Abstract
Objective
To detect occult micrometastatic tumor cells in pN0 lymph nodes of nonsmall cell lung cancer (NSCLC) by a combination of cytokeratin and p53 immunohistochemistry staining, and to evaluate the relation between the micrometastasis in pN0 lymph nodes and the prognosis of patients with completely resected stage 1 NSCLC.
Summary Background Data
The average 5-year survival rate for patients with completely resected stage 1 NSCLC is only about 70%; thus, about 30% of these patients have recurrent disease. This suggests that occult micrometastasis may exist at the time of surgery; the rate is clearly underestimated by current clinical staging examinations and conventional histopathologic methods.
Methods
A total of 474 hilar and mediastinal lymph nodes were removed during surgery from 49 patients with completely resected stage 1 NSCLC. The lymph nodes analyzed for micrometastasis using immunohistochemical staining with the biclonal anticytokeratin antibody, AE1/AE3. Of these 474 lymph nodes from 49 patients, 263 lymph nodes from 25 patients, whose primary tumors were positive for the p53 protein, were subjected to immunohistochemical staining with the monoclonal anti-p53 protein antibody DO-1.
Results
Cells positive for cytokeratin and p53 protein were found in 35 (7.4%) of 474 and 20 (7.6%) of 263 lymph nodes, respectively; 17 (34.7%) of 49 patients had cytokeratin-positive cells and 10 (40.0%) of 25 patients had p53-positive cells in their pN0 lymph nodes. By a combination of cytokeratin and p53 protein immunohistochemical staining, micrometastatic tumor cells were identified in pN0 lymph nodes in 22 (44.9%) of 49 patients. The patients with lymph node micrometastasis identified by a combination of cytokeratin and p53 protein immunohistochemical staining had a poorer prognosis than those without micrometastasis on both univariate and multivariate analyses (overall survival, P = .0003 and 0.013, respectively).
Conclusions
The detection of lymph nodal micrometastasis by cytokeratin and p53 protein immunohistochemical staining will be helpful to predict the recurrence and prognosis of patients with completely resected stage 1 NSCLC.
Lung cancer is the leading cause of cancer death in North America, and it became the leading cause of death among Japanese men and the second leading cause among Japanese women for all cancers in 1993. Lung cancer is also an aggressive carcinoma with a poor outcome. The TNM staging system of lung cancer is widely used as a guide for predicting the prognosis. The presence of lymph node metastases along with T and M status represents the most accurate factor currently available for the prediction of prognosis in patients who undergo complete surgical resection. However, about 30% of patients with pathologic stage 1 nonsmall cell lung cancer (NSCLC) have a recurrence of the tumor and die, despite complete surgical resection. 1,2 This suggests that occult micrometastatic tumor cells, which are not detected by current clinical staging examinations and conventional histopathologic methods such as hematoxylin and eosin staining, have already spread to the regional lymph nodes (lymphatic locoregional metastasis) or the distant mesenchymal organs (hematogenous systemic metastasis) at the time of surgery. Therefore, for an accurate prediction of prognosis, it is necessary to assess the lymph node status and to take account of micrometastasis.
Recently, we reported that micrometastatic p53 protein-positive cells in the lymph nodes of patients with NSCLC are associated with a poor prognosis. 3 This method can be used for patients with p53-positive staining in the primary tumor; however, the p53 tumor suppressor gene is mutated in only half of all patients with NSCLC. 4–6 Therefore, in patients with p53-negative primary tumors, we cannot detect the micrometastatic tumor cells by using p53 as a marker. In the past few years, several successful attempts have been made to detect micrometastatic tumor cells in lymph nodes, 7–10 bone marrow, and peripheral blood 11,12 by either immunohistochemical staining or genetic methods such as reverse transcriptase–polymerase chain reaction (RT-PCR) with cytokeratin as a marker for micrometastasis.
This study was designed to detect occult micrometastatic tumor cells in pN0 lymph nodes of NSCLC by a combination of cytokeratin and p53 immunohistochemical staining, and to evaluate the relation between the micrometastasis in pN0 lymph nodes and the prognosis of patients with completely resected stage 1 NSCLC.
