Abstract
Objective
Resting energy expenditure (REE) is commonly measured in critical illness to determine caloric “demands” and thus nutritive needs.
Summary Background Data
The purpose of this study was to 1) determine whether REE is associated with clinical outcomes and 2) determine whether an optimal caloric delivery rate based on REE exists to offset erosion of lean mass after burn.
Methods
From 1995 to 2001, REE was measured by indirect calorimetry in 250 survivors of 10 to 99%TBSA burns. Caloric intake and REE were correlated with muscle protein catabolism, length of stay, ventilator dependence, sepsis, and mortality. From 1998 to 2000, 42 patients (>60%TBSA burns) received continuous enteral nutrition at a spectrum of caloric balance between 1.0x REE kcal/d –1.8x REE kcal/d. Serial body composition was measured by dual energy x-ray absorptiometry. Lean mass, fat mass, morbidity, and mortality were determined.
Results
REE/predicted basal metabolic rate correlated directly with burn size, sepsis, ventilator dependence, and muscle protein catabolism (P < .05). Declining REE correlated with mortality (P < .05). 2) Erosion of lean body mass was not attenuated by increased caloric balance, however, fat mass increased with caloric supply (P < .05).
Conclusion
In surviving burned patients, caloric delivery beyond 1.2 × REE results in increased fat mass without changes in lean body mass. Declining energy expenditure appears to be a harbinger of mortality in severely burned patients.
A well-described array of metabolic derangements occurs after severe trauma or burn. This response includes hyperdynamic circulation, fever due to resetting of the hypothalamic temperature set point, immune deficiency, impaired wound healing, peripheral insulin resistance, alteration of hepatic protein synthesis, skeletal muscular protein catabolism, and elevated systemic energy expenditure. 1–9 During the flow phase after injury, survivors manifest a hyperdynamic circulation and begin to overtly display other systemic manifestations of global hypermetabolism. 10 Defects of both cellular and humoral immune function increase the likelihood of infection, hepatic protein synthesis shifts from storage and housekeeping proteins to acute phase response proteins, peripheral tissue insulin resistance with hyperglycemia occurs, and catabolism of proteins stores (predominantly skeletal muscle) leads to erosion of lean body mass. Fluxes of substrate fuels are altered with glucose, fat, and amino acids being supplied to the wound from hepatic gluconeogenesis, lipolysis, and auto-catabolized muscle protein.
With the exception of circulatory physiologic parameters, these diverse systemic alterations are difficult to quantify. Associated with the hypermetabolic response is elevation in systemic energy expenditure. 11 This has been shown to correlate with the degree of muscle protein catabolism, impaired wound healing, and immune deficiency. 12-14 Resting energy expenditure as a correlate to total energy expenditure can be measured relatively easily at the bedside utilizing indirect calorimetry. Resting energy expenditure is determined from inspired and expired gases, measuring oxygen consumption and carbon dioxide production. It is noninvasive, reproducible, and immediately quantifiable (results are usually obtained within minutes).
The general correlation between elevated energy expenditure and muscle catabolism has led to the notion that energy expenditure determines energy demand, which is required to offset catabolism. These demands, then, are met through the dietary delivery of calories. We hypothesize that muscle catabolism proceeds after severe burn regardless of caloric balance (caloric administration –measured energy expenditure). The goal of this study was twofold. First, we sought to determine the clinical relevance of elevated energy expenditure after burn, specifically whether elevated energy expenditure correlated with increased morbidity or mortality in the setting of severe burn. Second, we sought to determine whether energy expenditure could be used to define caloric demand to optimize attenuation of erosion of lean body mass. Our hypotheses were that 1) systemic energy expenditure is reflective of the degree of illness increasing to a point of exhaustion, after which decreases would correlate with mortality; and that 2) no optimal energy/caloric balance exists that abolishes catabolism of lean mass when at least 1.2 times the measured resting energy expenditure is delivered.
METHODS
The analyses presented are based on 250 burned patients who underwent treatment and metabolic study at the Shriners Burns Hospital for Children, Galveston, TX from January 1995 to January 2001. These patients were part of several discrete research projects carried out during this time. Each research project was performed under a University of Texas Medical Branch Institutional Review Board, with approved protocol. Informed written consent was obtained from each patient, or each patient’s guardian, before enrollment into the particular study.
Subjects and Clinical Care
All subjects were admitted to the Shriners Burns Hospital for Children in Galveston, TX, and treated in an identical manner by the same team of burn surgeons. Standard treatment included early excision of the burn wound, systemic antibiotic therapy, and continuous enteral feeding. 15 Within 48 hours of admission each patient underwent total burn wound excision and grafting with autograft skin and allograft (with the exception of face and hand burns). Patients returned to the operating room when autograft donor sites healed and became available for reharvest (usually 6 to 10 days). Serial staged surgical procedures for excision and grafting were undertaken until the wounds were healed.
Each subject received continuous enteral nutrition starting within hours of admission via nasoduodenal tubes with Vivonex TEN (Sandoz Nutritional Corporation, Minneapolis, MN). The composition of Vivonex is 82% carbohydrate, 15% protein, 3% fat. Daily caloric intake was given at a rate calculated to deliver 1500 kcal/m2 TBSA + 1500 kcal/m2 burned. This feeding regimen was continued until the wounds were healed. Patients were allowed to take supplemental oral nutrition as tolerated.
Patients were on bedrest for 5 days after grafting and excision, and ambulated daily thereafter until the next excision and grafting procedure. All patients underwent indirect calorimetry to determine resting energy expenditure weekly. A stable isotopic infusion study was performed on the fifth day after the primary burn excision in 177 of the 250 subjects to determine the rate of muscle protein catabolism (expressed as the net balance of skeletal muscle protein synthesis minus breakdown).
Study Design
We sought to answer two research questions: 1) does elevation of energy expenditure after severe burn predict clinically pertinent patient outcomes, such as death, sepsis, length of ICU stay, ventilator dependence, degree of wound contamination, or the degree of skeletal muscle protein catabolism; and 2) what amount of enteral feeding (relative to REE) is required to minimize catabolism of lean mass after burn? To answer these questions we grouped and analyzed the data in two fashions. In our first analysis (Analytic Group A), data from 250 subjects burned between 10% TBSA and 99.5% TBSA were examined. All were children < 19 years of age, and all required hospitalization at SBH-Galveston for at least 1 week. Resting energy expenditure was measured by indirect calorimetry weekly. Using this as our main outcome measure, we correlated energy expenditure (REE) with the following clinical parameters: age, sex, weight, body mass index, estimated basal metabolic rate, burn size, sepsis, inhalation injury, degree of wound contamination, time after injury, and time from injury to primary definitive surgical treatment. Then using REE as an independent variable, we evaluated for statistical correlation with dependent clinical outcomes: mechanical ventilator dependance, muscle protein catabolism, and mortality. Finally, we looked at the change in energy expenditure between consecutive weeks for each subject during the first 6 weeks of hospital stay (e.g., change in energy expenditure between first and second week, between second and third week, etc., for each subject). Again, we correlated these values with wound contamination, sepsis, number of ventilatory days during that study period, and the likelihood of death during that study period.
