Abstract
Objective
To assess the causes of failure of laparoscopic Heller myotomy and to verify whether endoscopic pneumatic dilation is a feasible treatment.
Summary Background Data
Laparoscopic Heller myotomy has proved an effective treatment for esophageal achalasia, with good or excellent results in 90% of patients. The treatment of failures remains controversial, however.
Methods
From 1992 to 1999, 113 patients underwent laparoscopic Heller myotomy for esophageal achalasia. Ten patients (8.7%) reported dysphagia (n = 7) or chest pain (n = 3) a median of 5 months after surgery (range 1–12) and were considered surgical failures. Pre- and postoperative radiologic, manometric, and 24-hour pH monitoring findings in patients with achalasia recurrence were compared with those of 74 asymptomatic subjects.
Results
The preoperative characteristics of the two groups were comparable. After surgery, a decrease in resting lower esophageal sphincter pressure was observed in both groups, whereas the abdominal and overall lengths were significantly shorter among the asymptomatic patients. No patients with recurrence had abnormal gastroesophageal reflux. Based on time to recurrence and manometric and fluoroscopic findings, the etiology of the recurrences was classified as incomplete myotomy upward (n = 1), incomplete myotomy or sclerosis of the myotomy downward (n = 7), or sigmoid megaesophagus (n = 1); in one patient the authors could not establish the etiology. Seven of nine patients were effectively treated with endoscopic pneumatic dilations (median 2 dilations, range 1–4); one refused to undergo further treatment. Two patients underwent redo surgery.
Conclusions
Recurrence of symptoms after myotomy is mainly related to incomplete myotomy or sclerosis of the distal site of the myotomy; it can be treated by dilations after surgery.
Laparoscopic myotomy of the distal esophagus and gastric cardia with antireflux anterior partial fundoplication has gained popularity in recent years and is now considered at most centers to be the treatment of choice for esophageal achalasia. 1,2 Although surgical division of the lower esophageal muscle fibers is very effective in treating achalasia, dysphagia or chest pain recurs in approximately 10% of patients. 3,4 The reasons for myotomy failure are difficult to determine and the appropriate management of these patients remains controversial, ranging from endoscopic dilation 5 to botulinum toxin injection, 6 redo myotomy, 7 and esophagectomy. 8 To investigate the causes of recurrent achalasia symptoms and clarify the etiology of myotomy failure, pre- and postoperative data from patients with recurrent symptoms were compared with findings in successfully treated patients. Based on our previous experience with open surgery, patients with recurrent symptoms were treated with endoscopic pneumatic dilation, and the timing, methods, and results of treatment were carefully analyzed.
METHODS
From January 1992 to December 1999, a total of 113 patients with a diagnosis of primary achalasia underwent laparoscopic Heller myotomy of the distal esophagus and cardia; in all patients an anterior partial fundoplication (Dor) was added to prevent gastroesophageal reflux. There were 64 male patients and 49 female patients, with a median age of 43 years (range 17–69). The median duration of symptoms was 24 months (range 2–240). Thirteen patients had undergone prior endoscopic treatment elsewhere: in 10 patients this involved esophageal balloon dilation with a Rigiflex dilator (Microvasive Boston Scientific Corp., Boston, MA) with a median of two dilations (range 1–6), 1 patient had been administered Botox injections, and 2 had undergone two dilations and one Botox injection.
Diagnostic Studies
The diagnosis of primary achalasia was based on clinical history, barium swallow, and esophageal manometry. Clinical data from each patient were prospectively collected by means of a questionnaire, and the patient’s symptoms were scored according to severity and frequency. The symptom score for dysphagia, chest pain, and regurgitation was calculated by adding the severity of each symptom (0, none; 2, mild; 4, moderate; 6, severe) to the frequency (0, never; 1, occasionally; 2, once a month; 3, every week; 4, twice a week; 5, daily); the highest score obtainable was 33.