METHODS
Patients, Lymph Nodes, Materials, and Follow-Up
Of 101 consecutive patients with NSCLC who underwent radical surgery of the primary tumor with dissection of the hilar and mediastinal lymph nodes (systematic nodal dissection) at the Department of Respiratory Surgery at National Oita Hospital, Japan, during the 4-year period from January 1987 to December 1990, 51 patients had stage 1 disease (pN0 lymph nodes) identified by routine histopathologic examination. Of these 51 patients, we were able to obtain adequate paraffin-embedded lymph nodes and tumor specimens from 49. The median number of lymph nodes available for examination from each patient was 10. The tumor stage was classified according to the Revisions in the International System for Staging Lung Cancer (1997). 13 There were 34 men and 15 women with a mean age of 62.8 years (range 36–81). The histologic types included 27 adenocarcinomas, 21 squamous cell carcinomas, and 1 adenosquamous carcinoma. Eighteen patients had stage 1A (T1NOMO) and 31 had stage 1B (T2NOMO) carcinomas.
A total of 474 hilar and mediastinal lymph nodes were removed during surgery from these 49 patients and were analyzed for micrometastasis using cytokeratin immunohistochemical staining. Of these 474 lymph nodes from 49 patients, 263 lymph nodes from 25 patients whose primary tumors were positive for p53 protein were subjected to p53 immunohistochemical staining. For the evaluation of the p53 protein immunohistochemical staining of the primary tumor, it was classified as a positive tumor when the proportion of stained cells was 10% or more. This criterion was based on the findings of our previous report, and the concordance rate between the DNA analysis and immunohistochemical staining was relatively constant when this cutoff level was used. 6 We performed p53 immunohistochemical staining for primary tumors in the same manner as the lymph node staining described below.
After the primary surgery, the patients were examined every month in the first year and thereafter at 2- to 4-month intervals as a rule. The evaluations included physical examination, chest radiography, blood chemistry, and carcinoembryonic antigen assay. Chest, abdominal, and brain computed tomographic scans and a bone scintiscan were performed every 6 months in the third year, and each year thereafter. If any symptoms or signs of recurrence appeared in these examinations, further frequent evaluations to detect the recurrent site were performed. All patients were followed up until 60 months after the primary surgery, and no patients were lost to follow-up. The median observation period was 44 months (range 4–60 months). At the last follow-up, 28 patients were alive and free of cancer, 9 patients had died of other causes without evidence of cancer, 1 patient was alive with recurrent cancer, and 11 patients had died of cancer.
Immunohistochemical Staining for Cytokeratin and p53 Proteins
Five 4-μm slices, representing every other slice from 10 slices of each paraffin-embedded lymph node section, were attached to glass slides. The slides were stained with primary antibodies against the cytokeratin and p53 proteins by using a labeled streptavidin-biotin method (DAKO LSAB kit, Dako Corp., Carpinteria, CA). The primary antibodies were the mouse biclonal antibody AE1/AE3 (Progen Biotechnik GmbH, Heidelberg, Germany) to the cytokeratins, which recognizes most of the type 1 (acidic type) and type 2 (basic type) cytokeratins, and the DO-1 antibody (Oncogene Science Inc., Cambridge, MA) to the p53 protein, which recognizes the codon regions 37 to 45 of the p53 protein. The staining procedures were as follows: deparaffinization with xylene and ethanol; antigen retrieval by five 5-minute microwave treatments for the p53 protein; incubation with the primary antibodies (dilutions: AE1/AE3, 1:200; DO-1, 1:100); incubation with the secondary antibody (either biotinylated goat antimouse IgG or goat antirabbit IgG); developing with peroxidase-labeled streptavidin and diaminobenzidine-H2O2; and counterstaining with hematoxylin. The presence of cytokeratin- or p53 protein-positive cells within the whole body section of the lymph nodes was accepted as evidence of micrometastatic tumor cells, even if only a single cytokeratin- or p53 protein-positive cell was detected. The specimens were examined and checked by three of the authors (C.D.G., T.Os., and K.D.) without knowledge of the patient data, including outcome.
