Abstract
Objective
To evaluate the efficacy of acute normovolemic hemodilution (ANH) and intraoperative cell salvage (ICS) in blood-conservation strategies for infrarenal aortic surgery.
Summary Background Data
Recent concerns over the risks of transfusion-related infection have resulted in sharp rises in the cost of blood preparations. Autologous transfusion may be a safe alternative to allogeneic transfusion, which has been associated with immune modulation and postoperative infection.
Methods
This multicenter prospective randomized trial compared standard transfusion practice with autologous transfusion combining ANH with ICS in 145 patients undergoing elective aortic surgery. The primary outcome measures were the proportion of patients requiring allogeneic blood and the volume of allogeneic transfusion. The secondary outcome measures were the frequency of complications, including postoperative infection, and postoperative hospital stay.
Results
The combination of ANH and ICS reduced the volume of allogeneic blood transfused from a median of two units to zero units. The proportion of patients transfused was 56% in allogeneic and 43% in autologous. There were no significant differences in complications or length of hospital stay.
Conclusions
Both ANH and ICS were safe and reduced the allogeneic blood requirement in patients undergoing elective infrarenal aortic surgery.
The United Kingdom is well served by the National Transfusion Service, with low risks of transfusion-related incidents. 1 Despite this, continuing concerns about transmission of human immunodeficiency virus, hepatitis C, and spongiform encephalopathies have undermined public confidence and forced transfusion services to adopt costly changes to blood products in the past 2 years. British donor plasma is no longer used for fractionation, and nucleic acid testing for hepatitis C is now required for all plasma components, with leukodepletion for all cellular products. The cost of allogeneic blood has risen drastically, 2 making autologous transfusion even more attractive. Allogeneic blood transfusion has also been associated with immune consequences such as tumor recurrence and postoperative infection. 3–5 Further, allogeneic blood transfusion may aggravate surgical bleeding by preventing the normal hypercoagulative response associated with hemorrhage and promoting “general ooze.”6 A transfusion strategy based on autologous blood may therefore improve outcomes and in Britain has been encouraged by the Department of Health in “Better Blood Transfusion,” a Health Service Circular: “By March 2000 all NHS trusts should have explored the feasibility of autologous transfusion and ensured that patients are aware of this option.”7
Transfusion services are already under strain from increasing surgical workloads, while the number of potential donors is diminishing because of the greater proportion of elderly in the population. Autologous options include preoperative autologous donation, cell salvage, and acute normovolemic hemodilution (ANH). Predonation has proved difficult in the United Kingdom because elective operations are frequently cancelled as a result of lack of beds or emergencies. Intraoperative cell salvage (ICS) has been widely used in cardiac surgery for many years and is used frequently in the United States. ICS is safe, with few serious complications, 8 but its routine use in aortic surgery remains controversial. 9–13 ANH is simple and inexpensive, 14,15 but its value in clinical practice, and in aortic surgery in particular, remains unproven by clinical trials. The combination of ANH and ICS in aortic surgery has several potential advantages: ANH may reduce transfusion requirements by diluting hemoglobin losses; ANH provides a source of fresh platelets and clotting factors at wound closure, when hemostasis is required; and ICS recovers red cells, which can be reinfused during surgery, delaying the need to reinfuse ANH blood.
This trial, known as ATIS, was a prospective, randomized, multicenter clinical trial comparing autologous transfusion (using a combination of ANH and ICS) with allogeneic transfusion in aortic surgery. The effects of the two transfusion strategies on the inflammatory response have been reported elsewhere. 16 An evaluation of the costs is in progress.
METHODS
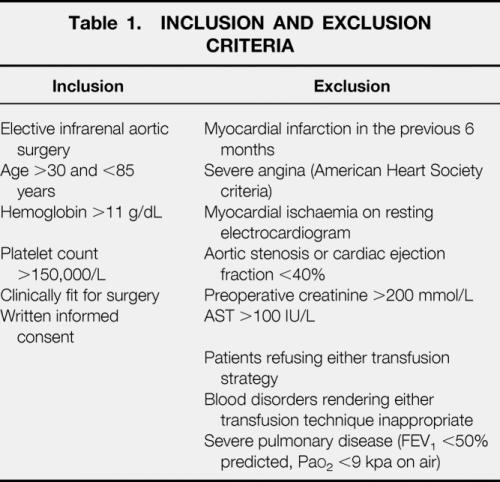
Sequential patients undergoing elective infrarenal aortic surgery for either aneurysmal or occlusive disease in eight hospitals in northwest England were recruited. Ethical approval was obtained for each participating site, and patients gave informed consent in writing. Inclusion and exclusion criteria are listed in Table 1. Exclusion criteria closely reflect the contraindications to ANH set by the British Committee for Standards in Haematology. 17 Patients were randomized to “allogeneic” or “autologous” by a central computer. Allocation was stratified using minimization for hospital, occlusive or aneurysmal aortic disease, antiplatelet or anticoagulant drugs, and estimated blood volume.
