 12 indicating that the presence of male sex steroids (i.e., testosterone) and the reduction of female sex steroids produce comparable immunodepressive effects on Mφ under such conditions. Previous studies from our laboratory have also shown that the immune advantage of young females versus young males after trauma-hemorrhage is lost with aging, as estrogen levels decline. 24,25 Our results, however, do not allow us to conclude whether those immunosuppressive effects are direct actions of sex hormones on Mφ or whether they are mediated via indirect mechanisms.
Ovariectomy, independent of trauma-hemorrhage, altered splenic and peritoneal Mφ cytokine production, consistent with studies by Deshpande et al 26 showing the suppressive effects of 17β-estradiol on splenic Mφ IL-1 and IL-6 production. The observed increase in proinflammatory cytokine production by splenic Mφ from ovariectomized females is most likely due to the decrease in systemic 17β-estradiol levels. The divergent responses observed between sham and trauma-hemorrhage animals support the concept that hormonal regulation of immune function in a traumatized host differs substantially from the regulation under normal conditions.
The antiinflammatory cytokine IL-10 has previously been identified as an important immunosuppressant of cell-mediated immune functions 27 and has been implicated in the suppression of splenocyte immune functions after trauma-hemorrhage. 28 Our findings suggest that female sex hormones suppress IL-10 production by splenic and peritoneal Mφ after trauma-hemorrhage, thereby allowing maintained production of proinflammatory mediators.
Kupffer cells have previously been shown to be a primary source of proinflammatory cytokines in males after trauma-hemorrhage. 29 Our results further indicate that Kupffer cell IL-1β and TNF-α release increased in ovariectomized females after trauma-hemorrhage, whereas no difference was seen in proestrus females. The activation of Kupffer cell TNF-α production in ovariectomized females was associated with significantly increased circulating concentrations of TNF-α after trauma-hemorrhage, whereas plasma TNF-α remained at sham concentrations in proestrus females. Previous studies have implicated TNF-α as an important mediator in the immune depression after trauma-hemorrhage. 30 The absence of a systemic TNF-α response in proestrus females after trauma-hemorrhage might in part explain the lack of immune depression and increased susceptibility to subsequent sepsis. There was no difference in Kupffer cell IL-6 production and plasma IL-6 concentrations between proestrus and ovariectomized females after trauma-hemorrhage; this may be related to the early time point used in this study. The elevation in plasma IL-6 concentrations after trauma-hemorrhage is more protracted than that of TNF-α. 31 Additional studies will be required to determine whether differences in Kupffer cell IL-6 and IL-10 production between proestrus and ovariectomized females might occur at later time points.
There was no difference in the death rates of proestrus females that underwent trauma-hemorrhage or sham operation before the induction of sepsis, consistent with previous findings. 10 In contrast, the death rate of ovariectomized females that underwent trauma-hemorrhage before induction of sepsis was higher than that of sham-operated ovariectomized females. Thus, physiologic levels of female sex steroids appear to prevent an increased death rate from sepsis in females after trauma-hemorrhage. The death rate of ovariectomized sham-operated animals was significantly higher than that of proestrus shams, further supporting a protective role for female sex hormones after sepsis.
Ovariectomy leads to complex alterations in the hormonal milieu; therefore, it cannot be determined whether a specific sex hormone or a combination of sex hormones is responsible for the observed effects on hemorrhage-induced immune dysfunction. Nonetheless, estrogen, which is elevated during the proestrus state of the cycle, 32 has significant immunomodulatory properties, and the administration of 17β-estradiol in males after trauma-hemorrhage exerts protective effects. 12,33 The decreased plasma concentrations of 17β-estradiol in ovariectomized females likely contributed to the depression of immune functions after trauma-hemorrhage. Nonestrogenic hormones, however, may also be involved in the immunoprotection observed in proestrus females, because prolactin administration in males after trauma-hemorrhage restored Mφ functions and reduced the death rate from subsequent sepsis. 11
It remains unclear whether estrogen therapy will be effective in the management of trauma patients. Recent clinical studies have suggested that females are both at a decreased risk for septic complications 16 and an increased risk for sepsis. 34 Although our findings suggest that estrogen is beneficial after trauma-hemorrhage, caution should be used in the application of estrogen therapy after trauma because of the potential deleterious side effects (increased risk of thromboembolism, 35,36 decreased gut motility, 37,38 suppression of apoptosis 39,40 leading to the induction of autoimmunity, 41 suppression of wound healing, 42 and potential suppression of cell-mediated immune responses 43,44). Further investigations, such as in vivo and in vitro hormone replacement studies, are needed to clarify the role of specific female sex hormones in maintaining immunocompetence after trauma-hemorrhage. Nonetheless, these results, along with previous experimental findings 9,12,33 and epidemiologic data, 14–18 support the notion of female sex hormones, specifically estrogen, as an adjunct in the management of trauma patients.