Statistical Analysis
Patient data were acquired by a retrospective chart review. The associations between the clinicopathologic characteristics and the micrometastatic status were analyzed by a contingency table. Statistical significance was evaluated using the chi-square test or the Fisher exact test. Survival curves were plotted according to the Kaplan-Meier method, 14 and differences between the curves were analyzed by the log-rank test. 15 The Cox proportional hazards model was applied to the multivariate survival analysis. 16 The difference was considered to be significant at P < .05. Data were analyzed with the use of Abacus Concepts, Survival Tools for StatView (Abacus Concepts, Inc., Berkeley, CA).
RESULTS
Detection of Micrometastatic Cytokeratin- and p53-Positive Cells
By using immunohistochemical staining with the biclonal antibody AE1/AE3 against cytokeratin and DO-1 against p53 protein, single tumor cells and small clusters of tumor cells were most often seen in the subcapsular or medullary sinuses and less frequently in the afferent lymphatics of the lymph node specimens (Fig. 1). The cytokeratin- and p53 protein-positive cells were found in 35 (7.4%) of 474 and 20 (7.6%) of 263 lymph nodes, respectively: 17 (34.7%) of 49 patients had cytokeratin-positive cells and 10 (40.0%) of 25 patients had p53-positive cells in their pN0 lymph nodes. By a combination of cytokeratin and p53 protein immunohistochemical staining, we identified micrometastatic tumor cells in the pN0 lymph nodes in 22 (44.9%) of 49 patients. The presence of micrometastatic tumor cells in the pN0 lymph nodes was not associated with age, sex, histologic type, pathologic stage, or T status (Table 1).
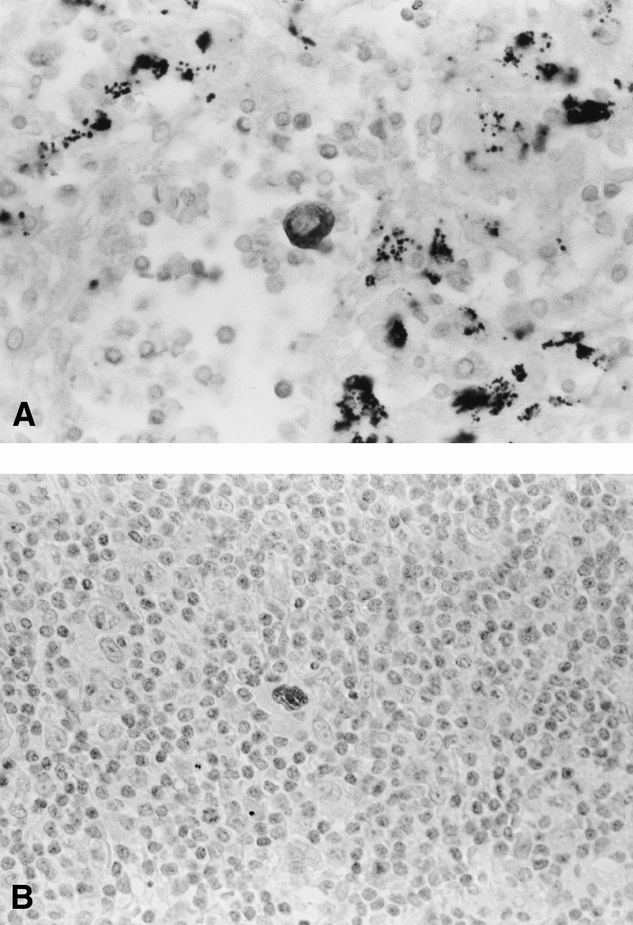
Figure 1. (A) A single micrometastatic cytokeratin-positive cell with deep brown-stained cytoplasm is seen against a background of benign lymphatic cells (original magnification ×400). (B) A single micrometastatic p53-positive cell with a deep brown-stained nucleus is seen against a background of benign lymphatic cells (original magnification ×400).