The maximum esophageal diameter was measured at the site of the barium air level in the standard anteroposterior image obtained during a barium swallow, performed before surgery and repeated a month after surgery and whenever a patient reported symptom recurrence. Patients were classified according to their maximum esophageal diameter and the shape of the esophagogastric passage at preoperative barium swallow as follows: grade I, less than 4 cm, 64 patients; grade II, 4 to 6 cm, 43 patients; grade III, more than 6 cm, 3 patients; and grade IV, more than 6 cm and sigmoid-shaped esophagus, 3 patients. After surgery, if the standard barium swallow failed to reveal any clear obstacle to the passage of the barium into the stomach, videofluoroscopy using barium mixed with solid food was performed. If the patient’s main complaint was pain, an attempt was made to reproduce this pain during the videofluoroscopy by administering the kind of food that usually elicited this symptom (i.e., bread, apples, or hamburger, mixed with barium).
Stationary manometry of the esophagus was performed before and 6 months after surgery and whenever a patient had recurrent symptoms, using a pneumohydraulic perfusion system. The lower esophageal sphincter (LES) pressure was calculated by averaging the pressures recorded by four side holes positioned at the same level, 90° apart, and withdrawing the catheter twice using a motorized pull-through technique at a constant speed of 1 mm/s, from the stomach to the esophageal body, passing through the high-pressure zone 9 (the LES pressure was therefore the average of eight pressure recordings). The LES pressure was calculated as the midexpiratory pressure at the respiratory inversion point. Abdominal and overall LES lengths were calculated as the average distance from the point where the pressure trace rises steadily, by at least 2 to 3 mm Hg with respect to the intragastric baseline pressure, the respiratory inversion point (abdominal part), and the point where the pressure trace falls below the esophageal baseline pressure (overall length). 10 Esophageal body motility and LES relaxations were assessed, recording the pressure changes elicited by 10 wet swallows with the side holes of the catheter positioned inside the LES and 5, 10, 15, and 20 cm higher up, according to the technique described elsewhere. 9 The residual LES pressure was defined as the minimum pressure (nadir) recorded in the LES during swallowing. Twenty-four-hour pH monitoring was performed only after surgery to evaluate any abnormal gastroesophageal reflux, positioning a glass electrode 5 cm above the upper border of the LES, according to the standard procedure used at our laboratory and reported elsewhere. 10 Tracings from patients with abnormal reflux on computerized analysis were carefully reviewed to distinguish true episodes of gastroesophageal reflux from false reflux resulting from stasis. 11
Surgical Technique
The surgical technique has been described in detail elsewhere. 12 Briefly, only the anterior part of the esophagus was dissected and a 6- to 8-cm-long myotomy was performed, extending 1.5 to 2 cm on the gastric side. A 30-mm Rigiflex balloon was positioned inside the esophageal lumen at cardia level during the myotomy, using an endoscopically positioned guidewire; during the myotomy the balloon was gently inflated and deflated with 60 to 80 ml air with the aid of a syringe. This maneuver exposed the circular fibers that were stretched and then easily cut or torn apart; the edges of the myotomy were separated and peeled away from the submucosal plane, and minimal bleeding from submucosal vessels was easily controlled by inflating the balloon, thus reducing the use of the cautery. An anterior partial hemifundoplication according to Dor completed the procedure.
Follow-Up
Patients were asked to come to the outpatient clinic 1, 6, and 12 months after surgery. A barium swallow was performed at the first checkup and manometry, pH monitoring, and endoscopy were performed immediately before the second. Patients were asked to come once a year after the first year. If patients did not show up for 12 months or more, they were interviewed by phone.
Endoscopic Dilation Technique
In patients with recurrent symptoms, pneumatic dilation of the cardia was performed using a Rigiflex balloon dilator under direct endoscopic visual control. Patients fasted for at least 8 hours before the procedure and were sedated with intravenous midazolam and, if necessary, with propofol. All patients were given topical anesthesia to the pharynx. A dilator was passed over a guidewire positioned in the stomach, and the correct location of the dilator was checked by maintaining the scope above the balloon and controlling its position across the cardia through the transparent membrane of the dilator. In the first three patients, a 30-mm balloon was initially used and it was necessary to repeat the procedure with a larger-diameter balloon in all three; in the other seven patients, a 35- or 40-mm balloon was used from the beginning. The balloon was inflated to a median 6 psi (range 5–10) for 1 minute. Further dilations with a 35-mm-diameter Rigiflex dilator or 40-mm Rigiflex balloon were performed if the patient’s symptoms persisted or recurred.