Table 1. INCLUSION AND EXCLUSION CRITERIA
Anesthetic Technique
Standard general anesthesia with intravenous induction, endotracheal intubation, and intermittent positive-pressure ventilation was used throughout. Maintenance of anesthesia was by either intravenous or volatile agents, with intraoperative fluids limited to crystalloids. Colloids were avoided because they may affect the acute-phase response. Epidural anesthesia with local anesthetic drugs was delayed to the postoperative period to avoid peripheral vasodilatation during surgery.
Transfusion Triggers
Allogeneic blood was administered when the hemoglobin concentration decreased to less than 8 g/dL or when ischemic electrocardiogram changes (2-mV ST-segment elevation or depression on 3-lead monitoring or 12-lead trace) persisted after correction of hypovolemia, unless salvaged red cells were available.
Autologous Transfusion
Blood volume was calculated before surgery based on height and weight using standard nomograms. 18 Before skin incision, sufficient blood was taken to reduce the hemoglobin concentration to 11 g/dL. The amount of blood to be removed was calculated using the following formula: ([Hb - 11]/[(Hb + 11)/2]) × blood volume.
Blood was collected into bags containing CPDA-1 anticoagulant and labeled according to published guidelines. 17 Blood volume was replaced simultaneously with crystalloids, maintaining a steady central venous pressure during blood collection. ANH blood, containing fresh platelets and clotting factors, was retained for reinfusion at wound closure when hemostasis was secure. Blood lost during the procedure was salvaged using one of three centrifugal cell salvage devices (Cell Saver 5, Haemonetics, Braintree, MA; BRAT2, Cobe Cardiovascular Inc., Arvada, CO; or CATS, Fresenius Kabi AG, Bad Homburg, Germany) with comparable efficacy in red cell recovery under standard conditions. 19,20 All autologous blood was reinfused within 6 hours of withdrawal.
Data Collection
Members of the research team attended all operations and recorded all data independently from the clinical team. These included clinical signs, complications, transfusion requirements, and laboratory assay results (blood count and immunology) at wound closure, 2 hours, 1, 2, and 7 days after surgery. Purpose-designed forms were used for all data, which were then entered on a dedicated database. A final visit on patient discharge allowed a systematic review of hospital records and laboratory results to verify all data.
Blinding
Because it was impossible to blind clinicians and researchers to either ANH or ICS, this was a single-blind trial. However, the decision to give allogeneic transfusion was made by a rigid protocol and was made by a physician independent from the research team.
Sample Size
The statistical power calculations were based on an audit of transfusion practice in South Manchester University Hospital. A total of 180 elective cases were reviewed: 78 patients underwent surgery without autologous blood techniques and 92 were subjected to a combination of ANH and ICS. Autologous transfusion techniques reduced exposure to allogeneic blood from 80.8% to 33.7%. Because these data reflected our practice and were not subject to an objective transfusion protocol, calculations were based on a more conservative estimate that 60% of “allogeneic” patients would require allogeneic transfusion and this proportion could be reduced to 35% using autologous transfusion. On these assumptions, 70 patients per group were required to show a statistically significant difference in exposure to allogeneic blood (β-1 = 0.8; α = 0.05; two-tailed, continuity-corrected chi-square test).
