Abstract
Objective
To determine the incidence of small bowel obstruction (SBO), to identify risk factors for its development, and to determine the most common sites of adhesions causing SBO in patients undergoing ileal pouch–anal anastomosis (IPAA).
Methods
All patients undergoing IPAA at Mount Sinai Hospital were included. Data were obtained from the institution’s database, patient charts, and a mailed questionnaire. SBO was based on clinical, radiologic, and surgical findings. Early SBO was defined as a hospital stay greater than 10 or 14 days because of delayed bowel function, or need for reoperation or readmission for SBO within 30 days. All patients readmitted after 30 days with a discharge diagnosis of SBO were considered to have late SBO.
Results
Between 1981 and 1999, 1,178 patients underwent IPAA (664 men, 514 women; mean age 40.7 years). A total of 351 episodes of SBO were documented in 272 (23%) patients during a mean follow-up of 8.7 years (mean 1.29 episodes/patient). Fifty-four patients had more than one SBO. One hundred fifty-four (44%) of the SBOs occurred in the first 30 days; 197 (56%) were late SBOs. The cumulative risk of SBO was 8.7% at 30 days, 18.1% at 1 year, 26.7% at 5 years, and 31.4% at 10 years. The need for surgery for SBO was 0.8% at 30 days, 2.7% at 1 year, 6.7% at 5 years, and 7.5% at 10 years. In patients requiring laparotomy, the obstruction was most commonly due to pelvic adhesions (32%), followed by adhesions at the ileostomy closure site (21%). A multivariate analysis showed that when only late SBOs were considered, performance of a diverting ileostomy and pouch reconstruction both led to a significantly higher risk of SBO.
Conclusions
The risk of SBO after IPAA is high, although most do not require surgical intervention. Thus, strategies that reduce the risk of adhesions are warranted in this group of patients to improve patient outcome and decrease healthcare costs.
Postoperative adhesions form as a result of trauma to the peritoneum and the ensuing biochemical and cellular response that occurs in an attempt to repair the peritoneal surface. 1 Intraabdominal adhesions develop in virtually all patients undergoing major abdominal and pelvic procedures. 2,3 Although adhesions do have beneficial effects, they are also the primary cause of small bowel obstructions (SBOs) after abdominal surgery. Not only do they cause a considerable number of complications and deaths, but the healthcare costs required to deal with these issues are also considerable. 4,5
The rate of SBO after abdominal and pelvic surgery varies considerably, depending mainly on the magnitude of the surgical procedure, the development of postoperative complications, and the length of follow-up. For instance, the risk of SBO has been reported to be 1% to 10% after appendectomy, 6,7 6.4% after open cholecystectomy, 7 and 10% to 25% after intestinal surgery. 8,9 The risk of SBO after gynecologic procedures has been reported to be 0.3% for benign conditions without hysterectomy, 10 2% to 3% after hysterectomy, 10,11 and as high as 5% after radical hysterectomy. 12
The ileal pouch–anal anastomosis (IPAA) has become the procedure of choice for patients with ulcerative colitis requiring surgery, as well as for selected patients with familial polyposis and other conditions. Patients who undergo IPAA may be at particularly high risk for the development of SBO because of the combined abdominal and pelvic dissection, the need for multiple operations, and possibly a higher septic complication rate than that of less complex procedures. The reported rate of SBO after IPAA has varied between 13% and 35% in various studies. 13–26 However, these studies have been limited by retrospective design, small numbers, and short and/or incomplete follow-up and have not been analyzed actuarially. Further, changes in surgical technique have been made and the impact of these modifications has not been assessed.
Thus, the purposes of this study were to determine the magnitude of the risk of SBO after IPAA in a large cohort of patients followed up prospectively, to identify perioperative risk factors that increase the likelihood of postoperative SBO, to identify the frequency that surgical intervention will be required to treat SBO, and to determine the specific locations of adhesions that most frequently cause SBO.