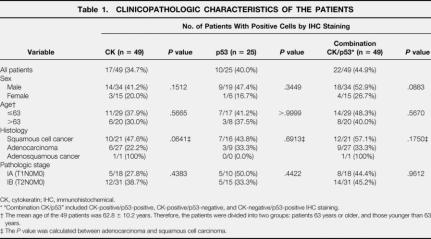
Table 1. CLINICOPATHOLOGIC CHARACTERISTICS OF THE PATIENTS
CK, cytokeratin; IHC, immunohistochemical.
* “Combination CK/p53” included CK-positive/p53-positive, CK-positive/p53-negative, and CK-negative/p53-positive IHC staining.
† The mean age of the 49 patients was 62.8 ± 10.2 years. Therefore, the patients were divided into two groups: patients 63 years or older, and those younger than 63 years.
‡ The P value was calculated between adenocarcinoma and squamous cell carcinoma.
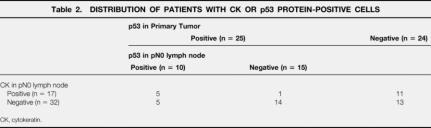
The distribution of patients with cytokeratin- and p53 protein-positive cells in the pN0 lymph nodes is shown in Table 2. Eleven (45.8%) of 24 patients whose primary tumors were negative for the p53 protein had cytokeratin-positive cells in the pN0 lymph nodes, whereas 5 (15.6%) of 32 patients without cytokeratin-positive cells had p53-positive cells in the pN0 lymph nodes.
Table 2. DISTRIBUTION OF PATIENTS WITH CK OR p53 PROTEIN-POSITIVE CELLS
CK, cytokeratin.
A restaging of the nodal status, based on the combination of cytokeratin and p53 immunohistochemical staining, was performed. Among the patients with pN0 disease identified by conventional hematoxylin and eosin histopathologic study, 9(18.4%) patients were restaged as N1 and 13 (26.5%) patients were restaged as N2.
Influence of Micrometastasis on Recurrence and Survival
We evaluated the influence of lymph node micrometastasis on the patient’s recurrence and overall survival using the combination of cytokeratin and p53 protein immunohistochemical staining. Within the 5-year complete follow-up on all 49 patients, 12 patients (24.5%) had recurrences during this period. The median duration from surgery to recurrence in these 12 patients was 28.5 months (range 5–60). As shown in Table 3, 10 (45.5%) of 22 patients with cytokeratin- or p53 protein-positive cells in their pN0 lymph nodes had recurrences, whereas 2 (7.4%) of 27 patients who were free of these cells had recurrences. The patients with micrometastatic tumor cells in the pN0 lymph nodes had a significantly higher recurrence rate than those without such cells (P = .003). The presence of micrometastasis in the pN0 lymph nodes was also predictive of the pattern of recurrence. Of the 10 patients with micrometastasis and recurrent diseases, 8 had hematogenous distant recurrences.
Table 3. RECURRENCE RATES
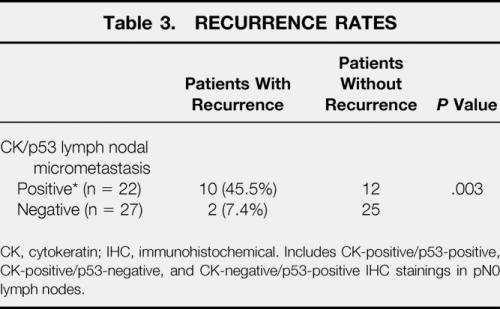
CK, cytokeratin; IHC, immunohistochemical. Includes CK-positive/p53-positive, CK-positive/p53-negative, and CK-negative/p53-positive IHC stainings in pN0 lymph nodes.