Nude weight was determined on the day of each metabolic study by placing subjects in a sling scale. Burn size was determined by means of age-appropriate diagrams and verified by one of the attending surgeons. Wound area was defined as total cutaneous injury (burn area + donor area) and also determined by means of diagrams at each serial surgical procedure. Inhalation injury was defined as evidence of airway injury on bronchoscopy done before the first operation. Body mass index is a value derived from the subject’s weight (kg) divided by the height 2 (m2) and designed to correlate with obesity. This variable was included to give a general estimation of an individual’s relative abundance of fat mass. The degree of wound colonization was determined by quantitative cultures of tissue taken within two days of indirect calorimetry—tissue was procured either surgically during a serial excision and grafting procedure or by punch biopsy at the bedside. The diagnosis of “burn sepsis” was based on a typical constellation of signs and symptoms of grossly infected, toxic-appearing burn patients. 15,16 These include disorientation, hyper- or hypothermia, tachycardia, tachypnea, leukocytosis or leukopenia (>12,000 or < 4,000 WBC/mL), hyperglycemia (>200mg/dL), thrombocytopenia (persistent platelet count of < 100,000/mL), enteral feeding intolerance (inability to tolerate enteral feedings because of abdominal distention, high gastric residuals of > 150 cc3/h, or uncontrollable diarrhea), and hypotension refractory to administration of volume (as blood, crystalloid, or colloid). At least three of these clinical derangements were required concurrently with identification of a pathologic tissue source for the diagnosis of burn sepsis to be made.
Our second analysis (Analytic Group B) was designed to determine whether measured energy expenditure could be used to predict a caloric (or energy) balance that would offset catabolism of lean mass. In order to have a homogenous study population, victims of massive burn (greater than 60% TBSA burned) were chosen. In all of these patients, >90% BSA was cutaneous wound—either thermal injury or surgically created donor area. Between March 1998 and November 2000, there were 108 such subjects admitted to the Shriners Burns Hospital. Fifty-seven received anabolic agent therapy at some point during their acute hospital stay and thus excluded from analysis. Of the remaining 51 subjects, none received anabolic agents during acute treatment. Nine patients died. All of the 42 survivors completed serial body composition studies at approximately 1 week after primary burn excision and again at the time of full healing and discharge from the intensive care unit. Resting energy expenditure was determined weekly (always at least 5 days after an operative procedure, if the patient underwent surgery that week) by indirect calorimetry and expressed in units of Kcal/d. Caloric intake was calculated by the burn dietitian from dietary intake, intravenous infusions, and blood product transfusions. Caloric balance was calculated by dividing the total caloric intake over the entire acute hospitalization by the extrapolated caloric energy expenditure over the entire hospitalization. Caloric balance was correlated with changes in lean body mass and fat mass derived from serial DEXA (dual energy x-ray absorptiometry) body composition scanning.
Indirect Calorimetry
Indirect calorimetry was performed between midnight and 5:00 am while the subject was asleep. Resting energy expenditure was measured with a Sensor-Medics 2900 metabolic cart (Sensor-Medics, Yorba Linda, CA). The composition of inspired and expired gases was sampled and analyzed at sixty-second intervals. Values of carbon dioxide production, volume of and oxygen consumption were accepted when they were at a steady state for five minutes. The average REE was calculated from these steady state measurements.
All indirect calorimetry measurements were made at 30°C, which is a standard environmental setting for all patient rooms in our acute burn intensive care unit. For statistical comparison, energy expenditure was expressed as the percentage of the basal metabolic rate predicted by the Harris-Benedict equation. 17
Body Composition
Total body lean mass and fat mass were measured by dual energy x-ray absorptiometry. A Hologic model QDR-4500W DEXA (Hologic Inc., Waltham, MA) was used to measure body composition for the majority of the study, but was replaced when nonfunctional by a QDR-4500A Absorptiometer (Hologic Inc., Waltham, MA). To minimize systematic deviations, the Hologic system was calibrated daily against a spinal phantom in the anteroposterior, lateral, and single-beam modes. Individual pixels were calibrated against a tissue bar phantom to determine whether the pixel was reading bone, fat, lean tissue, or air.
Stable Isotope Study
The degree of protein catabolism was quantified using stable isotope tracers. Protein kinetic studies were performed beginning between 5:00 and 7:00 am, on postoperative day 5 after the first excision and grafting procedure. All stable isotope studies consisted of a 5-hour infusion of d5-Phenylalanine, as previously described. 18 Because phenylalanine is neither synthesized nor degraded in the peripheral tissues (it is metabolized only in the liver) measurement across the leg reflects the net balance of protein synthesis and breakdown. Blood samples were taken simultaneously from an ipsilateral femoral artery and vein for this determination. Indocyanine green was used to determine leg blood flow. 19
Analysis of Blood Samples
The blood concentration of unlabeled phenylalanine was determined by gas chromatography- mass spectrometry (GCMS) using the internal standard approach and the nitrogen-acetol-n-propyl esters, as previously described. 20 The isotopic enrichment of free amino acids in blood was determined on a HP model 5989 (Hewlett-Packard Co., Palo Alto, CA) by chemical ionization and selected ion monitoring at mass-to-charge ratios of 250:1, 255:1, 256:1. Indocyanine green concentrations were determined spectrophotometrically at (=805 mm on a spectronic 1001 (Bausch and Lomb, Rochester, NY)
Calculations
As phenylalanine is neither synthesized nor degraded in the periphery, the difference in concentration of this substrate in the femoral arterial and venous plasma pools reflects the net balance of leg skeletal muscle protein synthesis and breakdown. The net balance (NB) was calculated and standardized for leg volume by the following equation:
where CA and CV are the blood free amino acid concentrations of the femoral artery and vein, and BF is leg blood flow in cc/min/100 mL leg. Leg blood flow was determined from the following modification of Fick’s equation,
where CF is the femoral venous concentration of ICG and CC is the central (contralateral femoral) venous concentration of ICG. With the infusion rate set at 0.5 mg/min, the equation was solved for leg blood flow (BF). As is indicated above, BF was normalized for each patient by leg volume. Subject weight, leg circumference at prescribed points relative to anatomical landmarks, and the distances between these points were used to mathematically model leg volume.