Knowledge of the magnitude of the risk of SBO and of the particular sites of adhesions causing obstruction is necessary to evaluate the need for and optimization of strategies to prevent postoperative adhesions and SBO.
METHODS
All patients who underwent IPAA at Mount Sinai Hospital between 1981 and September 1999 were identified using the institution’s database. All data regarding the surgery and follow-up were collected prospectively. In addition, a mailed questionnaire was sent to all patients in case additional admissions for SBO occurred at other hospitals. Discharge summaries and operative reports from outside hospitals were obtained to verify information reported on the questionnaires. In all patients in whom SBO developed, charts were reviewed to determine the cause, management, and outcome. For those who required laparotomy, the operative note was reviewed to determine the cause of obstruction. For those SBOs found to be due to adhesions, the site of the adhesions that caused the SBO was recorded.
Data were collected for age, sex, preoperative diagnosis, whether colectomy was performed before or in conjunction with the pelvic pouch, use of a diverting ileostomy at the time of IPAA construction, anastomotic leakage, need for pouch reconstruction, and the occurrence of early or late SBO.
The diagnosis of SBO was based on the history, physical examination, and abdominal radiographic findings. At least two of three signs of SBO (absence of passage of flatus/acute constipation, high-pitched bowel sounds, air–fluid levels on radiography) had to be present.
Early SBO was defined as a postoperative hospital stay greater than 14 days after the IPAA, or 10 days after closure of ileostomy, because of delayed bowel function, when no other cause for delayed bowel function could be identified; or if a patient was readmitted or required reoperation for an obstruction occurring within 30 days of the surgery. Late obstructions were those occurring more than 30 days after the pelvic pouch procedure or ileostomy closure.
Statistical Analysis
All data were entered into the Mount Sinai Hospital IBD database using Access software (Microsoft, Redmond, WA). The data were analyzed using SAS software (SAS Institute Inc., Cary, NC). Data are presented as proportions or means plus or minus standard deviation. Differences were tested using chi-square or Student t test. The risk of SBO and the need for surgical intervention for SBO over time were calculated using the product-limit method of Kaplan and Meier. Confidence intervals (95% CI) were constructed using the Greenwood formula for standard error. 27 The Cox proportional hazards model 28 was used for all multivariate models. Probability values for each variable in the model were calculated from the Wald chi-square test. Factors analyzed included prior subtotal colectomy, use of a diverting-loop ileostomy, occurrence of an anastomotic leak (pouch or ileoanal), and the need for pouch reconstruction. Pouch reconstruction was defined as combined abdominal and perineal approach with or without construction of a new pouch. A loop ileostomy was always performed in conjunction with the procedure. In addition, the occurrence of an early SBO was analyzed as a risk factor for subsequent late SBO.
RESULTS
A total of 1,178 patients underwent IPAA at Mount Sinai Hospital between 1981 and 1999. Their demographic and clinical details are shown in Table 1. Most of the patients had ulcerative colitis, about half had their colectomy performed before the IPAA, and approximately two thirds had a diverting-loop ileostomy. Sixteen patients died during follow-up; no deaths were related to SBO. Ninety-six patients were lost to follow-up before 1999, and data on these patients were included until the date of their last admission or follow-up visit. The questionnaire was returned by 83% of patients. The mean follow-up of the cohort was 8.7 ± 4.8 years.
Table 1. PATIENT CHARACTERISTICS

A total of 351 episodes of SBO were documented in 272 patients during follow-up (Table 2). Early SBO occurred in 145 patients and accounted for 43.9% of all episodes. Of these, only eight (5.2%) required laparotomy. The cause was adhesions in six and internal hernia or volvulus in two. A total of 197 episodes of late SBO occurred in 149 patients (average 1.3 episodes/patient). Of these, 72 (36.5%) required laparotomy. Adhesions were the cause of the SBO in 65 (90.3%) of the 72 patients.