Statistical Analysis
Data are expressed as medians and range. Nonparametric tests were used to compare groups (Mann-Whitney and Wilcoxon, as appropriate). The Fisher exact test was used to compare categorical data. A probability of less than 5% was assumed to be statistically significant (P < .05).
RESULTS
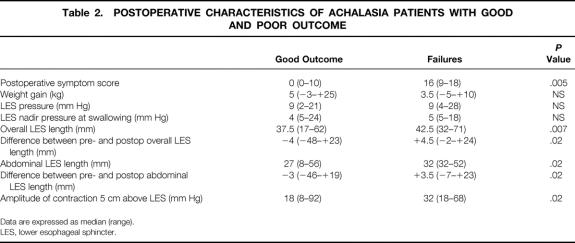
None of the 113 patients were lost to follow-up after surgery. Three patients died during the follow-up period of causes unrelated to achalasia. The median follow-up was 24 months (range 1–83). At follow-up, 103 of 113 patients were symptom-free. Figure 1 shows the probability of their remaining cured by surgery after 5 years. Ten patients (8.7%) had a postoperative symptom score higher than the 10th percentile of the preoperative score (>8), with a median score of 16 (range 9–18), and they were considered as failures of the procedure. For comparison, the median postoperative score among patients considered healed was 0 (range 0–7). The main symptoms in the patients with recurrence were dysphagia in seven patients and chest pain on swallowing in three. All but two of the patients with recurrent symptoms reported some benefit from surgery, and the median of their postoperative scores was statistically lower than before surgery (before surgery 20 [range 9–27]; after surgery 16 [range 9–18], P < .05). The timing of the recurrence varied considerably: four patients had recurrence within the first 3 months after surgery, four between 4 and 7 months afterward, one at 7 months, and one at 11 months. Figure 2 summarizes the timing of symptom recurrences.
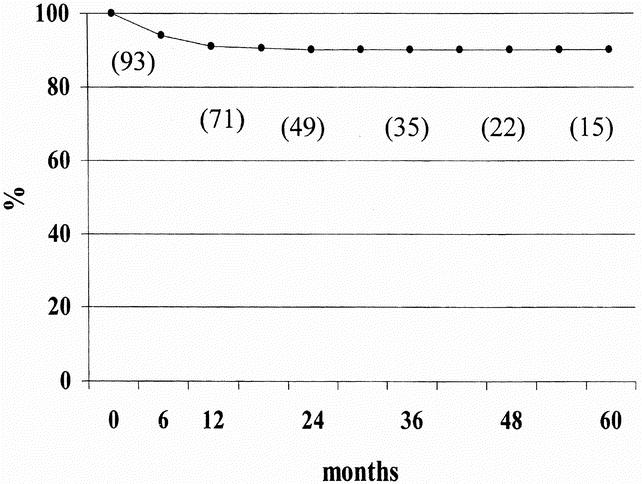
Figure 1. Actuarial curve for symptom control showing probability of patients being symptom-free at 5-year follow-up. The number of patients evaluated is given in brackets. No patients were lost to follow-up; three died of unrelated causes 18, 44, and 56 months, respectively, after the Heller myotomy.
Figure 2. Timing of recurrence and main symptom. The arrows indicate the months since surgery when patients had recurrent symptoms, the main symptoms being P (chest pain) and D (dysphagia). Four patients had early symptom recurrence (within 3 months of surgery) and six had late recurrence (4 months or more after surgery).
When the two groups (i.e., with and without recurrent symptoms) were compared, no differences were found in the preoperative characteristics (Table 1). However, the finding of a large, sigmoid-shaped esophagus was more common in the group with recurrent symptoms than among the other patients (1/10 [10%] vs. 2/105 [2%]), although the difference was not significant.
Table 1. PREOPERATIVE CHARACTERISTICS OF ACHALASIA PATIENTS WITH GOOD AND POOR OUTCOME
Data are given as median (range).
LES, lower esophageal sphincter.