Statistical Analysis
Data were analyzed on an intention-to-treat basis. Normally distributed variables were compared using the unpaired two-tailed t test and skewed distributions with the Mann-Whitney test. Proportions were analyzed with the chi-square test, with the Yates corrections for 2×2 tables. Multiple linear regression identified factors influencing the volume of allogeneic blood transfused (in units). Independent variables in this analysis were preoperative hemoglobin and platelet count, antiplatelet medication intake, hospital, aortic disease, randomization group, intraoperative blood loss, and operating time. Because more cardiac events occurred in “autologous” patients, logistic regression was carried out to identify independent predictors of this complication. Covariates in the analysis were age, aortic disease, diabetes, hypertension, cardiac or respiratory disease, antiplatelet medications, randomization group, operating time, blood loss, and volume of allogeneic blood transfused during surgery. Multiple logistic regression was also used to estimate the odds of requiring allogeneic blood in different subgroups. Predictors of larger transfusion requirements, identified by linear regression (preoperative hemoglobin, transfusion strategy, blood volume, and blood loss) were the covariates in this analysis.
RESULTS
Recruitment and Randomization
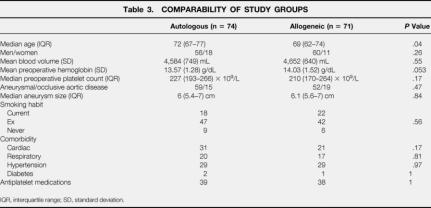
During a 30-month period (July 1997 to December 1999), 197 patients were screened for inclusion. Fifty-two were excluded for various reasons, mostly before randomization and always before surgery (Table 2). Seventy-four patients were randomized to autologous and 71 to allogeneic transfusion with similar distributions of gender, blood volume, platelet count, type of aortic disease, aneurysm size, and general health. However, despite randomization, “allogeneic” patients had slightly higher mean preoperative hemoglobin concentrations (14.03 vs. 13.57 g/dL;P = .053) and “autologous” patients were slightly older (72 vs. 69 years;P = .04) (Table 3).
Table 2. REASONS FOR EXCLUSION

Table 3. COMPARABILITY OF STUDY GROUPS
IQR, interquartile range; SD, standard deviation.
Transfusion Requirements
The mean number of 450-mL units removed from each “autologous” patient during hemodilution was 1.66 ± 0.71. The actual mean post-ANH hemoglobin concentration was 11.3 ± 0.93 g/dL. Median (interquartile range) blood loss during surgery was 1,000 (688–1,734) mL in “allogeneic” and 921 (661–1,374) mL in “autologous” patients (P = .37). The median packed red cell volume reinfused after cell salvage was 415 (225–543) mL, equivalent to more than one unit of allogeneic blood because the hematocrit of reinfused red cells was approximately 65%. Forty (56%) “allogeneic” and 32 (43%) “autologous” patients required allogeneic transfusion (P = .12). However, a median of two (zero to four) units was transfused to “allogeneic” patients versus 0 (zero to two) units in “autologous” patients (P = .008) (Table 4). Fifty “allogeneic” patients and only 19 “autologous” patients were transfused during or within 24 hours of surgery (P < .001), a total of 200 and 31 units, respectively (P < .001). Transfusion strategy did not influence hemoglobin concentration or platelet count, except during hemodilution (Fig. 1).
Table 4. TRANSFUSION REQUIREMENTS
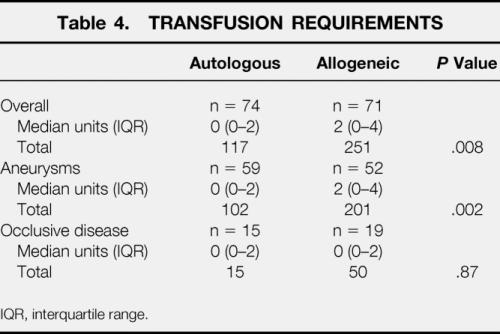
IQR, interquartile range.