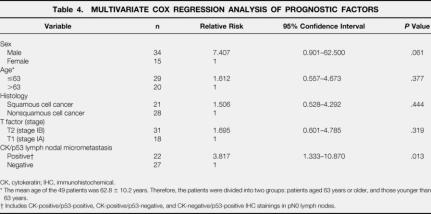
The overall 5-year survival was 58.8% in the 49 patients in this study. The Kaplan-Meier survival curves showed that the patients with micrometastatic tumor cells in the pN0 lymph nodes had significantly shorter survival periods than those without such cells (P = .0003) (Fig. 2). Among the patients with stage 1A disease (n = 18), the overall 5-year survival rates in the patients with and without micrometastatic tumor cells were 50.0% and 90.0%, respectively (P = .057). Among the patients with stage 1B disease (n = 31), the overall 5-year survival rates in the patients with and without micrometastatic tumor cells were 21.4% and 76.0%, respectively (P = .0016). Further, a Cox multivariate survival analysis showed that the micrometastatic nodal status was a significant independent predictor of a poorer prognosis from the T status in patients with completely resected stage 1 NSCLC (P = .013, relative risk = 3.817) (Table 4). There was no difference in survival between the patients with N1 and those with N2 status restaged by the combination of cytokeratin and p53 immunohistochemistry (P = .507).
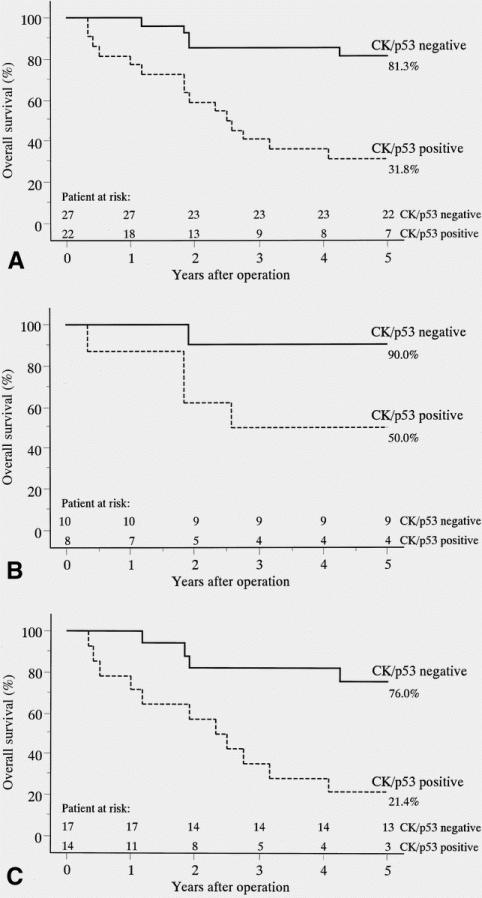
Figure 2. (A) Survival of patients with stage 1 disease (n = 49) with or without micrometastatic tumor cells in the pN0 lymph nodes detected by the combination of cytokeratin and p53 protein immunohistochemical staining (Kaplan-Meier analysis, log-rank test, P = .0003). (B) Survival of patients with stage 1A disease (n = 18) with or without micrometastatic tumor cells in the pN0 lymph nodes detected by the combination of cytokeratin and p53 protein immunohistochemical staining (P = .057). (C) Survival of patients with stage 1B disease (n = 31) with or without micrometastatic tumor cells in the pN0 lymph nodes detected by the combination of cytokeratin and p53 protein immunohistochemical staining (P = .0016). “CK/p53 positive” included cytokeratin-positive/p53-positive, cytokeratin-positive/p53-negative, and cytokeratin-negative/p53-positive immunohistochemical stainings. “CK/p53 negative” included cytokeratin-negative/p53-negative immuno-histochemical staining.
Table 4. MULTIVARIATE COX REGRESSION ANALYSIS OF PROGNOSTIC FACTORS
CK, cytokeratin; IHC, immunohistochemical.
* The mean age of the 49 patients was 62.8 ± 10.2 years. Therefore, the patients were divided into two groups: patients aged 63 years or older, and those younger than 63 years.
† Includes CK-positive/p53-positive, CK-positive/p53-negative, and CK-negative/p53-positive IHC stainings in pN0 lymph nodes.