Statistical Analysis
Univariate regression analyses were used to determine factors influencing systemic energy expenditure over the course of acute burn injury and treatment. All variables that did not significantly correlate with protein net balance by univariate linear regression were further analyzed. For each of these patient factors, a simple scatter plot was generated (independent variable of interest vs. protein net balance) to see if significant, but non-linear associations with the rate of catabolism were present. If no correlations were apparent or no differences were detected after this further analysis, the variable of interest was deemed to be unrelated to the catabolic response after burn. When appropriate, t-tests were used to compare between identified outcome groups. Statistical software (SigmaStat and SigmaPlot, SPSS, Chicago, IL) was used to perform all analyses. Significance was accepted at P < .05.
RESULTS
Analytic Group A
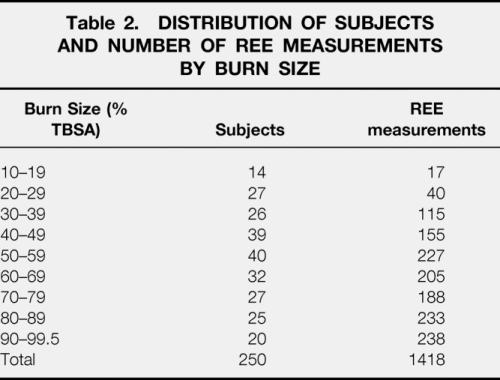
Data from the first set of 250 patients were analyzed over the course of acute surgical treatment and wound healing to determine which clinical factors significantly influence energy expenditure. Demographics for this group are shown in Table 1. One thousand four hundred and eighteen metabolic cart measurements of REE were analyzed. Measurements were not consistently made in intubated patients receiving > 70% FiO2 due to technologic limitations of indirect calorimetry at high inspired concentrations of oxygen. All measurements were made on the fifth day after a serial excision and grafting procedure. The distribution of subjects and REE measurements by burn size are shown in Table 2.
Table 1. ANALYTIC SET A DEMOGRAPHICS
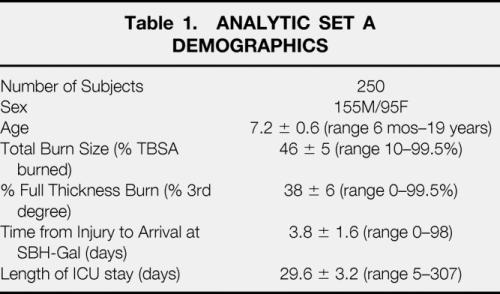
Table 2. DISTRIBUTION OF SUBJECTS AND NUMBER OF REE MEASUREMENTS BY BURN SIZE
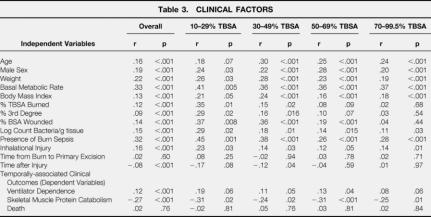
The results from univariate linear regressions correlating the degree of hypermetabolism (expressed as the ratio of measured REE/predicted Basal Metabolic Rate from the Harris-Benedict equation) with important clinical variables are shown in Table 3.
Table 3. CLINICAL FACTORS
Age, sex, and weight were all significant predictors of the degree of hypermetabolism. It is not surprising then, that predicted BMR also strongly correlated with elevated energy expenditure. Body mass index (weight/height, 2 a gross anthropomorphic measure of body fat content) linearly correlated with increasing energy expenditure after massive burn (r = 0.13).
Total burn size, percent of full thickness burn, and body surface area wound (burned area + area of excised donor site) correlated with increasing metabolic rate. This association was strongest in smaller burns. The presence of burn sepsis predicted greater energy expenditure; as did the degree of wound contamination (though to a lesser degree than clinical sepsis). Inhalational injury was also associated with greater systemic energy expenditure.
Total time from injury had a significant negative correlation with energy expenditure–this was most significant in burns less than 50% TBSA. In burns over 50% TBSA, there was no correlation between time after burn (during the acute hospitalization) and declining energy expenditure.
When evaluated as an independent variable to determine potential influence of elevated energy expenditure on clinical markers of morbidity, the relative degree of hypermetabolism correlated well with skeletal muscle catabolism (the negative correlation coefficient reflects a direct relationship with a negative net balance of protein synthesis and breakdown). We also found a significant, but weak, correlation between elevated energy expenditure and ventilator dependence (number of ventilator days during the weekly metabolic study period). No association was found between the degree of hypermetabolism and death.
Serial metabolic cart measurements were then evaluated by consecutive two-week intervals through the first 6 weeks to determine the significance of changes in measured energy expenditure (Table 4). A strong, direct association between increasing energy expenditure and heavy wound contamination was found. Increasing energy expenditure was also associated with sepsis, but not until after the first two weeks postburn. After the first month of injury, elevation of energy expenditure correlated with greater likelihood of mechanical ventilation. Finally, throughout the first 6 weeks of treatment, declining energy expenditure was significantly associated with mortality; this correlation was particularly strong initially after burn and after the first month of injury.
Table 4. ASSOCIATION OF CHANGE IN ENERGY EXPENDITURE WITH CLINICAL FACTORS
Analytic Group B
One hundred eight subjects were admitted over this time period with > 60% TBSA burns. Fifty-seven received anabolic agents during some part of their acute hospitalization. Of the fifty-one subjects who received no anabolic or anticatabolic therapy, forty-two survivors remained. Demographics for the analytic group are shown in Table 5. All underwent DEXA body composition study approximately one week after the first surgery and at the time of full healing and discharge. Caloric balance over the course of hospitalization was calculated in these 42 subjects and correlated with changes in lean and fat mass (Fig. 1). Caloric balance was also compared to the changes in total body weight from the first week after admission to that at discharge (Fig. 2). Catabolism of lean mass was unchanged over the entire spectrum of nutritional administration. However, with increased feeding relative to energy expenditure, accretion of fat mass linearly increased. The slope of change in fat accretion was relatively similar to that for increases in total body weight. Net fat synthesis balanced lean mass erosion to result in no net change in weight when caloric intake was approximately 1.5x REE.