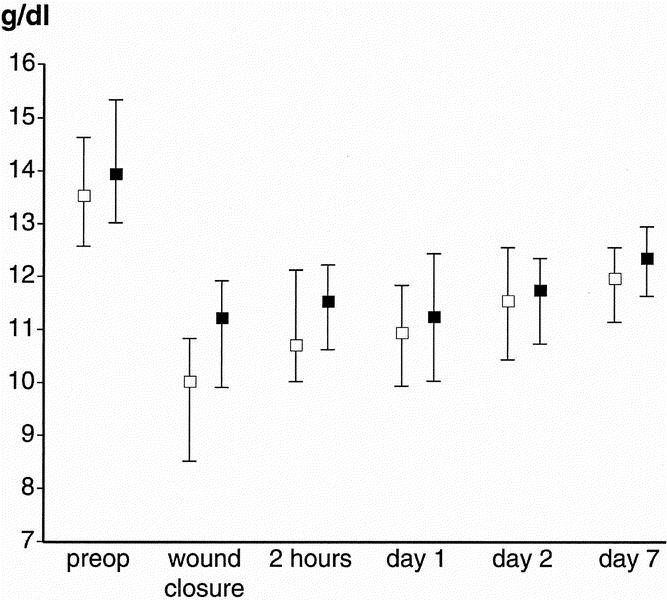
Figure 1. Median (interquartile range) hemoglobin concentration before and after surgery in patients in the “autologous” (open square) and “allogeneic” (closed square) groups.
Factors Influencing Blood Transfusion
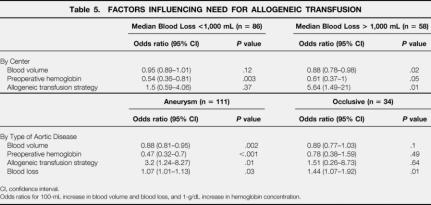
Multiple linear regression confirmed the influence of transfusion strategy on volume transfused after adjusting for confounding variables (P < .001). Other independent predictors of a larger allogeneic transfusion requirement were hospitals with median blood loss more than 1,000 mL (P = .001) and low blood volume (P = .001) and low preoperative hemoglobin (P = .02). Variables not significantly associated with transfusion were aneurysmal versus occlusive aortic disease (P = .07), antiplatelet/anticoagulant medications (P = .58), platelet count (P = .28), and operating time (P = .13).
Allogeneic transfusion strategy (P = .01) and low blood volume (P = .02) predicted the need for transfusion in hospitals where the median blood loss was more than 1,000 mL, but not elsewhere. In all hospitals, preoperative hemoglobin was a predictor of transfusion (Table 5).
Table 5. FACTORS INFLUENCING NEED FOR ALLOGENEIC TRANSFUSION
CI, confidence interval.
Odds ratios for 100-mL increase in blood volume and blood loss, and 1-g/dL increase in hemoglobin concentration.
Median blood loss was 668 (400–861) mL for occlusive disease and 1,100 (754–1,696) mL in surgery for aneurysm (P < .001). In patients with occlusive disease, transfusion was associated only with high blood loss (P = .01). Allogeneic transfusion in aneurysm repair was predicted by allogeneic transfusion strategy (P = .02), low blood volume (P = .002), low preoperative hemoglobin (P < .001), and high blood loss (P = .003).
Clinical Outcome
Thirty-three (46%) “allogeneic” and 32 (43%) “autologous” patients had complications. In particular, 19 “allogeneic” and 16 “autologous” patients had some form of postoperative infection (P = .6). One “allogeneic” patient had a minor transfusion reaction. Although the complications in the two groups were generally comparable, there were 13 cardiac events in “autologous” and 8 in “allogeneic” patients (P = .4). Logistic regression showed that the only predictor of cardiac complications was age (P < .001), with an odds ratio of 1.2 (95% confidence interval, 1.07–1.3) per 1-year increase and that autologous transfusion did not independently predict cardiac problems (odds ratio 1.05, 95% confidence interval, 0.31–3.5, P = .94). Hemorrhagic complications occurred in five “autologous” patients, two of whom required a laparotomy (one for massive bleeding from the proximal aortic anastomosis, one for upper gastrointestinal hemorrhage). In the “allogeneic” group, three patients had intraoperative hemorrhage and a further five required reoperation for intraabdominal bleeding. Hemorrhagic complications occurred in five of the eight hospitals, randomizing a total of 70 patients (19% incidence), and resulted in nine deaths (69%). Reoperation after nonhemorrhagic complications was necessary in eight “autologous” patients (five thromboembolectomies, two laparotomies for bowel obstruction, one groin wound resuturing) and two “allogeneic” patients (two thromboembolectomies). Eleven deaths occurred in “allogeneic” patients compared with 13 in “autologous” patients (P = .91), with 13 deaths (54%) from cardiac causes. Median hospital stay was 9 days (7–12) for “allogeneic” patients and 10 (8–13) days for “autologous” patients (P = .17).