A postoperative barium swallow was obtained in 80 patients (10 with and 70 without symptoms). The postoperative diameter of the esophagus was larger in patients with symptoms (25.5 mm [range 18–60] vs. 20 mm [range 10–55], P < .05); a significant reduction with respect to the preoperative diameter was observed in both groups, however (Fig. 3).
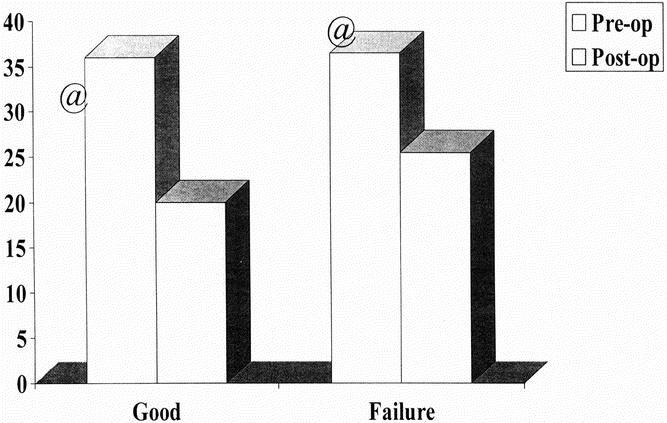
Figure 3. Pre- and postoperative esophageal diameters, as measured at barium swallow. Patients with and without recurrent symptoms had a similar preoperative esophageal diameter (white column) and a significant reduction after surgery (gray column), but patients with recurrent symptoms had a larger esophageal diameter at follow-up than those without symptoms (P < .05).
Postoperative functional studies (esophageal manometry and 24-hour pH monitoring) were performed in 74 asymptomatic patients and 10 patients with persistent or recurrent symptoms. Ten patients had abnormal distal esophagus acid exposure at 24-hour pH monitoring. Only five of them had genuine gastroesophageal reflux, however: three reported mild heartburn and one had erosive esophagitis at endoscopy. None of the patients with recurrent dysphagia or chest pain showed any abnormal gastroesophageal reflux.
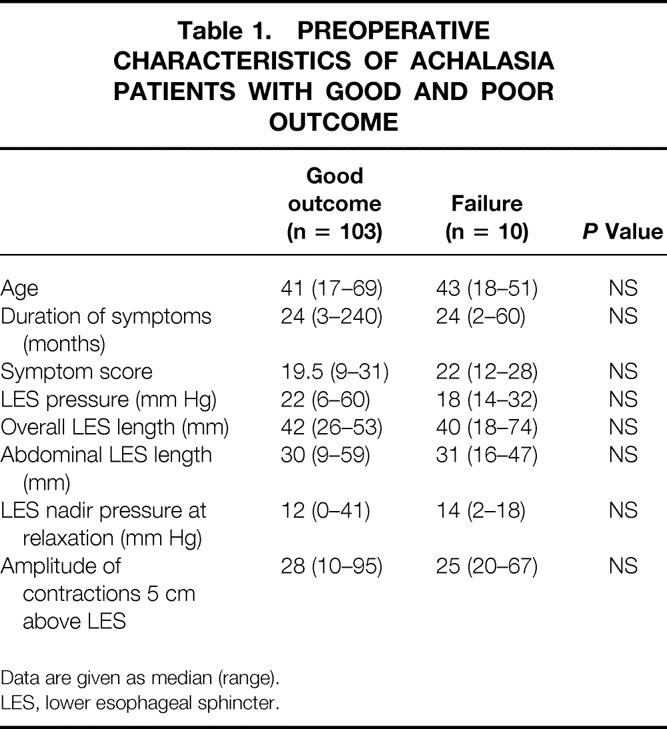
The postoperative results of the esophageal motility studies are summarized in Table 2. The overall and abdominal LES lengths, the difference between pre- and postoperative LES lengths, and the amplitude of the distal esophageal contractions were significantly different between the two groups (P < .05).
Table 2. POSTOPERATIVE CHARACTERISTICS OF ACHALASIA PATIENTS WITH GOOD AND POOR OUTCOME
Data are expressed as median (range).
LES, lower esophageal sphincter.