DISCUSSION
Lung cancer is now thought to arise from the accumulation of several genetic changes, such as mutations and deletions. Recent advances in molecular biology and genetics have created new diagnostic and treatment possibilities for clinical oncology. The potential prognostic implications of several biologic and molecular parameters, including oncogenes, such as K-ras mutation 17 and c-erb B-2 overexpression, 18 and tumor suppressor genes, such as p53 abnormalities, 6,19,20 and cancer cell proliferative activity 21 have been reported in patients with NSCLC. Nevertheless, pathologic tumor staging represents the most accurate factor currently available for predicting the prognosis of patients who have undergone radical resection. However, the average 5-year survival rate for patients with completely resected stage 1 NSCLC is only about 70%;1,22 thus, about 30% of these patients have recurrent disease. This suggests that occult micrometastasis may exist at the time of surgery; the rate is clearly underestimated by current clinical staging examinations and conventional histopathologic methods. In this study, we identified occult micrometastatic tumor cells in pN0 lymph nodes in half of the patients with completely resected stage 1 NSCLC by using a cytokeratin and p53 protein immunohistochemical staining assay. Further, the patients with lymph node micrometastasis had a poorer prognosis than the patients without micrometastasis by both univariate and multivariate analyses; the prognostic impact was independent from the TNM staging system.
Several attempts have been made to detect micrometastatic tumor cells in lymph nodes, 7–10 bone marrow, and peripheral blood 11,12 by immunohistochemical staining or genetic methods such as RT-PCR, using several markers for epithelial cells such as cytokeratin and Ber-EP4, and oncogenes/oncoproteins such as K-ras and p53. We previously reported the use of p53 immunohistochemical staining in the detection of occult tumor cells in regional lymph nodes of patients with NSCLC, and occult micrometastasis was identified in 45.2% of this population. 3 The p53 tumor suppressor gene is altered in a high proportion of human neoplasms;23 however, it is mutated in about half of the various types of malignant diseases, including NSCLC. 24,25 This means that using oncogenes and oncoproteins, such as p53 and K-ras, as micrometastatic markers is disadvantageous for the detection of occult tumor cells. However, cytokeratin, which forms the intermediate filaments of the cytoskeleton within both normal and malignant epithelial cells, 27 is widely used as a marker of epithelial cells. The wide distribution of cytokeratins in all epithelial tumors means that antibodies to cytokeratin can detect occult metastasis from different kinds of cancers. 7 However, the cytokeratins are not specific to only tumor cells but are also present in normal epithelial cells, suggesting that a false-positive reaction may also be seen within nontumor cells in the lymph nodes, 7,8,27 although we can distinguish the cytokeratin-positive tumor cells from the nontumor cells by comparing their morphologic characteristics. In this study, we used the biclonal antibody AE1/AE3 to cytokeratin and the monoclonal antibody DO-1 to p53 protein as a marker for micrometastasis in the lymph node. Seventeen (34.7%) of the 49 patients had micrometastatic tumor cells using cytokeratin immunohistochemical staining alone, whereas we were able to identify micrometastasis in the pN0 lymph nodes in 22 (44.9%) patients by adding p53 immunohistochemical staining to that of cytokeratin. The diagnostic efficiency was increased by combining cytokeratin and p53 protein in the immunohistochemical staining, although performing both immunohistochemical stainings increased the cost of analysis.
The analysis of the tumor relapse pattern in this study revealed it to be hematogenous. Among 10 patients with micrometastasis and recurrent disease, 8 had hematogenous distant recurrences. Lymph node micrometastasis, as well as overt lymph node metastasis, does not necessarily reflect lymphogenous spread, but it may signal the early phase of hematogenous systemic tumor cell dissemination. In our laboratory, we also detected micrometastatic tumor cells in bone marrow aspirate samples of patients with NSCLC, which represents systemic tumor cell dissemination, using the anticytokeratin monoclonal antibody (CK2), which recognizes cytokeratin 18. 11 The results of micrometastasis in the bone marrow also indicated that hematogenous recurrence was more prevalent than locoregional recurrence. However, there was no relation between micrometastasis in the lymph nodes and in the bone marrow. Among 24 patients with lymph node micrometastasis, 7(29.2%) had bone marrow micrometastasis, whereas in 37 patients without lymph node micrometastasis, bone marrow micrometastasis was found in 11 (29.7%) (P > .999) (unpublished data, with subjects different from those of this study). This finding suggests that different determinants or mechanisms may exist in micrometastasis to the lymph node and to the bone marrow.