DISCUSSION
The ATIS trial is the largest prospective randomized clinical trial addressing transfusion strategy in aortic surgery and the first to investigate ANH in this setting. This autologous transfusion strategy clearly reduced allogeneic blood requirements but had little influence on clinical outcome.
In three smaller randomized trials, ICS alone has been evaluated for use in aortic surgery. In two, ICS reduced transfusion requirements, 21,22 with one also reporting a reduced hospital stay and a trend toward lower postoperative infection rates. 22 However, a third trial, in which transfusion triggers were more permissive and blood losses smaller, found no benefit from cell salvage. 23 The transfusion triggers we used may seem restrictive but are in line with recent published literature. 24–26
The benefit of ANH has been reported in several small-scale studies in cardiac, 27 orthopedic, 28 gynecologic, 29 and aortic surgery. 30,31 The technique appears to be safe in the elderly and in patients with left main coronary artery stenosis. 32,33 Further, ANH is inexpensive, and it is reassuring for the anesthesiologist to have two or three units of blood available for use if necessary. The ATIS trial confirmed the safety of these techniques in combination, because cardiac events on logistic regression analysis appeared to be due to age and not to transfusion strategy. Despite randomization, “autologous” patients were significantly older, with 13 older than 80 years compared with only 6 in the “allogeneic” group.
On linear regression analysis, preoperative hemoglobin concentration, blood volume, hospitals with a median blood loss of more than 1,000 mL, and intraoperative blood loss independently predicted larger allogeneic blood requirements. Some hospitals consistently had lower blood losses, emphasizing the importance of surgical technique and the variations in practice that exist. Hemorrhagic complications were also more common in hospitals where the median intraoperative blood loss exceeded 1,000 mL.
Two “autologous” patients who required reoperation for massive bleeding needed transfusion of 20 and 25 units, respectively, almost doubling the transfusion requirements for the whole group. Because data were analyzed on an intention-to-treat basis, all patients were included in the analysis. However, excluding all patients requiring reoperation for bleeding reduced allogeneic blood requirements in both groups and improved the efficacy of an autologous strategy in reducing transfusion.
The high reoperation rate for bleeding and the death rate in some of our participating hospitals reduced the sensitivity of the study to detect a clinical benefit from autologous transfusion. Numbers for each center were too small for meaningful statistical analysis. Most deaths were due to cardiac causes, but reoperation for hemorrhage was strongly associated with later death. Although this death rate might have masked any protective effect of autologous transfusion on outcome, it could not have had a large influence on transfusion requirements.
In patients undergoing surgery for occlusive disease, ICS did not appear to be necessary. Patients with larger blood volumes and a high hemoglobin concentration were also less likely to require transfusion and may benefit from ANH alone, because more blood can be collected; this should be sufficient to avoid allogeneic transfusion. ICS should be added for patients with small blood volumes or a low hemoglobin concentration, 34 or when complicated surgery is anticipated. If in doubt, cell salvage devices may be used as suction reservoirs, delaying the decision to process saved blood until a critical amount of blood is lost. This reduces the cost of disposables. Because there was significant variability in the results from different centers, surgeons should audit their practice both to identify where surgical technique could be improved and to plan an appropriate strategy for autologous transfusion.
We could not show any impact of autologous transfusion on rates of complications, infections, or death or postoperative hospital stay in this number of patients. The unexpectedly high reoperation rates in some hospitals may have diluted the sensitivity of this study for the detection of complications associated with transfusion. Further, in the United Kingdom, social circumstances have an important influence on the decision to discharge elderly patients.
The combination of ANH and ICS was safe and significantly reduced allogeneic blood requirements in aortic surgery, but it had little impact on clinical outcome. Further studies are necessary to define the role of ANH alone in patients undergoing surgery where moderate blood loss is anticipated.
ATIS Investigators
Dr. M. McKavney (South Manchester University Hospital); Mr. S. Hardy, Dr. S. Gilligan (Blackburn Royal Infirmary); Mr. A.B. Woodyer, Dr. J. Mazumder (Tameside General Hospital); Mr. R. Hughes, Mr. G. Thomson, Dr. E.P. McKiernan, Dr. S. Keens, Dr. P. Lee, Dr. A. Bewly (Preston Royal Hospitals); Mr. M. Welch, Mr. H. Al-Khaffaf, Dr. G. Bond (Burnley General Hospital); Mr. G. Williams, Mr. W.F. Tait, Dr. S. Chadwick (North Manchester General Hospital); Mr. M. Lambert, Dr. M. Chamberlain (Blackpool Victoria Hospital); Dr. M. James (Keele University); Dr. E. Love (Manchester Blood Center); Dr. B. Faragher (University of Manchester).