Based on the timing of the recurrence and the radiologic and manometric findings, patients who had symptom recurrence within 3 months of surgery were thought to have an incomplete myotomy resulting from the persistence of LES muscle fibers (n = 3), whereas those whose symptoms recurred later were considered as having an incomplete myotomy resulting from healing with fibrosis (n = 4); the cause of failure was thought to be the presence of a decompensated megaesophagus in one patient, a motor disorder of the esophageal body was detected in one, and we could not draw any etiologic conclusions in one.
Nine of the 10 patients with persistent or recurrent symptoms accepted further treatment; one refused to be treated at our department and received Botox injections elsewhere without benefit. All nine patients treated at our institution received pneumatic dilations. A total of 24 pneumatic dilations were performed with Rigiflex balloons (median 2 per patient, range 1–5) at a median interval of 4 months (range 1–15). Figure 4 summarizes the dilations, the diameter of the balloons, and the intervals between dilations. No complications of these esophageal dilations were observed. The median follow-up after the endoscopic dilations was 14.5 months (range 3–60). At present, five patients are completely asymptomatic, two report occasional dysphagia (although both had normal passage of liquids and solids at barium swallow), and two have remained symptomatic: one patient has undergone a thoracoscopic long myotomy and one a redo laparotomic abdominal myotomy.
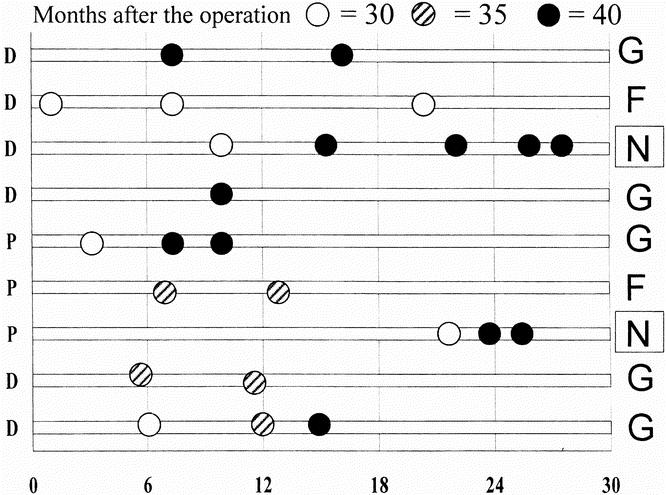
Figure 4. Timing and diameter of pneumatic dilations in nine patients agreeing to further treatment at our institution. The left X coordinate shows the patient’s main symptom (P, chest pain; D, dysphagia) and the right X coordinate shows the final outcome of dilation (G, good; F, fair; N, none). The white dots indicate the 30-mm Rigiflex dilator, the striped dots the 35-mm dilator, and the gray dots the 40-mm dilator. All patients required multiple dilations and only one remained symptomatic.
Esophageal manometry was repeated after the dilation or dilations in eight of nine patients (Fig. 5). After dilation, a decrease in esophageal sphincter lengths was observed in all the patients who responded well to the dilation treatment, whereas a further increase was detected in the patients with persistent dysphagia. The medians of the overall and abdominal LES lengths in the patients who responded well to dilation ranged from 47 mm (range 71–32) to 36.1 mm (range 47–34) for the overall length (P < .05) and from 35.8 mm (range 52–32) to 25.7 mm (range 33–14) for the abdominal length (P < .05). Only a minimal reduction in LES pressure was observed (i.e., 9 mm Hg [range 16–5] vs. 8 mm Hg [range 28–4];P = NS).
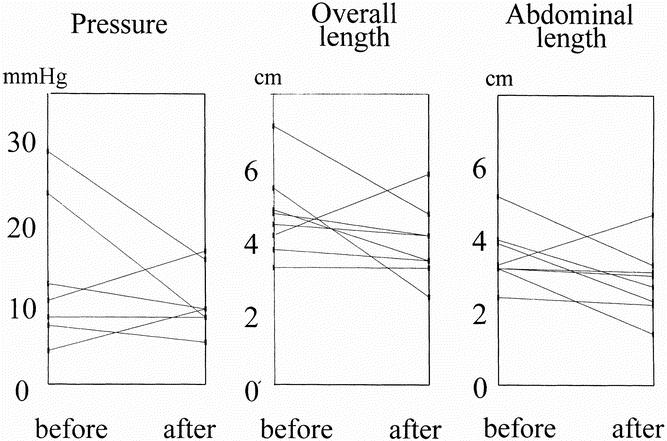
Figure 5. Manometric characteristics of the lower esophageal sphincter (LES) before and after pneumatic dilation. Resting pressure was not modified by the dilations (A), whereas all but one patient had a decrease in LES lengths (B, C); the patient whose LES lengths further increased is one of the two symptomatic patients.