Table 5. ANALYTIC SET B DEMOGRAPHICS
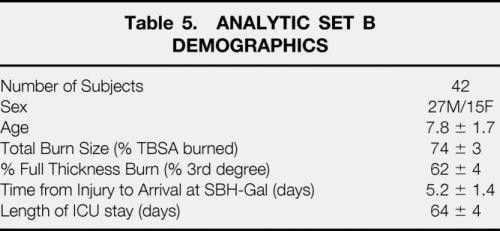
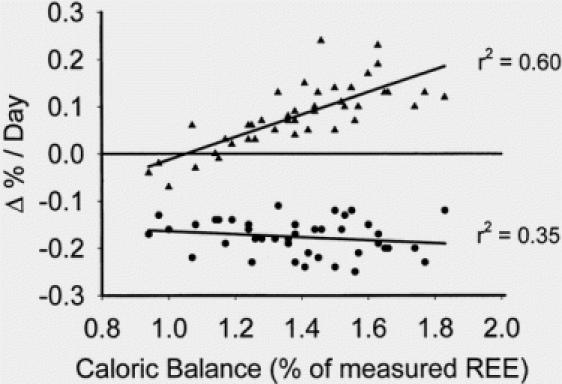
Fig. 1. Linear correlation between fat mass and lean body mass with caloric delivery indexed to measured resting energy expenditure. Increasing caloric delivery relative to resting energy expenditure increased fat accretion without effects on lean body mass.
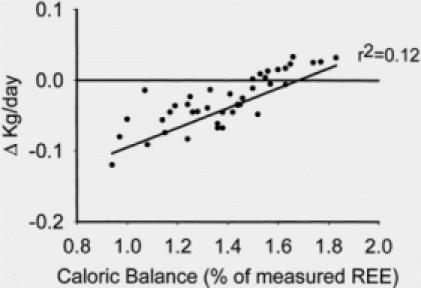
Fig. 2. Linear correlation between total body weight and caloric delivery indexed to measured resting energy expenditure. Increasing caloric delivery increased total body weight to the extent that no change in total body weight was seen at approximately 1.5 × REE. From the data on lean body mass, we conclude that the total body weight at higher caloric intakes was maintained by the addition of fat mass in the face of lean body mass loss.
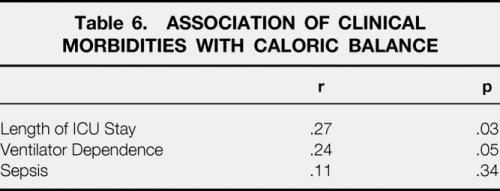
Associations between other clinical morbidities and caloric balance are shown in Table 6. Increased feeding relative to energy expenditure was associated with significantly lengthened total ICU stay and total percentage of time spent on a ventilator. No correlation between incidence of sepsis and caloric administration was found.
Table 6. ASSOCIATION OF CLINICAL MORBIDITIES WITH CALORIC BALANCE
Measured serial energy expenditures for both survivors and nonsurvivors are shown in Figure 2. Of the nine nonsurvivors, four required maximal ventilatory support (with FiO2>70%) during their entire hospital course and were not successfully studied by indirect calorimetry. Weekly REE measurements for the remaining five nonsurvivors (all of which succumbed to sepsis) are depicted by the solid lines while REE measurements from the survivors are shown as scatter points. Interestingly, all five nonsurvivors experienced large declines in REE before death. In four of these five, the final metabolic cart measurement was made within 24 hours of death.
DISCUSSION
Numerous nutritional formulas have been advocated for feeding of the severely burned by various groups within the burn community. 21-28 This is a testament not only to the difficulty of feeding severely burned patients, but also to the lack of clear superiority of any proposed regimen. Catabolism of body protein stores is synonymous with acute injury. 8 This highly conserved teleologic process is thought to supply substrate to the liver (allowing production of protective acute phase response proteins) and to the wound for cutaneous protein synthesis. Like other inflammatory cascades, when this process persists too long or becomes too severe, detrimental effects may perhaps supplant its initial protective purposes. After severe burn (> 40% TBSA), muscle protein catabolism persists at least 9 months after injury. 29 This continuous erosion of lean body mass retards rehabilitation and frequently delays re-entry into society. 30
Early, aggressive enteral nutritional support has been shown to attenuate auto-catabolism after thermal injury. 31 Caloric delivery rates from 0.8x REE to 1.8 to 2.0x REE have been advocated in the literature. At our pediatric burn hospital, Hildreth and Carvajal, 27 originally proposed caloric administration at a rate which supplied most victims of > 40% TBSA burn with approximately 1.6x REE (Kcal/d). Over several hundred patients, this rate was documented to maintain gross total body weight throughout hospital course in an era of serial burn debridement. With the popularization of early excisional therapy, the SBH-Galveston pediatric feeding formula was modified to the current 1500 kcal/m2 BSA and 1500 kcal/m2 TBSA burned. 32 Today, this delivers approximately 1.35 to 1.45x REE to most severe burn victims. It must be emphasized that these formulas are caloric delivery goals that are often affected by the clinical course of injury. On occasion, patients will not tolerate this rate of feeding, which has been associated with the onset of sepsis. 33 Thus, these goals are not always attained, accounting in part for some of the variability of caloric delivery compared to resting energy expenditure. Nevertheless, by burn community standards, this is a high caloric rate. In general however, it has been very well tolerated. Despite these successes, we have seen sporadic cases of hepatic dysfunction and even respiratory compromise due to massive fatty infiltration of the liver. The analyses reported here were initiated as an internal review of our own practice to determine if increased feeding (relative to energy expenditure) was associated with protein sparing or catabolism and general clinical health or morbidity.
The principal finding of this report, then, is that over a broad spectrum of nutritional energy delivery relative to systemic energy expenditure, no differences in catabolism of body protein stores (lean body mass) were found. As expected, most of the subjects involved in the Group B analysis received between 1.2 and 1.6 × REE kcal/d (mean 1.39 ± 0.03 × REE) over the course of hospitalization in accordance with our published feeding formulas. We feel that the variability in delivery was due either to enteral feeding intolerance during sepsis as previously mentioned that caused a decrease in caloric delivery, and also due to inherent variability in resting energy expenditure among individual burned patients. Patients on tube feedings at the desired goal rate were also allowed to consume oral diets as tolerated as well, which increased the caloric delivery. The regression line derived from the changes in percent of lean body mass from these 42 subjects was essentially flat, indicating no alteration of lean mass catabolism over the entire spectrum of caloric balance. We did, however, find a strong linear association between increased feeding and deposition of fat mass (r2= 0.60).
The feeding formulas we used were devised to maintain total body weight in the severely burned. In this study, we found that total body weight was generally maintained in the second analytic group, but based on the lean body mass measurements and the above demonstrated linear association of fat mass accretion with higher amounts of feeding, we can only conclude that weight was maintained by the addition of fat mass in the face of continued lean muscle mass loss.