Footnotes
Supported by a grant from the National Institutes of Health (R01 GM 37127 to I.H.C.) and a fellowship from the Deutsche Forschungsgemeinschaft (Kn 475/1-1 to M.W.K.).
Presented in part at the 85th Annual Clinical Congress, American College of Surgeons, San Francisco, California, October 1999
M.W.K. is currently at the University of Ulm, Ulm, Germany.
Correspondence: Dr. Irshad H. Chaudry, Center for Surgical Research, Department of Surgery, The University of Alabama at Birmingham, Volker Hall, G094 1670 University Blvd., Birmingham, AL 35294-0019.
E-mail: irshad.chaudry@ccc.uab.edu
Accepted for publication April 5, 2001.
References
- 1.Chaudry IH, Ayala A. Immunological aspects of hemorrhage. Austin, TX: Medical Intelligence Unit; R.G. Landes Co., 1992: 1–132.
- 2.Xu YX, Ayala A, Chaudry IH. Prolonged immunodepression after trauma and hemorrhagic shock. J Trauma 1998; 44: 335–341. [DOI] [PubMed] [Google Scholar]
- 3.Ayala A, Perrin MM, Chaudry IH. Increased susceptibility to sepsis following hemorrhage: Defective Kupffer cell mediated antigen presentation. Surg Forum 1989; 40: 102–104. [Google Scholar]
- 4.Ayala A, Perrin MM, Wagner MA, et al. Enhanced susceptibility to sepsis after simple hemorrhage: Depression of Fc and C3b receptor mediated phagocytosis. Arch Surg 1990; 125: 70–75. [DOI] [PubMed] [Google Scholar]
- 5.Ayala A, Perrin MM, Wang P, et al. Hemorrhage induces enhanced Kupffer cell cytotoxicity while decreasing peritoneal or splenic macrophage capacity: involvement of cell-associated TNF and reactive nitrogen. J Immunol 1991; 147: 4147–4154. [PubMed] [Google Scholar]
- 6.Wichmann MW, Ayala A, Chaudry IH. Male sex steroids are responsible for depressing macrophage immune function after trauma-hemorrhage. Am J Physiol 1997; 273: C1335–C1340. [DOI] [PubMed] [Google Scholar]
- 7.Angele MK, Wichmann MW, Ayala A, et al. Testosterone receptor blockade following hemorrhage in males. Restoration of the depressed immune functions and improved survival after subsequent sepsis. Arch Surg 1997; 132: 1207–1214. [DOI] [PubMed] [Google Scholar]
- 8.Wichmann MW, Angele MK, Ayala A, et al. Flutamide. A novel agent for restoring the depressed cell-mediated immunity following soft-tissue trauma and hemorrhagic shock. Shock 1997; 8: 1–7. [PubMed] [Google Scholar]
- 9.Wichmann MW, Zellweger R, DeMaso CM, et al. Enhanced immune responses in females as opposed to decreased responses in males following hemorrhagic shock. Cytokine 1996; 8: 853–863. [DOI] [PubMed] [Google Scholar]
- 10.Diodato MD, Knöferl MW, Schwacha MG, et al. Gender differences in the inflammatory response and survival following haemorrhage and subsequent sepsis. Cytokine 2000; 14: 162–169. [DOI] [PubMed] [Google Scholar]
- 11.Zellweger R, Zhu X-H, Wichmann MW, et al. Prolactin administration following hemorrhagic shock improves macrophage cytokine release capacity and decreases mortality from subsequent sepsis. J Immunol 1996; 157: 5748–5754. [PubMed] [Google Scholar]
- 12.Knöferl MW, Diodato MD, Angele MK, et al. Do female sex steroids adversely or beneficially affect the depressed immune responses in males after trauma-hemorrhage? Arch Surg 2000; 135: 425–433. [DOI] [PubMed] [Google Scholar]