In conclusion, the detection of lymph node occult micrometastatic tumor cells by using cytokeratin and p53 protein immunohistochemical staining is helpful in predicting the recurrence and prognosis of patients with completely resected stage 1 NSCLC. These patients need to be carefully followed up after surgery. At present, postoperative adjuvant chemotherapy is not a routine standard therapy for patients with completely resected NSCLC because of its unreliable results for the improvement of prognosis. However, numerous trials, including two randomized trials, 28,29 of induction chemotherapy for stage 3A NSCLC have shown that it was feasible and indicated higher response rates and a survival advantage. When we consider the application of induction chemotherapy, the detection of micrometastasis in the mediastinal lymph nodes sampled before surgery by mediastinoscopy may be useful to identify patients with occult stage 3A disease.
Footnotes
Supported in part by a Grant-in-aid for Scientific Research (09771021) from the Ministry of Education, Science and Culture, Japan (T.Os.).
Correspondence: Toshihiro Osaki, MD, Department of Surgery II, School of Medicine, University of Occupational and Environmental Health, 1-1 Iseigaoka, Yahatanishi-ku, Kitakyushu 807-8555, Japan.
E-mail: t-osaki@med.uoeh-u.ac.jp
Accepted for publication May 3, 2001.
References
- 1.Martini N, Bains MS, Burt ME, et al. Incidence of local recurrence and second primary tumors in resected stage I lung cancer. J Thorac Cardiovasc Surg 1995; 109: 120–129. [DOI] [PubMed] [Google Scholar]
- 2.Strauss GM, Kwiatkawski DJ, Harpole DH, et al. Molecular and pathologic markers in stage I non-small cell carcinoma of the lung. J Clin Oncol 1995; 13: 1265–1279. [DOI] [PubMed] [Google Scholar]
- 3.Dobashi K, Sugio K, Osaki T, et al. Micrometastatic p53-positive cells in the lymph nodes of non-small-cell lung cancer: prognostic significance. J Thorac Cardiovasc Surg 1997; 114: 339–346. [DOI] [PubMed] [Google Scholar]
- 4.Harris CC, Hollstein M. Clinical implication of the p53 tumor-suppressor gene. N Engl J Med 1993; 329: 1318–1327. [DOI] [PubMed] [Google Scholar]
- 5.Kishimoto Y, Murakami, Shiraishi M, et al. Aberration of the p53 tumor suppressor gene in human non-small cell carcinoma of the lung. Cancer Res 1992; 52: 4799–4804. [PubMed] [Google Scholar]
- 6.Misudomi T, Oyama T, Nishida K, et al. P53 nuclear immunostaining and gene mutations in non-small-cell lung cancer and their effects on patient survival. Ann Oncol 1995; 6 (suppl 3): S9–S13. [DOI] [PubMed] [Google Scholar]
- 7.Chen ZL, Perez S, Holmes EC, et al. Frequency and distribution of occult micrometastases in lymph nodes of patients with non-small-cell lung carcinoma. J Natl Cancer Inst 1993; 85: 493–498. [DOI] [PubMed] [Google Scholar]
- 8.Passlick B, Izbicki JR, Kubuschok B, et al. Detection of disseminated lung cancer cells in lymph nodes: impact on staging and prognosis. Ann Thorac Surg 1996; 61: 177–183. [DOI] [PubMed] [Google Scholar]
- 9.Izbicki JR, Passlick B, Hosch SB, et al. Mode of spread in the early phase of lymphatic metastasis in non-small-cell lung cancer: significance of nodial micrometastasis. J Thorac Cardiovasc Surg 1996; 112: 623–630. [DOI] [PubMed] [Google Scholar]
- 10.Maruyama R, Sugio K, Misudomi T, et al. Relationship between early recurrence and micrometastases in the lymph nodes of patients with stage I non-small-cell lung. J Thorac Cardiovasc Surg 1997; 114: 535–543. [DOI] [PubMed] [Google Scholar]