Diminished protein delivery is also likely to contribute to reduction of lean mass loss in the severely burned. We have previously shown that increasing protein intake above 1.5 g/kg/d does not increase muscle protein synthesis and thus lean body mass in burned children, and that additional delivery only results in higher urinary urea excretion. 34 In the current analysis, all children received 3.7 ± 0.7 g/kg/d of protein (range from 2.8 g/kg/d to 4.8 g/kg/d). The amount of protein was so high because the protein content in our feeding formula is constant (15% of calories). We adjusted the amount of feeding to be given to meet estimated caloric needs based on total body surface area and total body surface area burned. Protein delivery per kilogram then increases in conjunction with caloric delivery. Nonetheless, the amount of protein given to the children in this study was sufficient to meet protein needs in terms of muscle metabolism (>1.5 g/kg/d) in all cases. We also point out that the same conclusions made about increased caloric delivery for increased resting energy expenditure can also be made for protein delivery; i.e., increased protein delivery over and above estimates based on resting energy expenditure does not engender increases in lean body mass.
While this certainly suggests no benefit to increased nutritional supplementation, it does not directly suggest increased morbidity as a result. To determine whether increased feeding was harmful, other markers of morbidity were identified and tracked in these patients. We found direct associations between high caloric balance and both increased ventilatory dependence and overall ICU stay (Table 6). From our database, we could not evaluate mortality in a similar fashion since postmortem DEXA body composition scans were not performed in non-survivors. During systematic review of metabolic cart measurements made on non-survivors, however, it was noted that in all instances when indirect calorimetry was successful before death, the measured REE seemed to be relatively low. Our sample size (n = 5 nonsurvivors successfully studied by metabolic cart before demise) did not permit meaningful statistical evaluation of this trend; when depicted graphically (Fig. 2), however, this trend is visually striking. Caloric balance data were reviewed in these five patients and it was noted that they did appear to have a high caloric balance relative to survivors (1.58 ± 0.04x REE vs. 1.39 ± 0.03x REE;P = .11). Interestingly, none of these patients exceeded the caloric administration specified by our standard institutional feeding formula. The high caloric balance was primarily due to relatively hypometabolic REE’s measured in all five subjects. This germinated further examination of energy expenditures in a larger, more diverse burn patient population.
When REE was directly evaluated, we found that larger body habitus (both general size and also relative abundance of fat mass) and larger burn size predicted greater hypermetabolism. Interestingly, burn size was incrementally more weakly correlated with energy expenditure as burn size increased. We were not able to determine whether this represents a ceiling of human physiologic reserve or greater injury homogeneity among larger burns (for example, among all burn injuries > 70% TBSA, we found > 90% of the body surface area was either injury or donor site; this narrow spectrum may be statistically responsible for inability to discern a linear association in regression analysis).
Sepsis and the degree of wound contamination were universal predictors of elevated energy expenditure. In burns of < 50% TBSA, the presence of sepsis is the single most strongly correlated clinical factor with elevated energy expenditure. Sepsis is difficult to define in burn patients, but this finding is consistent with previous reports in the literature. 15,35
The addition of an inhalation injury to severe burn exacerbates hypermetabolism over the entire spectrum of burn. This is consistent with previously published laboratory reports by groups at our institution and elsewhere. 36,37
Time after burn weakly influenced energy expenditure. This negative correlation (implying declining energy expenditure was associated with greater time from injury) was strongest in small burns, but dissipated as burn size increased. Data from both the Fort Sam Houston and our burn unit have suggested that hypermetabolism persists throughout the entire acute treatment and wound healing period—in fact, hypermetabolism and catabolism seem to persist at least 6 months after full healing in severely burned patients (>40% TBSA). 29,38
Time from injury to definitive surgical treatment did not seem to affect hypermetabolism throughout the remainder of the acute hospitalization. We have previously reported that delayed excision of burn wounds exacerbates muscle protein catabolism. 13 While energy expenditure and catabolism are usually associated processes, circumstances certainly exist where they are not linked. We receive a large percentage of our patients from South and Central America after an extended period from injury. As a rule, these children have not received excisional surgery or aggressive nutritional support. While they do seem to be very catabolic, we have noted that they have depressed energy expenditure. We believe this is reflective of nutritional depletion and perhaps even frank starvation. Regardless, inclusion of these hypometabolic patients along with typical, hypermetabolic responders in our regression analysis precluded discovery of a significant linear association.
When we used REE as an independent variable to assess its influence on clinical morbidity, we found associations between energy expenditure and both ventilator dependence and muscle protein catabolism (the statistical regression between REE and muscle protein net balance was performed in 177 of the 250 subjects—all that underwent concurrent indirect calorimetry and stable isotope infusion studies). We expected to find a significant negative correlation between energy expenditure and death, however, this was not evident. This is likely due to the heterogeneity of this sample group (i.e., “relative” hypometabolism in a 90% TBSA burn victim may have had an identical absolute value to the expected response of a 20% TBSA burn victim or a patient with delayed definitive surgical treatment and nutritional support).
To specifically evaluate for potential associations between changes in energy expenditure and morbidity or mortality, sequential evaluation of consecutive weekly REE measurements was made over the first 6 weeks of treatment. We found a direct linear relationship between increasing degree of wound contamination and increasing energy expenditure. This association was strongest over the first 2 to 3 weeks after burn and less tightly correlated thereafter (Fig. 3). After 2 to 3 weeks postburn, both sepsis and ventilator dependance correlated with rising energy expenditure. We speculate that the delay in this association becoming evident is that the general rule for all burn patients is to increase energy expenditure until a peak is reached at 2 to 3 weeks (regardless of presence of sepsis or pulmonary dysfunction). 39 When the influence of change in REE on mortality was assessed, a significant negative correlation was present. This was strongest early after injury (during the typical time frame of the characteristic rise and peak in energy expenditure) and then several weeks after injury–presumably when only severely burned patients (whose REE should not decline) remained hospitalized and were available for study.
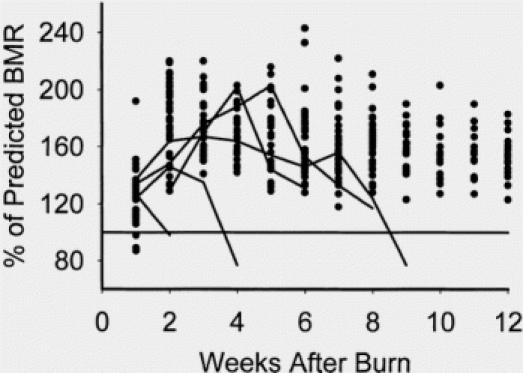
Fig. 3. Plot of resting energy expenditures expressed as percentage of predicted basal metabolic rate from the Harris-Benedict equation. Points are from survivors, and lines are from nonsurvivors. Nonsurvivors were characterized by a drop in resting energy expenditure before death.