- 13.Ogle TF, Kitay JI. Ovarian and adrenal steroids during pregnancy and the oestrous cycle in the rat. J Endocrinol 1977; 74: 89–98. [DOI] [PubMed] [Google Scholar]
- 14.Bone RC. Toward an epidemiology and natural history of SIRS (systemic inflammatory response syndrome). JAMA 1992; 268: 3452–3455. [PubMed] [Google Scholar]
- 15.McGowan JE, Barnes MW, Finland N. Bacteremia at Boston City Hospital: occurrence and mortality during 12 selected years (1935–1972) with special reference to hospital-acquired cases. J Infect Dis 1975; 132: 316–335. [DOI] [PubMed] [Google Scholar]
- 16.Schröder J, Kahlke V, Staubach KH, et al. Gender differences in human sepsis. Arch Surg 1998; 133: 1200–1205. [DOI] [PubMed] [Google Scholar]
- 17.McLauchlan GJ, Anderson ID, Grant IS, et al. Outcome of patients with abdominal sepsis treated in an intensive care unit. Br J Surg 1995; 82: 524–529. [DOI] [PubMed] [Google Scholar]
- 18.Watanakunakorn C. Staphylococcus aureus endocarditis at a community teaching hospital, 1980 to 1991. An analysis of 106 cases. Arch Intern Med 1994; 154: 2330–2335. [PubMed] [Google Scholar]
- 19.Baker CC, Chaudry IH, Gaines HO, et al. Evaluation of factors affecting mortality rate after sepsis in murine cecal ligation and puncture model. Surgery 1983; 94: 331–335. [PubMed] [Google Scholar]
- 20.Ayala A, Perrin MM, Chaudry IH. Defective macrophage antigen presentation following haemorrhage is associated with the loss of MHC class II (Ia) antigens. Immunology 1990; 70: 33–39. [PMC free article] [PubMed] [Google Scholar]
- 21.Ayala A, Perrin MM, Ertel W, et al. Differential effects of haemorrhage on Kupffer cells: decreased antigen presentation despite increased inflammatory cytokine (IL-1, IL-6 and TNF) release. Cytokine 1992; 4: 66–75. [DOI] [PubMed] [Google Scholar]
- 22.Ertel W, Morrison MH, Ayala A, et al. Chloroquine attenuates hemorrhagic shock induced suppression of Kupffer cell antigen presentation and MHC class II antigen expression through blockade of tumor necrosis factor and prostaglandin release. Blood 1991; 78: 1781–1788. [PubMed] [Google Scholar]
- 23.Ayala A, Perrin MM, Kisala JM, et al. Polymicrobial sepsis selectively activates peritoneal but not alveolar macrophage to release inflammatory mediators (IL-1, IL-6 and TNF). Circ Shock 1992; 36: 191–199. [PubMed] [Google Scholar]
- 24.Kahlke V, Angele MK, Ayala A, et al. Immune dysfunction following trauma-haemorrhage: influence of gender and age. Cytokine 2000; 12: 69–77. [DOI] [PubMed] [Google Scholar]
- 25.Kahlke V, Angele MK, Schwacha MG, et al. Reversal of sexual dimorphism in splenic T lymphocyte responses after trauma-hemorrhage with aging. Am J Physiol Cell Physiol 2000; 278: C509–C516. [DOI] [PubMed] [Google Scholar]
- 26.Deshpande R, Khalili H, Pergolizzi RG, et al. Estradiol down-regulates LPS-induced cytokine production and NFκB activation in murine macrophages. Am J Reprod Immunol 1997; 38: 46–54. [DOI] [PubMed] [Google Scholar]
- 27.Howard M, O’Garra A, Ishida H, et al. Biological properties of interleukin 10. J Clin Immunol 1992; 12: 239–247. [DOI] [PubMed] [Google Scholar]
- 28.Ayala A, Lehman DL, Herdon CD, et al. Mechanism of enhanced susceptibility to sepsis after hemorrhage: Interleukin (IL)-10 suppression of T-cell response is mediated by eicosanoid induced IL-4 release. Arch Surg 1994; 129: 1172–1178. [DOI] [PubMed] [Google Scholar]