Table 2. DETAILS OF SMALL BOWEL OBSTRUCTION (SBO)
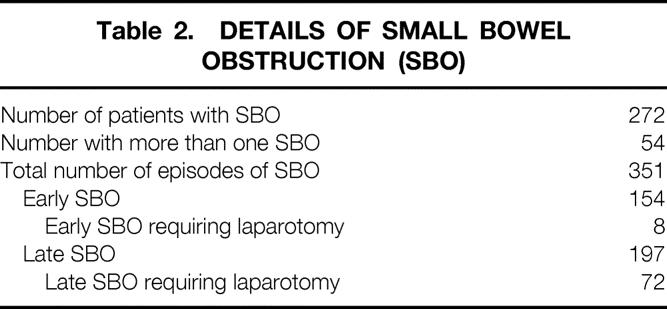
The cumulative risk of SBO and the need for surgical intervention for SBO after IPAA are shown in Figure 1. The risk of SBO was 8.7% (95% CI 7.1–10.2) at 30 days, 18.1% (95% CI 15.9–20.3) at 1 year, 26.7% (95% CI 24.1–29.3) at 5 years, and 31.4% (95% CI 28.4–34.4) at 10 years. The need for surgical intervention was 0.8% (95% CI 0.3–1.3) at 30 days, 2.6% (95% CI 1.8–3.7) at 1 year, 5.4% (95% CI 5.2–8.2) at 5 years, and 7.5% (95% CI 5.8–8.2) at 10 years.
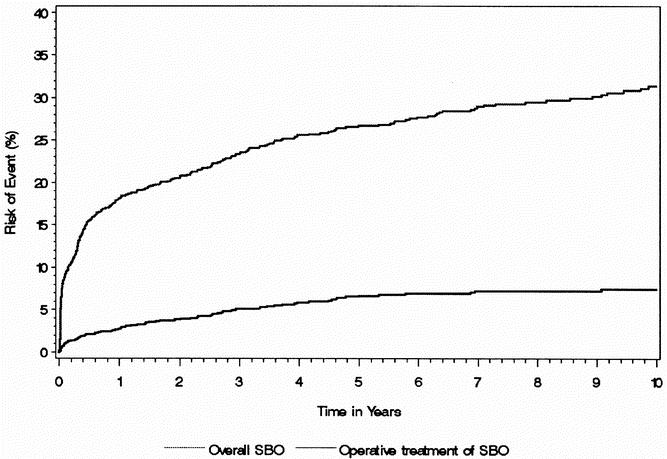
Figure 1. Overall risk of small bowel obstruction and risk of need for surgical treatment of small bowel obstruction after IPAA.
There were 134 episodes of early SBO in the 790 patients who had their IPAA defunctioned with an ileostomy. Forty-four of these occurred after closure of the ileostomy. Thus, 90 of 790 (11.4%) patients had an early SBO after IPAA with an ileostomy, compared with 20 of 383 (5.2%) (P < .001) after IPAA with no ileostomy.
The risk for late SBO when all early obstructions were excluded is shown in Figure 2. The risk of late SBO was 6.4% (95% CI 5.0–7.8) at 1 year, 14.5% (95% CI 12.4–16.6) at 5 years, and 19.2% (95% CI 16.5–21.9) at 10 years. Of the patients who developed a late SBO, 23.5% developed another SBO and 4.8% required surgery for SBO. For those who required laparotomy and lysis of adhesions for SBO, the risk of recurrent SBO was 21%, and the risk of requiring another laparotomy for recurrent SBO was 5%.