DISCUSSION
The etiology of esophageal achalasia remains elusive, and the treatments currently available are designed only to palliate the typical symptoms of the disease (dysphagia and/or chest pain on swallowing) by weakening, disrupting, or dividing the unrelaxing LES muscle responsible for the disorder. There is consensus that surgery is probably the most effective method to achieve this goal 13 and that laparoscopic myotomy is as effective as open surgery, 14 although this treatment may also fail in a substantial number of patients.
As noted by Ellis, 15 the possible causes of cardiomyotomy failure are (in order of increased frequency) the late occurrence of carcinoma, the presence of a decompensated “sigmoid-shaped” megaesophagus before surgery, gastroesophageal reflux, a tight fundoplication, an inadequate myotomy (caused either by incomplete section of the muscle fibers or by healing with fibrosis of the myotomy edges).
Cardiomyotomy certainly has a greater likelihood of failing in patients with a large sigmoid and decompensated megaesophagus (grade IV achalasia), and such patients should be informed of this possibility. Myotomy is still appropriate in these patients, however, given the low risk of the procedure, especially by comparison with the alternative option, which is esophagectomy.
Gastroesophageal reflux disease after laparoscopic myotomy is a potential problem in patients with achalasia who have impaired esophageal motility, but it was only a marginal problem in our series, as in other reports. 2,16 Laparoscopic cardiomyotomy can be performed with a minimal dissection of the anterior part of the esophagus alone, preserving all of its posterior attachments, and partial anterior hemifundoplication is probably sufficient to prevent gastroesophageal reflux in the vast majority of patients. In the few with an abnormal gastroesophageal reflux, this causes only a mild form of gastroesophageal reflux disease that is easily controlled by medication.
In the present series, an excessively tight fundoplication was unlikely to be the cause of esophageal flow obstruction because all patients with early recurrence responded well to pneumatic dilation (which is unlikely to be effective in the case of a tight anterior fundoplication). 16
The main problem that we, like other authors, 16,17 encountered was persistent and/or recurrent dysphagia or chest pain in patients with achalasia grades I to III resulting from incomplete myotomy.
As for diagnosis, barium swallow and videofluoroscopy were the most useful tests for correlating symptoms and obstruction and for locating the obstruction in these patients. Measuring the LES pressure at rest and on swallowing added little information on the presence and cause of dysphagia after myotomy. In most patients with recurrent or persistent symptoms, however, an increase in sphincter lengths was recorded: this probably reflects the persistence of a short segment with a pressure high enough to obstruct the passage of food, or the onset of scar tissue around the myotomy.
In three of four patients with early symptom recurrence (and considered as having had an incomplete myotomy), barium swallow showed a short narrowing at the lower end of the myotomy (in the fourth patient, who also had an esophageal body motor disorder, the obstruction was in the upper part of the myotomy). Extending the myotomy downward on the gastric side of the cardia remains a critical part of the operation: as shown by Mattioli et al, 18 dividing the muscle on the gastric side of the cardia over a length of at least 1.5 cm is essential to deal thoroughly with the unrelaxing sphincter problem. This part of the operation is still the most difficult, however, because the plane between the submucosa and the muscle layer is less evident and bleeding is more likely. These difficulties may explain why, in most series in which recurrences after surgery were due to incomplete myotomy, the myotomy was incomplete on the gastric side rather than upward.