- 11.Ohgami A, Misudomi T, Sugio K, et al. Micrometastatic tumor cells in the bone marrow of patients with non-small cell lung cancer. Ann Thorac Surg 1997; 64: 363–367. [DOI] [PubMed] [Google Scholar]
- 12.Kruger W, Krzizanowski C, Holweg M, et al. Reverse transcriptase/polymerase chain reaction detection of cytokeratin-19 mRNA in bone marrow and blood of breast cancer patients. J Cancer Res Clin Oncol 1996; 122: 679–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest 1997; 111: 1710–1717. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–481. [Google Scholar]
- 15.Peto R, Pike MC, Armitage P, et al. Design and analysis of randomised clinical trials requiring prolonged observation of each patient. Br J Cancer 1977; 35: 1–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cox DR. Regression models and life tables. J R Stat Soc 1972; 34: 187–220. [Google Scholar]
- 17.Sugio K, Ishida T, Yokoyama H, et al. ras gene mutations as a prognostic marker in adenocarcinoma of the human lung without lymph node metastasis. Cancer Res 1992; 52: 2903–2906. [PubMed] [Google Scholar]
- 18.Kern J, Slebos R, Top B, et al. c-erb B-2 expression and codon 12 K-ras mutations both predict shortened survival for patients with pulmonary adenocarcinomas. J Clin Invest 1994; 93: 516–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishio M, Koshikawa T, Kuroishi T, et al. Prognostic significance of abnormal p53 accumulation in primary, resected non-small-cell lung cancers. J Clin Oncol 1996; 14: 497–502. [DOI] [PubMed] [Google Scholar]
- 20.Mitsudomi T, Oyama T, Kusano T, et al. Mutations of the p53 gene as a predictor of poor prognosis in patients with non-small-cell lung cancer. J Natl Cancer Inst 1993; 85: 2018–2023. [DOI] [PubMed] [Google Scholar]
- 21.Isida T, Kaneko S, Akazawa K, et al. Proliferating cell nuclear antigen expression and argyrophilic nucleolar organizer regions as factors influencing prognosis of surgically treated lung cancer patients. Cancer Res 1993; 53: 5000–5003. [PubMed] [Google Scholar]
- 22.Nesbitt JC, Putnam JB, Walsh GL, et al. Survival in early-stage non-small cell lung cancer. Ann Thorac Surg 1995; 60: 466–472. [DOI] [PubMed] [Google Scholar]
- 23.Greenblatt MS, Bennett WP, Hollstein M, Harris CC. Mutations in the p53 tumor suppressor gene: clues to cancer etiology and molecular pathogensis. Cancer Res 1994; 54: 4855–4878. [PubMed] [Google Scholar]
- 24.Soussi T, Legros Y, Lubin R, et al. Multifactorial analysis of p53 alteration in human cancer: a review. Int J Cancer. 1994; 57: 1–9. [DOI] [PubMed] [Google Scholar]
- 25.Brambilla E, Guzzeri S, Brambilla C, et al. Immunohistochemical study of p53 in human lung carcinomas. Am J Pathol 1993; 143: 199–210. [PMC free article] [PubMed] [Google Scholar]
- 26.Moll R, Franke WW, Schiller DL, et al. The catalog of human cytokeratins: patterns of expression in normal epithelia. Cell 1982; 31: 11–24. [DOI] [PubMed] [Google Scholar]
- 27.Traweek ST, Liu J, Battifora H. Keratin gene expression in nonepithelial tissues: detection with polymerase chain reaction. Am J Pathol 1993; 142: 1111–1118. [PMC free article] [PubMed] [Google Scholar]
- 28.Roth JA, Fossella F, Komaki R, et al. A randomized trial comparing perioperative chemotherapy and surgery with surgery alone in resectable stage IIIA non-small cell lung cancer. J Natl Cancer Inst 1994; 86: 673–680. [DOI] [PubMed] [Google Scholar]
- 29.Rosell R, Gomez-Codina J, Camps C, et al. A randomized trial comparing preoperative chemotherapy plus surgery with surgery alone in patients with non-small- cell lung cancer. N Engl J Med 1994; 330: 153–158. [DOI] [PubMed] [Google Scholar]