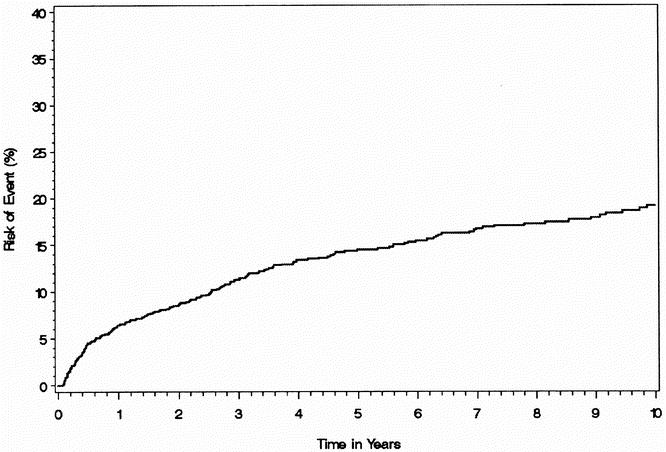
Figure 2. Risk of small bowel obstruction, excluding all early obstructions, after IPAA.
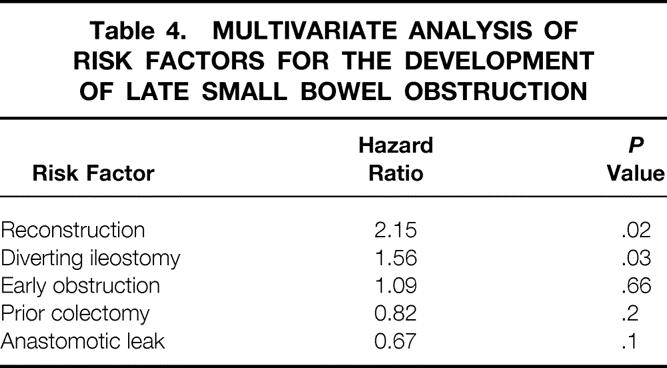
Construction of an ileostomy at the time of the pelvic pouch procedure and pouch reconstruction were associated with a significantly increased risk of late SBO (Table 3). Previous subtotal colectomy and the occurrence of an ileoanal anastomotic leak were associated with a decreased risk of SBO, especially in the early postoperative period, when the risk of SBO was significantly decreased. For patients who had developed early SBO, the risk of subsequent SBO was not significantly increased, having a hazard ratio of only 1.09 (Table 4).
Table 3. MULTIVARIATE ANALYSIS OF RISK FACTORS FOR THE DEVELOPMENT OF SMALL BOWEL OBSTRUCTION
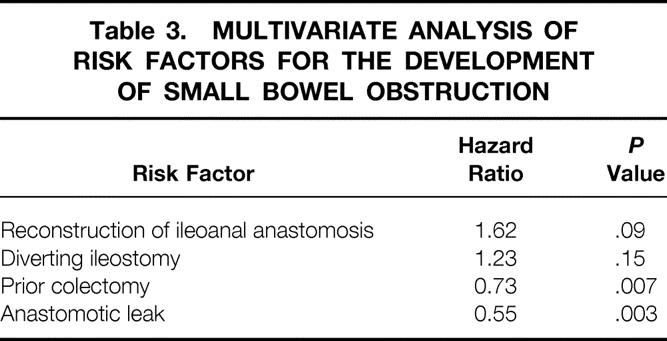
Table 4. MULTIVARIATE ANALYSIS OF RISK FACTORS FOR THE DEVELOPMENT OF LATE SMALL BOWEL OBSTRUCTION
Two hundred seventy-one of the 351 episodes of SBO were treated without surgery; 80 patients required laparotomy. Adhesions were the cause of the obstruction in 71 (89%) of the 80 patients (Table 5). Of the 71 patients who were found to have an adhesional SBO at laparotomy, the site of the obstruction is shown in Figure 3. Adhesions between the small bowel and the pelvis or the prior ileostomy site were the most common findings in these patients, accounting for more than 50% of the adhesive obstructions.