The main finding of this study, however, is that pneumatic dilation of the cardia was a safe and effective procedure for treating recurrent dysphagia and chest pain after laparoscopic myotomy. Forceful endoscopic dilation of the esophagus after unsuccessful Heller myotomy is by no means new, however: nearly 30 years ago, Palmer 5 described eight patients with failed Heller myotomy who were treated successfully and without complications by brusque perioral dilations using a Stark probang. In more recent years, esophageal pneumatic dilations have occasionally been used with varying results. Donahue et al 17 treated seven subjects with recurrent symptoms after laparoscopic myotomy by “stretching” the cardia passage using a TTS 18- to 26-mm balloon and a Rigiflex 30-mm balloon. Again, the diagnosis of periesophageal fibrosis was an assumption based on clinical and radiographic evidence of an esophageal obstruction and the absence of esophagitis or stricture. Patti et al 16 reported contrasting results using esophageal pneumatic dilation: dysphagia improved in only one third of the dilated patients (no details were given as to the type of dilator, the pressure exerted, and the duration of the procedure).
It seems obvious that surgeons are concerned about the use of pneumatic dilations in patients who have undergone surgery for achalasia because they fear perforating a cardia whose mucosa has been exposed by the previous myotomy. To reduce this risk, patients are never dilated sooner than 4 months after surgery, a lower pressure (6 psi) is used than the one generally recommended for untreated patients (10–20 psi), 19 and the dilation time is shorter (1 minute instead of the usual 2–3 minutes). 20 In untreated patients with achalasia, the risk of perforation has been found to relate to the pressure reached in the balloon, and a previous study showed that an inflation pressure of more than 11 psi was an independent risk factor for complications of pneumatic dilation. 21 We therefore preferred to use several “gentle” dilations rather than one very forceful dilation, so most of our patients needed two or more dilations to deal with their symptoms; this policy was rewarded, however, by the absence of complications. In the last five patients dilations were performed in an outpatient setting, as is common policy for untreated patients. As for the size of the dilator, a 30-mm Rigiflex balloon was initially used in our patients with recurrent achalasia, but all the patients dilated with this balloon gained little benefit and required repeat dilations with 35- and 40-mm balloons. The reason for this probably lies in the routine use of a 30-mm Rigiflex balloon to stretch the cardia during myotomy. If myotomy is unsuccessful, a larger balloon is needed to stretch the fibers even further, or to disrupt the periesophageal fibrosis. The success rate of the pneumatic dilations was nearly 80%, and this should now be considered the treatment of choice for patients with achalasia who have persistent or recurrent symptoms after Heller myotomy.
Manometry after dilation showed a reduction in LES lengths in all patients who responded well to the treatment, confirming that the manometric parameters most affected in patients with recurrent achalasia are abdominal and overall LES lengths. As expected, any LES pressure changes were less marked and provided no practical information.
In conclusion, the most common cause of unsuccessful laparoscopic Heller myotomy is either incomplete myotomy or fibrosis at the distal end of the myotomy. These conditions, diagnosed clinically and radiographically, cause an increase in LES length rather than in pressure and can be effectively treated with pneumatic dilations in nearly 80% of patients.
Footnotes
Supported in part by grant #9906198133 from the Italian Ministry for Research and the University of Padova.
Correspondence: Giovanni Zaninotto, MD, Department of Medical and Surgical Sciences, Clinica Chirurgica IV, University of Padova, School of Medicine, Via Giustiniani 4, 35128 Padova, Italy.
E-mail: giovanni.zaninotto@unipd.it
Accepted for publication June 19, 2001.