In conclusion, we believe our data provide good evidence that increased feeding relative to energy expenditure does not preserve lean body mass. Optimal caloric balance should be determined from a prospective randomized trial–however, from this data we would recommend that such a trial not include a feeding regimen that supplies > 1.4x REE kcal/d. Such a trial would ideally include measures of morbidity and of functional outcome. We propose again that caloric delivery alone cannot assuage the loss of lean body mass after severe injury. To maintain lean body mass after severe injury, pharmacologic treatment with an anabolic agent such as insulin or oxandrolone will likely be required. Our other hypothesis was that the primary utility of REE was as a measure of physiologic reserve. This study does not conclusively prove this. We did find a clinically significant relationship between declining energy expenditure and mortality early (first 2–3 weeks) and late (>5 weeks) after thermal injury. It is not clear, however, that this phenomenon could be identified early enough in the hospital course to direct clinical therapy or positively affect outcome. To some degree, our findings may be a function of our patient population—children as a rule, do have extensive physiologic reserve and manifest physiologic normalcy until acute decompensation. We wish to report this view of energy expenditure as a potential, but as yet unproved, measure of physiologic reserve.
Discussion
DR. BASIL A. PRUITT, JR. (San Antonio, Texas): Dr. Hart, Dr. Herndon and their colleagues have added another chapter to their extensive body of work in which they have described the metabolic alterations evoked by injury and identified the response to a wide variety of nutritional and pharmacological interventions. Today they report that increased calorie loading – i.e., to levels above 1.2 times resting energy expenditure – does not reduce loss of lean body mass but does increase fat mass. This is a disappointing but perhaps not too surprising finding, since 82% of the calories of the enteric feeding regimen were supplied as carbohydrates and protein intake was fixed at 15% of the total calorie supply. Since nitrogen balance is heavily dependent on nitrogen intake and nitrogen loss as well as non-protein calories, was it possible to increase lean body mass by increasing the protein fractional content of the diet?
The answers to several other questions will help us interpret your findings. The studied patients were markedly heterogeneous with respect to age, 6 months to 19 years, burn size, 10% to 99.5%, time of admission, from the day of injury to 98 post-injury days, and spontaneous oral intake above and beyond the enteral feedings. Also, the number of measurements of resting energy expenditure progressively rose from 1.2 per patient in those with small burns to 11.9 per patient in those with burns of over 90%. Could that patient and experimental heterogeneity account for the apparent lack of effective calories on lean body mass and would more homogeneous groups identify a beneficial effect?
Since both exercise and narcotics used for analgesia can influence muscle wasting, how are those two variables controlled or standardized? Did the increase in fat mass correlate with an elevated or rising respiratory quotient, and was the increase in fat mass associated with hepatic steatosis and evidence of hepatic dysfunction?
In the five Group B patients who died with sepsis, was that sepsis caused by gram negative organisms that produced endotoxin and could endotoxin have been responsible for the progressive pre-terminal decrease in resting energy expenditure that you observed in those patients rather than what you term metabolic exhaustion?
Lastly, do you consider these data to justify the use of hormone therapy to increase lean body mass without increasing fat mass as a central component of the nutritional support of hypermetabolic injured patients?
PRESENTER DR. DAVID W. HART (Galveston, Texas): Thank you, Dr. Pruitt, for your usually insightful comments. We have previously reported the attempts to increase nitrogen content in overall diet. When one exceeds 2 grams per kilogram per day of nitrogen, giving 3 grams per kilogram per day of nitrogen, Dr. Patterson published in the American Journal of physiology with us, there was no improvement in protein synthesis in muscle by that maneuver. The amount of the protein calories that we are delivering in this series of patients is in excess of 3 grams per kilogram per day. The only improvement by increasing protein delivery in that patient population was in wound protein synthesis. It is still possible that adding more protein to diets in relation higher than the 15% that we have set at this time may have benefits in wound or anastomotic healing, and that remains to be looked at in the future. In terms of the more homogeneous groups, the analytic Group B that we looked at, which were patients with burns over 40% of the total body surface whose mean burn size was quite homogeneous at 75% of the total body surface, their age also was quite homogeneous, and that second group did adjust for and I think validates the fact that feeding over 1.2 times REE does result in increased fat secretion and does not affect lean body mass in this patient population. We did see patients with hepatic steatosis. We did see patients with development of gross central fat, with buffalo humps and moonlight facies in overfed groups. Narcotics and pain control were standardized in this group entirely and did not affect the results of this ongoing study.
The sepsis that was present in this patient population included gram negative, gram positive, and fungi. Because of the few numbers, figuring which one of those was effective on metabolic rate is not possible from these data today. Perhaps with larger series over time we can address that insightful question. We do believe that this study justifies the use of hormonal therapy. And though the hormonal data that was done in many studies for which these patients are controls are not presented here, clearly, lean body mass cannot be supported by feeding alone, in my opinion.
DR. DOUGLAS W. WILMORE (Boston, Massachusetts): In the early 1980s, Graham Hill, a New Zealander who was working in England, examined body composition in a group of patients requiring gastrointestinal operations and receiving parenteral nutrition. He found from these sophisticated investigations that patients receiving high caloric feedings increased body fat and extracellular fluid, but that this therapy did not maintain or expand lean tissue, the structural and functional components of the body. Kudos go to Drs. Hart, Herndon and associates for using clinically relevant data to arrive at a similar conclusion and thus to hone in on the amount of calories that are really needed to support patients and hopefully to improve or support their lean body mass.
I would like to ask the following questions: What is the effect of feeding on metabolic rate? As I understand your protocol, VMR, or RR, resting metabolic rate, was really measured during the feeding. If you fast these people and then measure metabolic rate, how does that compare to the metabolic rate that was captured during the fed state? How are the different levels of feeding assigned to the different patients? You have a broad range of feeding schedules. Was there a protocol that actually assigned feedings? Or was this a clinically relevant study that took feeding schedules as they occurred in your intensive care unit?
The third question is really where the rubber hits the road, and that is: Over the range of caloric intakes from 1 to 1.6 times metabolic rate, we saw no change in protein accretion. Have you now dropped feeding schedules below 1? Does .8 get you the same sort of protein accretion as 1? Or could we everyone drop feeding schedules down to 60% of metabolic rate? Do you have that data available? Likewise, could we change composition of feeding? Could we take out carbohydrate and add some fat and get some efficiency that way?