Table 5. CAUSE OF SMALL BOWEL OBSTRUCTION FOUND AT LAPAROTOMY

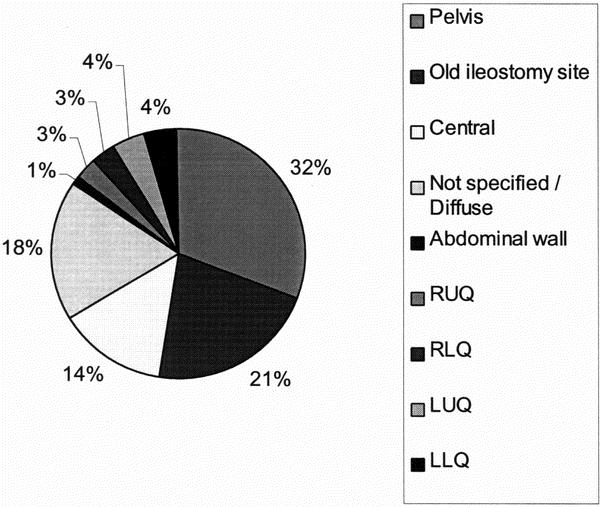
Figure 3. Location of adhesions found at laparotomy.
DISCUSSION
Small bowel obstruction is a common complication after major abdominal surgery. The risk of SBO has been reported to be 1% to 10% after appendectomy, 6,7 6.4% after open cholecystectomy, 7 and 10% to 25% after intestinal surgery. 8,9 The risk of SBO after gynecologic procedures seems lower. Rates of 0.3% for benign conditions without hysterectomy, 10 2% to 3% after hysterectomy, 10,11 5% after radical hysterectomy, 12 20% after radical hysterectomy and radiation, 12 and 7.5% after pelvic exenteration 29,30 have been reported. However, these studies are retrospective, lack long-term follow-up, and may suffer from underreporting because there was no mechanism to trace patients who would have sought care by general surgeons at other institutions; in the report by Orr et al 30 looking at pelvic exenteration, only those SBOs requiring surgical management were recorded.
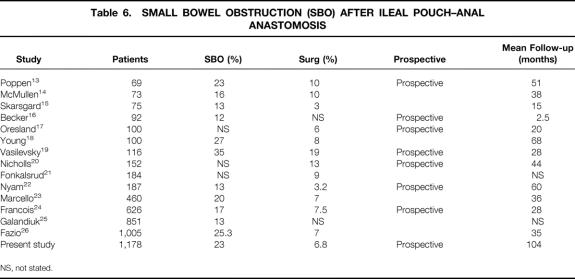
The reported risk of SBO after IPAA in previous studies ranged from 13% to 35% (Table 6). 13,15–17,19–26,31 The largest study was by Fazio et al at the Cleveland Clinic. 26 Their study included a large cohort of 1,008 patients and the overall risk of SBO was 25.3%, with a 7.5% risk of early SBO. However, the data were complete in only 645 patients, criteria for SBO were not defined, and the median follow-up was only 2.3 years. In addition, the data were not analyzed actuarially. The Mayo Clinic 24 reported rates of 17% overall, with a surgical risk of 7.5% in 626 patients, with a short mean follow-up of 2.3 years. They found that those with a Brooke ileostomy had a higher risk of SBO than those with a loop ileostomy, and those with previous operations were at higher risk than those who had no prior surgery. The Lahey Clinic 23 analyzed 460 patients with a median follow-up of 3 years. The overall risk of SBO was 20%, with 7% requiring surgery. Stomal rotation was the only independent risk factor for subsequent SBO. In our cohort, early SBO occurred in 15% of patients. This high rate may be in part due to the criteria used to define SBO. Early SBO was defined as a hospital stay of greater than 14 days after IPAA or 10 days after ileostomy closure, solely because of delayed bowel function. In our series, the median length of stay for all patients after IPAA was 10 days, and 5 days after ileostomy closure. Thus, the postoperative stays chosen in our definitions were clearly abnormal, and every effort to distinguish ileus from obstruction was made by excluding patients in whom another cause of delayed bowel function was found. In doing so, however, some patients may have been excluded who did have obstruction, and vice versa.
Table 6. SMALL BOWEL OBSTRUCTION (SBO) AFTER ILEAL POUCH–ANAL ANASTOMOSIS
NS, not stated.