- 29.O’Neill PJ, Ayala A, Wang P, et al. Role of Kupffer cells in interleukin-6 release after trauma- hemorrhage and resuscitation. Shock 1994; 1: 43–47. [DOI] [PubMed] [Google Scholar]
- 30.Ertel W, Morrison MH, Ayala A, et al. Biological significance of elevated TNF levels: in vivo administration of monoclonal antibody against TNF after haemorrhage shock increases the capacity of macrophages to release TNF while restoring immunoresponsiveness. Cytokine 1994; 6: 624–632. [DOI] [PubMed] [Google Scholar]
- 31.Schwacha MG, Knöferl MW, Samy TSA, et al. The immunologic consequences of hemorrhagic shock. Crit Care Shock 1999; 2: 42–64. [Google Scholar]
- 32.Smith MS, Freeman ME, Neill JD. The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology 1975; 96: 219–226. [DOI] [PubMed] [Google Scholar]
- 33.Angele MK, Knöferl MW, Schwacha MG, et al. Sex steroids regulate pro- and anti-inflammatory cytokine release by macrophages after trauma-hemorrhage. Am J Physiol 1999; 277: C35–C42. [DOI] [PubMed] [Google Scholar]
- 34.Eachempati SR, Hydo L, Barie PS. Gender-based differences in outcome in patients with sepsis. Arch Surg 1999; 134: 1342–1347. [DOI] [PubMed] [Google Scholar]
- 35.Rossouw JE. Hormone replacement therapy and cardiovascular disease. Curr Opin Lipidol 1999; 10: 429–434. [DOI] [PubMed] [Google Scholar]
- 36.Douketis JD, Gordon M, Johnston M, et al. The effects of hormone replacement therapy on thrombin generation, fibrinolysis inhibition, and resistance to activated protein C: prospective cohort study and review of literature. Thromb Res 2000; 99: 25–34. [DOI] [PubMed] [Google Scholar]
- 37.Everson GT. Gastrointestinal motility in pregnancy. Gastroenterol Clin North Am 1992; 21: 751–776. [PubMed] [Google Scholar]
- 38.Pines A, Eckstein N, Dotan I, et al. Effect of estradiol on rat ileum. Gen Pharmacol 1998; 31: 735–736. [DOI] [PubMed] [Google Scholar]
- 39.Gohel A, McCarthy MB, Gronowicz G. Estrogen prevents glucocorticoid-induced apoptosis in osteoblasts in vivo and in vitro. Endocrinology 1999; 140: 5339–5347. [DOI] [PubMed] [Google Scholar]
- 40.Evans MJ, MacLaughlin S, Marvin RD, et al. Estrogen decreases in vitro apoptosis of peripheral blood mononuclear cells from women with normal menstrual cycles and decreases TNF-alpha production in SLE but not in normal cultures. Clin Immunol Immunopathol 1997; 82: 258–262. [DOI] [PubMed] [Google Scholar]
- 41.Bynoe MS, Grimaldi CM, Diamond B. Estrogen up-regulates Bcl-2 and blocks tolerance induction of naive B cells. Proc Natl Acad Sci USA 2000; 97: 2703–2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frazier-Jessen MR, Mott FJ, Witte PL, et al. Estrogen suppression of connective tissue deposition in a murine model of peritoneal adhesion formation. J Immunol 1996; 156: 3036–3042. [PubMed] [Google Scholar]
- 43.Purtilo DT, Hallgren HM, Yunis EJ. Depressed maternal lymphocyte response to phytohaemagglutinin in human pregnancy. Lancet 1972; 1: 769–771. [DOI] [PubMed] [Google Scholar]
- 44.Gregory MS, Duffner LA, Faunce DE, et al. Estrogen mediates the sex difference in post-burn immunosuppression. J Endocrinol 2000; 164: 129–138. [DOI] [PubMed] [Google Scholar]