Finally, to follow up on what has previously been said, do you see that we can reduce feedings to 80% or 75% of resting metabolic rate and add anabolic factors and therefore preserve lean body mass while reducing the potential complications of high substrate infusions?
DR. DAVID W. HART (Galveston, Texas): Thank you very much, Dr. Wilmore. Feeding does increase resting energy expenditure, as demonstrated by Dr. G. G. Bores many years ago. These studies were all done in the fed condition. Fasting would grossly affect them. However, I think this is the clinically appropriate method of measurement to conclude clinical regimens for feeding. These were clinically relevant feeding levels. All patients were begun on a goal of 1,500 kilocalories per meter squared plus 1,500 kilocalories of calorie delivery. Whether they met those goals or not was dependent on clinically relevant issues – whether they have ileus which prevented them from receiving those enteral calories, whether they could eat from trays, which would allow them to have slightly more than those clinically relevant amounts of calories. The real variable here is resting energy expenditure, not the amount of delivered calories. Some patients are relatively hypometabolic relative to others that are relatively hyper metabolic. That was what varied the most. And that was what caused the change in ratio between delivery and expenditure that allowed us to bring these conclusions. There can be biases from this kind of interpretation – i.e., those patients who were hypometabolic may not have been able to tolerate diet as well as those that were more hypermetabolic. But over the large range of patients described here, we think that that is not the case.
Can we get fat? Other studies that we have performed showed that lipolysis is massively elevated because of catecholamine production in this patient population. In fact, the amount of endogenous lipolysis calculated is far greater than can be that exported by the liver. So fatty
infiltration of the liver developed even from endogenous fat release in these highly stressed patients. To add fat to that would cause greater morbidity. So we believe that the future is to get hypocaloric feeding as you describe with anabolic support with agents such as axanderlone, testosterone, insulin, IGF-1, and also agents which block lipolysis and hypermetabolic responses such as supranalol or beta blocker.
DR. WILLIAM G. CIOFFI (Providence, Rhode Island): I would like to congratulate Dr. Herndon and his colleagues on helping us to more precisely define the care of these patients both to decrease the morbidity and hasten their rehabilitation. But before we accept this study as proof that maybe we overfeed our patients, I have a few questions.
One, this is a non-randomized trial. Dr. Wilmore hinted a little bit about the feeding schedule. But basically the way the ratio was calculated was to take total calories over the entire hospitalization and divide by their expected energy expenditure. Do you have any data, however, on how often and what percentage of the time the patients actually received their predicted requirements and how much did it vary so sometimes the patients receive only 30%, 40%, and other times over 200%. If you took the data and analyzed it by calorie nitrogen ratio instead of just gross caloric intake, would the data look different?
The conclusions of the study rest on the DEXA scans. And although DEXA scanning has been validated in other patient populations, what data do you have for us that shows it been validated and is accurate in injured patients with massive fluid shifts and the like?
Your outcome with fat deposition – and you hint that you think that this leads to increased morbidity, which you presented no data other than a few patients which developed hepatic steatosis. Do you have any data on their rehabilitation in terms of getting back to exercise, et cetera, than the children who have had some increase in fat deposition actually did worse during their rehabilitative phase?
Finally, how do you feed your patients now? In the Group B patients, you excluded 50 patients who had received anabolic agents. Can you share with us some of the DEXA data on those patients? And did it actually result in increased protein synthesis and decreased fat deposition?
DR. DAVID W. HART (Galveston, Texas): Thank you very much, Dr. Cioffi. You are right, this is a non-randomized trial and total calories were calculated over expected resting energy expenditure. We did, however, achieve the predicted caloric intakes in the vast majority of the patients plus-minus 5% overall and did maintain body weight by so doing.
However, with DEXA validated by K-counter – we used two different techniques. Understanding the criticism of possible interpretational errors due to fluid shifts, we used DEXA scans as well as total body K-counter techniques to look at total fat content. Feeding over 1.2 times REE results in an increase in fat deposition. And that accounts for the maintenance of weight that was maintained by maintaining total caloric intake and what we had predicted it will require to maintain total body weight.
This study in fact greatly validates previous studies that we have performed that the formula of 1,500 kilocalories per meter squared of total body surface plus 1,500 kilocalories of meter squared burn is what is required to maintain total body weight. But it adds additionally that if we do that in this way, we put on fat and we do not increase muscle mass.
We do believe that new studies need to be done giving less, as Dr. Wilmore alluded to, giving less than 1.0 times REE, perhaps going down to .8 times REE. The patients that we gave anabolic agents to in this series did have accretion of lean body mass.
Which anabolic agent should be chosen to do these future studies that are suggested by you and Dr. Wilmore, we have not come to complete conclusion about. Oxandrolone is the chief agent, hormone growth has certain problems with it, but is the one that is best validated.
Certainly we need to continue to do this. We will use dual image photometry as well as K-counters to answer your objections, and, further, have purchased a protein monitoring machine for a third validation technique which we will use in future studies.
Footnotes
Presented at the American Surgical Association, April 2001, Colorado Springs, Colorado
Supported by National Institutes of Health Grants RO1-GM56687, P50-GM60338, T32-GM08256, and Shriners Hospitals for Children Grants 8490, 8520, 8660.
Correspondence: Steven E. Wolf, MD, Shriners Hospitals for Children, 815 Market Street, Galveston, Texas 77550-1220.
E-mail: swolf@utmb.edu
Accepted for publication April 2001.
References
- 1.Asch MJ, Feldman RJ, Walker HL, et al. Systemic and pulmonary hemodynamic changes accompanying thermal injury. Ann Surg 1973; 178 (2): 218–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilmore DW, Mason AD, Johnson DW, et al. Effect of ambient temperature on heat production and heat loss in burn patients. J Appl Physiol 1975; 38: 593–597. [DOI] [PubMed] [Google Scholar]
- 3.Alexander JW. Nutrition and infection—A new perspective on and old problem. Arch Surg 1986; 121: 966–972. [DOI] [PubMed] [Google Scholar]
- 4.Yurt RW. McManus AT. Mason AD Jr. et al. Increased susceptibility to infection related to extent of burn injury. Archives of Surgery 1984 Feb; 119(2):183–8. [DOI] [PubMed]
- 5.Miller CL, Baker CC. Changes in lymphocyte activity after thermal injury. J Clin Invest 1979; 63: 202–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolfe RR, Durkot MJ, Allsop JR, et al. Glucose metabolism in severely burned patients. Metabolism 1979; 28: 1031–1039. [DOI] [PubMed] [Google Scholar]
- 7.Xia Z-F, Coolbaugh MI, Feng HE, et al. The effects of burn injury on the acute phase response. J Trauma 1992; 32 (2): 245–251. [DOI] [PubMed] [Google Scholar]
- 8.Moore FD. Response to starvation and stress. In: Moore FD, ed. Metabolic Care of the Surgical Patient. Philadelphia, Pa: WB Saunders; 1959: 202–275.