When early obstructions, which are probably most often due to the ileostomy or postoperative edema, were excluded, the rate of postoperative obstruction (late SBO) was only 16.8%. Of those patients who developed one late SBO, the risk of having a second obstruction was 23.5%. The latter is on the lower end of the spectrum of previous reports. For instance, Barkan et al 32 reported a recurrence rate of 53% after an initial episode of SBO and 85% or more after a second, third, or later episode. Further, in our series of patients who developed an obstruction, the risk of subsequently requiring surgery for a later SBO was only 4.8%, suggesting that the initial nonoperative therapy is warranted.
The use of a diverting ileostomy was associated with an increased risk of SBO. This was true for both early and late obstructions. Possible reasons for this are that the small bowel might rotate around the ileostomy, and after closure adhesions may occur at this site, possibly as a result of difficulties in fully mobilizing the ileostomy. Several other studies 23,24,31 have also shown ileostomy to be a risk factor for the development of SBO. Although construction of an ileostomy is associated with an increased risk of SBO, a leak from the ileoanal anastomosis can cause significant complications and is still the most significant complication leading to pouch failure. In some situations, it may be safe to omit the ileostomy. However, if the risk of an ileoanal anastomosis leak is significant, the ileostomy should not be omitted. Instead, in these situations, strategies aimed at reducing adhesions should be adopted. The second risk factor associated with development of late SBO was pouch reconstruction. This was likely due to the multiple operations that these patients undergo.
This study also showed a lower risk of SBO in patients who had their colectomy performed before the IPAA procedure, as well in those who developed an anastomotic leak. This was particularly true in the early postoperative period. Performing the colectomy before IPAA may have led to a decreased risk of SBO because it is our practice to omit an ileostomy when a colectomy has been performed previously. In addition, it is our practice to do a colectomy and delay IPAA in patients receiving high-dose steroids. Because high-dose steroids may reduce the risk of adhesions, 33 this may have contributed to the lower incidence of SBO seen in this group.
The reason that an anastomotic leak was associated with a decreased risk of subsequent SBO is more perplexing. It is likely, however, related at least in part to our definition. Because we chose to define early SBO as there not being another cause for delayed bowel function, in those patients who had a leak, delayed bowel function would have been attributed to the leak, whereas some may have in fact been due to obstruction, and unrelated to the leak. When the data were analyzed for late obstructions only, the association was no longer statistically significant.
The need for surgical intervention was much more common for late than early SBO. It may be due to our policy of tending to treat early obstructions conservatively and allowing postoperative edema to subside, whereas we take a more aggressive approach to late obstructions. This appears to be a safe policy in that only 4 patients of the 80 who required laparotomy needed a bowel resection because of ischemia. All of these were in the late SBO group. Further, early SBO did not predict the occurrence of a late SBO, so surgical intervention with early SBO would not have prevented subsequent SBO.
In contrast to other complications, such as anastomotic leak and pouch failure, which have a decreased frequency with increasing surgical experience, the occurrence of SBO has remained high and fairly constant over time. The rates of SBO seen in the previous studies shown in Table 6 vary between 13% and 35%, but with no relation to the time of the study. In this study, there was no significant change in the rate of SBO in those cases performed before 1990 from those performed after 1990.
Given the frequency, complications, and cost of SBO, strategies to decrease SBO are warranted. Based on the pathogenesis of adhesions, six main strategies have been used (Table 7). However, although there is some evidence from animal studies that adhesion formation is decreased with these agents, there is conflicting evidence in humans. Also, there are concerns that side effects, such as depression of the immune system, reduced wound healing, infections, and allergic reactions, may limit their use. Until recently, 32% dextran 70 has been the most promising agent. The Adhesion Study Group 34 found that the instillation of 250 mL 32% dextran 70 before closure decreased adhesion formation. However, Jansen 35 showed no benefit. In addition, concerns regarding the potential side effects of 32% dextran 70, including infection, anaphylactic reactions, 36 increased bleeding time, and increased central venous pressure, although unproven or rare, may limit its potential use.