Footnotes
Funded by the NHS Executive Research & Development (UK).
Correspondence: Charles N. McCollum, FRCS, Academic Surgery Unit, Education and Research Unit, Wythenshawe Hospital, Manchester M23 9LT, United Kingdom.
E-mail: cnmcc@fs1.with.man.ac.uk
Accepted for publication May 3, 2001.
References
- 1.Regan F, Hewitt P, Barbara J, et al. Prospective investigation of transfusion transmitted infection in recipients of over 20000 units of blood. Br Med J 2000; 320: 403–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Provan D. Better blood transfusion. Br Med J 1999; 318: 1435–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mezrow CK, Bergstein I, Tartter PI. Postoperative infections following autologous and homologous transfusions. Transfusion 1992; 32: 27–30. [DOI] [PubMed] [Google Scholar]
- 4.Triulzi DJ, Variek K, Ryan DH, et al. A clinical and immunological study of blood transfusion and postoperative bacterial infection in spinal surgery. Transfusion 1992; 32: 517–524. [DOI] [PubMed] [Google Scholar]
- 5.Duffy G, Neal KR. Differences in post-operative infection rates between patients receiving autologous and allogeneic blood transfusion: a meta-analysis of published randomized and nonrandomized studies. Transfusion Med 1996; 6: 325–328. [DOI] [PubMed] [Google Scholar]
- 6.Blair SD, Janvrin SB, McCollum CN, et al. Effect of early blood transfusion on gastrointestinal hemorrhage. Br J Surg 1986; 73: 783–785. [DOI] [PubMed] [Google Scholar]
- 7.Winyard G (NHS Executive). Better blood transfusion. Appendix I to NHS Executive Clinical Effectiveness circular HSC 1998/24, 11 December 1998. http://www.open.gov.uk/doh/coinh.htm
- 8.Faught C, Wells P, Fergusson D, et al. Adverse effects of methods for minimizing perioperative allogeneic transfusion: A critical review of the literature. Transfusion Med Rev 1998; 12: 206–225. [DOI] [PubMed] [Google Scholar]
- 9.Thomas GI, Jones TW, Stavney LS, et al. Experiences with autotransfusion during abdominal aortic aneurysm resection. Am J Surg 1980; 139: 628–633. [DOI] [PubMed] [Google Scholar]
- 10.Hallett JW Jr. Minimizing the use of homologous blood products during repair of abdominal aortic aneurysms. Surg Clin North Am 1989; 69: 817–826. [DOI] [PubMed] [Google Scholar]
- 11.Ouriel K, Shortell CK, Green RM, et al. Intraoperative autotransfusion in aortic surgery. J Vasc Surg 1993; 18: 16–22. [DOI] [PubMed] [Google Scholar]
- 12.O’Hara PJ, Hertzer NR, Santilli PH, et al. Intraoperative autotransfusion during abdominal aortic reconstruction. Am J Surg 1988; 145: 215–220. [DOI] [PubMed] [Google Scholar]
- 13.Kelley-Patteson C, Ammar AD, Kelley H. Should the Cell Saver Autotransfusion Device be used routinely in all infrarenal abdominal aortic by pass operations? J Vasc Surg 1993; 18: 261–265. [PubMed] [Google Scholar]
- 14.Roberts WA, Kirkley SA, Newby M. A cost comparison of allogeneic and preoperatively or intraoperatively donated autologous blood. Anesth Analg 1996; 83: 129–133. [DOI] [PubMed] [Google Scholar]
- 15.Rosenblatt MA, Cantos EM Jr, Mohandas K. Intraoperative hemodilution is more cost-effective than preoperative autologous donation for patients undergoing procedures associated with a low risk for transfusion. J Clin Anesth 1997; 9: 26–29. [DOI] [PubMed] [Google Scholar]
- 16.Haynes S, Wong J, Dalrymple K, et al. The immune response and blood transfusion in aortic surgery. Br J Surg 2000; 87: 639–640. [Google Scholar]