References
- 1.Rosati R, Fumagalli U, Bona S, et al. Evaluating results of laparoscopic surgery for esophageal achalasia. Surg Endosc 1998; 12: 270–273. [DOI] [PubMed] [Google Scholar]
- 2.Ancona E, Anselmino M, Zaninotto G, et al. One-year follow-up after laparoscopic Heller-Dor operation for esophageal achalasia. Surg Endosc 1997; 11: 3–7. [DOI] [PubMed] [Google Scholar]
- 3.Hunter JG, Trus TL, Branum GD, Waring P. Laparoscopic Heller myotomy and fundoplication for achalasia. Ann Surg 1997; 225: 655–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zaninotto G, Costantini M, Molena D, et al. Treatment of esophageal achalasia with laparoscopic Heller myotomy and Dor partial fundoplication: prospective evaluation of 100 consecutive patients. J Gastrointest Surg 2000; 4: 282–289. [DOI] [PubMed] [Google Scholar]
- 5.Palmer ED. Treatment of achalasia when Heller operation has failed. Am J Gastroenterol 1972; 57: 255–259. [PubMed] [Google Scholar]
- 6.Annese V, Basciani M, Lombardi G, et al. Perendoscopic injection of botulinum toxin is effective in achalasia after failure of myotomy or pneumatic dilatation. Gastrointest Endosc 1996; 44: 461–465. [DOI] [PubMed] [Google Scholar]
- 7.Kiss J, Voros A, Szirany E, et al. Management of failed Heller’s operation. Surg Today 1996; 26: 541–545. [DOI] [PubMed] [Google Scholar]
- 8.Miller DL, Allen MS, Trastek VF, et al. Esophageal resection for recurrent achalasia. Ann Thorac Surg 1995; 60: 922–926. [DOI] [PubMed] [Google Scholar]
- 9.Passaretti S, Zaninotto G, DiMartino N, et al. Standards for oesophageal manometry. A position statement from the Gruppo Italiano di Studio Motilità Apparato Digerente (GISMAD). Dig Liv Dis 2000; 32: 46–55. [DOI] [PubMed] [Google Scholar]
- 10.Zaninotto G, Demeester TR, Schwizer W, et al. The lower esophageal sphincter in health and disease. Am J Surg 1988; 155: 104–111. [DOI] [PubMed] [Google Scholar]
- 11.Crookes PF, Corkill S, DeMeester TR. Gastroesophageal reflux in achalasia: when is reflux really a reflux. Dig Dis Sci 1997; 42: 1354–1361. [DOI] [PubMed] [Google Scholar]
- 12.Ancona E, Peracchia A, Zaninotto G, et al. Heller laparoscopic cardiomyotomy with antireflux anterior fundoplication (Dor) in the treatment of esophageal achalasia. Surg Endosc 1993; 7: 459–461. [DOI] [PubMed] [Google Scholar]
- 13.Bonavina L, Nosadini A, Bardini R, et al. Primary treatment of esophageal achalasia. Arch Surg 1992; 127: 222–227. [DOI] [PubMed] [Google Scholar]
- 14.Ancona E, Anselmino M, Zaninotto G, et al. Esophageal achalasia: laparoscopic versus conventional open Heller-Dor operation. Am J Surg 1995; 170: 265–270. [DOI] [PubMed] [Google Scholar]
- 15.Ellis HF. Failure after esophagomyotomy for esophageal motor disorders. Causes, prevention, and management. Chest Surg Clin North Am 1997; 7: 476–488. [PubMed] [Google Scholar]
- 16.Patti MG, Pellegrini CA, Horgan S, et al. Minimally invasive surgery for achalasia. An 8-year experience with 168 patients. Ann Surg 1999; 230: 587–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donahue PE, Teresi M, Patel S, et al. Laparoscopic myotomy in achalasia: intraoperative evidence for myotomy of the gastric cardia. Dis Esoph 1999; 12: 30–36. [DOI] [PubMed] [Google Scholar]
- 18.Mattioli S, Pilotti V, Felice V, et al. Intraoperative study on the relationship between the lower esophageal sphincter pressure and the muscular components of the gastro-esophageal junction in achalasia patients. Ann Surg 1993; 218: 635–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khan A, Shah SWQ, Alam A, et al. Pneumatic balloon dilation in achalasia: a prospective comparison of balloon distention time. Am J Gastroenterol 1998; 93: 1064–1067. [DOI] [PubMed] [Google Scholar]
- 20.Eckardt VF, Kanzler G. Complications and their impact after pneumatic dilations: prospective long-term follow-up study. Gastrointest Endosc 1997; 45: 349–353. [DOI] [PubMed] [Google Scholar]
- 21.Nair LA, Reynolds JC, Parkman HP, et al. Complications during pneumatic dilation for achalasia or diffuse esophageal spasm. Analysis of risk factors, early clinical characteristics and outcome. Dig Dis Sci 1993; 38: 1893–1904. [DOI] [PubMed] [Google Scholar]