- 9.Reiss W, Pearson E, Artz CP. The metabolic response to burns. J Clin Invest 1956; 35: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilmore D, Aulick L. Metabolic changes in burned patients. Surg Clin North Am 1978; 58: 1173–1280. [DOI] [PubMed] [Google Scholar]
- 11.Harrison TS, Seaton JF, Feller I. Relationship of increased oxygen consumption to catecholamine excretion in thermal burns. Ann Surg 1967; 165: 169–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herndon DN, Parks DH. Comparison of serial debridement and autografting and early massive excision with cadaver skin overlay in the treatment of large burns in children. J Trauma 1986; 26 (2): 149–152. [DOI] [PubMed] [Google Scholar]
- 13.Hart DW, Wolf SE, Chinkes DL, et al. Determinants of skeletal muscle catabolism after severe burn. Ann Surg 2000; 232: 455–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bessey PQ. Metabolic response to critical illness. IN: Care of the Surgical Patient 1993. ed. DW Wilmore. Scientific American, New York, NY.
- 15.Wolf SE, Rose JK, Desai MH, et al. Mortality determinants in massive pediatric burns. Ann Surg 1997; 225: 554–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neely AN, Smith WL, Warden GD. Efficacy of a rise in C-Reactive Protein serum levels as an early indicator of sepsis in burned children. J Burn Care Rehabil 1998; 19: 102–105. [DOI] [PubMed] [Google Scholar]
- 17.Harris JA, Benedict FG. Biometric studies of basal metabolism in man. Carnegie Institute of Washington, Publ. No. 279. Washington, DC 1919
- 18.Hart DW, Wolf SE, Ramzy PI, et al. Anabolic effects of oxandrolone after severe burn. Ann Surg 2001; 233: 556–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biolo G, Chinkes D, Zhang X, et al. A new model to determine in vivo the relationship between amino acid transmembrane transport and protein kinetics in muscle. JPEN 1992; 16: 305–315. [DOI] [PubMed] [Google Scholar]
- 20.Biolo G, Maggi SP, Williams BD, et al. Increased rates of muscle protein turnover and amino acid transport following resistance exercise in humans. Am J Physiol (Endocrin Metab) 1995; 268 (31): E514–520. [DOI] [PubMed] [Google Scholar]
- 21.Wolfe RR. Caloric requirements of the burned patient. J Trauma 1981; 712–714. [Google Scholar]
- 22.Bartlett RH, Allyn PA, Medley T, et al. Nutritional therapy based on positive caloric balance in burn patients. Arch Surg 1977; 112: 974–980. [DOI] [PubMed] [Google Scholar]
- 23.Curreri PW, Richmond D, Marvin J, et al. Dietary requirements of patients with major burns. J Am Diet Assn 1974; 415–417. [PubMed] [Google Scholar]
- 24.Long CL, Schaffel N, Geiger JW, et al. Metabolic response to injury and illness: estimation of energy and protein needs from indirect calorimetry and nitrogen balance. JPEN 1979; 3: 452–455. [DOI] [PubMed] [Google Scholar]
- 25.Saffle JR, Medina E, Raymond J., et al. Use of indirect calorimetry in the nutritional management of burned patients. J Trauma 1985; 25: 32–39. [DOI] [PubMed] [Google Scholar]
- 26.Wilmore DW. Nutrition and metabolism following thermal injury. Clin Plast Surg 1974; 1: 603–615. [PubMed] [Google Scholar]
- 27.Hildreth M, Carvajal HF. A simple formula to estimate daily caloric requirements in burned children. J Burn Care Rehab 1982; 3: 78–80. [Google Scholar]
- 28.Allard JP, Jeejheebhoy KN, et al. Factors influencing energy expenditure in patients with burns. J Trauma 1988; 28 (2): 199–202. [DOI] [PubMed] [Google Scholar]
- 29.Hart DW, Wolf SE, Mlcak RP, et al. Persistence of muscle catabolism after severe burn. Surgery 2000; 128: 312–319 [DOI] [PubMed] [Google Scholar]
- 30.Wolfe RR. Metabolic responses to burn injury: nutritional implications. In: Herndon DN, ed. Total Burn Care. Philadelphia: WB Saunders; 1996: 217–222.
- 31.Mochizuki H, Trocki O, Dominioini L, et al. Mechanism of prevention of postburn hypermetabolism and catabolism by early enteral feeding. Ann Surg 1984; 200 (3): 297–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hildreth MA, Herndon DN, Desai MH, et al. Reassessing caloric requirements in pediatric burn patients. J Burn Care Rehab 1988; 9: 616. [DOI] [PubMed] [Google Scholar]
- 33.Wolf SE, Jeschke MG, Rose JK, et al. Enteral feeding intolerance: An indicator of sepsis associated mortality in burned children. Arch Surg 1997; 132: 1310–1314. [DOI] [PubMed] [Google Scholar]
- 34.Patterson BW, Nguyen T, Pierre E, et al. Urea and protein metabolism in burned children: effect of dietary protein intake. Metabolism 1997 May; 46(5):573–578. [DOI] [PubMed]
- 35.Jacobs D, Kobayashi T, Imagire J, et al. Sepsis alters skeletal muscle energetics and membrane function. Surgery 1991; 110: 318–326. [PubMed] [Google Scholar]
- 36.Demling R, Lalonde C, Youn YK, et al. Effect of graded increases in smoke inhalation injury on the early systemic response to a body burn. Crit Care Med 1995; 23 (1): 171–178. [DOI] [PubMed] [Google Scholar]
- 37.Theissen JL, Prien T, Maguire J, et al. Respiratory and hemodynamic sequelae of unilateral inhalation injury of the lung. Anaesthesist 1989; 38: 531–535. [PubMed] [Google Scholar]
- 38.Milner EA, Cioffi, WG, Mason AD, et al. A longitudinal study of resting energy expenditure in thermally injured patients. J Trauma 1994; 37: 167–170. [DOI] [PubMed] [Google Scholar]
- 39.Noordenbos J, Hansbrough JF, Gutmacher H, et al. Enteral nutritional support and wound excision and closure do not prevent postburn hypermetabolism as measured by continuous metabolic monitoring. J Trauma 2000; 49: 667–671. [DOI] [PubMed] [Google Scholar]