- 17.British Committee for Standards in Haematology Blood Transfusion Task Force. Guidelines for autologous transfusion. II. Perioperative haemodilution and cell salvage. Br J Anaesth 1997; 78: 768–771. [DOI] [PubMed] [Google Scholar]
- 18.Nadler SB, Hidalgo JU, Bloch T. Prediction of blood volume in normal human adults. Surgery 1962; 51: 224–232. [PubMed] [Google Scholar]
- 19.Bengtson JP, Bengtsson A, Rydberg L. Blood spillage during salvage. Transfus Med 1997; 7: 101–106. [DOI] [PubMed] [Google Scholar]
- 20.Shulman G. Quality of processed blood for autotransfusion. J Extracorpor Technol 2000; 32: 11–19. [PubMed] [Google Scholar]
- 21.Thompson JF, Webster JHH, Chant ADB. Prospective randomized evaluation of a new cell saving device in elective aortic reconstruction. Eur J Vasc Surg 1990; 4: 507–512. [DOI] [PubMed] [Google Scholar]
- 22.Spark JI, Chetter IC, Kester RC, et al. Allogenic versus autologous blood during abdominal aortic aneurysm surgery. Eur J Vasc Endovasc Surg 1997; 14: 482–486. [DOI] [PubMed] [Google Scholar]
- 23.Clagett GP, Valentine RJ, Jackson MR, et al. A randomized trial of intraoperative autotransfusion during aortic surgery. J Vasc Surg 1999; 29: 22–30. [DOI] [PubMed] [Google Scholar]
- 24.Carson JL, Duff A, Berlin JA, et al. Perioperative blood transfusion and postoperative mortality. JAMA 1998; 279: 199–205. [DOI] [PubMed] [Google Scholar]
- 25.Bracey AW, Radovancevic R, Riggs SA, et al. Lowering the hemoglobin threshold for transfusion in coronary artery bypass procedures: effect on patient outcome. Transfusion 1999; 39: 1070–1077. [DOI] [PubMed] [Google Scholar]
- 26.Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. Hebert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. N Engl J Med 1999; 340:409–417. [DOI] [PubMed]
- 27.Kahraman S, Altunkaya H, Celebioglu B, et al. The effect of acute normovolaemic haemodilution on homologous blood requirements and total estimated red blood cell volume lost. Acta Anaesthesiol Scand 1997; 41: 614–617. [DOI] [PubMed] [Google Scholar]
- 28.Olsfanger D, Fredman B, Goldstein B, et al. Acute normovolaemic haemodilution decreases postoperative allogeneic blood transfusion after total knee replacement. Br J Anaesth 1997; 79: 317–321. [DOI] [PubMed] [Google Scholar]
- 29.Oberhauser M, Bardenheuer HJ, Bernasconi H, et al. Isovolemic hemodilution for avoiding homologous blood transfusions: effectiveness in large gynecologic interventions. Infusions Ther Transfusions Med 1996; 23: 15–23. [PubMed] [Google Scholar]
- 30.Kramer AH, Hertzer NR, Beven EG. Intraoperative hemodilution during elective vascular reconstruction. Surg Gynecol Obstet 1979; 149: 831–836. [PubMed] [Google Scholar]
- 31.Tulloh BR, Brakespear CP, Bates SC, et al. Autologous predonation, haemodilution and intraoperative blood salvage in elective abdominal aortic aneurysm repair. Br J Surg 1993; 80: 313–315. [DOI] [PubMed] [Google Scholar]
- 32.Herregods L, Moerman A, Foubert L, et al. Limited intentional normovolemic hemodilution: ST-segment changes and use of homologous blood products in patients with left main coronary artery stenosis. J Cardiothorac Vasc Anesth 1997; 11: 18–23. [DOI] [PubMed] [Google Scholar]
- 33.Hobisch-Hagen P, Schobersberger W, Falkensammer J, et al. No release of cardiac troponin I during major orthopedic surgery after acute normovolemic hemodilution. Acta Anaesthesiol Scand 1998; 42: 799–804. [DOI] [PubMed] [Google Scholar]
- 34.Brecher ME, Rosenfeld M. Mathematical and computer modeling of acute normovolemic hemodilution. Transfusion 1994; 34: 176–179. [DOI] [PubMed] [Google Scholar]