Table 7. STRATEGIES FOR ADHESION PROPHYLAXIS

There has been recent interest in barrier methods. The ideal barrier should have a low risk of side effects and complications, should be easy to use, should adhere on its own, should be absorbable, and should be effective at preventing adhesions. Barriers that have been studied include Silastic, 37 amnion, 38 peritoneum, omentum, Surgicel, 39 fibrin, and Interceed. 40,41 These substances have shown either no or limited efficacy at reducing adhesions. Interceed in particular showed promise in animal models, but its efficacy is markedly reduced in the presence of blood. 42,43 More recently, Seprafilm, a sodium hyaluronate-based bioresorbable membrane, has been shown in a randomized controlled trial 16 to significantly decrease the frequency of adhesions to the anterior abdominal wall after IPAA. Adhesions to the midline incision were seen in 94% of control subjects but in only 49% of patients in the Seprafilm group.
Theoretically, a laparoscopic approach to surgery might result in decreased adhesion formation and lower risk of subsequent SBO. However, long-term follow-up will be required to determine whether that is the case. The Operative Laparoscopic Study Group 44 showed that adhesions do develop after laparoscopic surgery. After undergoing laparoscopic adhesiolysis, adhesions were observed in 97% of patients at second-look laparoscopy within 90 days. The risk of subsequent SBO was not determined.
Although research into preventing postoperative adhesions is exciting, the most relevant question is whether a decrease in adhesions will lead to a decreased risk of subsequent SBO. To date, none of these strategies have been shown to decrease the risk of SBO. Seprafilm has been shown to decrease adhesion formation, 16,45 but long-term follow-up will be required to determine whether such a decrease will result in a decreased incidence of SBO. In addition, a cost-effectiveness analysis will be important because the cost of Seprafilm is significant.
The current study also indicates that adhesions in the pelvis and at the ileostomy closure site are the most likely causes of SBO. Thus, realizing it may not be possible to eliminate all postoperative adhesions, trying to decrease adhesions at these sites may be a more cost-effective option for experimenting with Seprafilm.
The development of SBO is associated with significant patient complications and some deaths and is a burden on our healthcare system. For patients who required surgical intervention for SBO, the obstruction was most commonly due to pelvic adhesions, followed by adhesions at the ileostomy closure site. This has implications with regard to adhesion prevention strategies. In this group of patients, these two sites represented more than half of the obstructions requiring surgery. Strategies that reduce the risk of adhesions, particularly in these two sites, are warranted to improve patient outcome and decrease healthcare costs. Trials studying hyaluronic acid-based bioresorbable membranes are in progress, but it remains to be seen whether these will decrease the incidence of postoperative SBO in a cost-effective manner. 46-56
Footnotes
Supported by a research fellowship from Axcan Pharma (A.R.McL.).
Correspondence: Robin S. McLeod, MD, Mount Sinai Hospital, 600 University Ave., Suite 449, Toronto, Ontario, Canada, M5G-1X5
E-mail: rmcleod@mtsinai.on.ca
Accepted for publication June 5, 2001.
There will be no reprints available.
References
- 1.Buckman RF, Woods M, Sargent L, et al. A unifying pathogenetic mechanism in the etiology of intraperitoneal adhesions. J Surg Res 1976; 20: 1–5. [DOI] [PubMed] [Google Scholar]
- 2.Weibel MA, Majno G. Peritoneal adhesions and their relation to abdominal surgery. A postmortem study. Am J Surg 1973; 126: 345–353. [DOI] [PubMed] [Google Scholar]
- 3.Menzies D, Ellis H. Intestinal obstruction from adhesions—how big is the problem? Ann R Coll Surg Engl 1990; 72: 60–63. [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson MS, Hawkswell J, McCloy RF. Natural history of adhesional small bowel obstruction: counting the cost. Br J Surg 1998; 85: 1294–1298. [DOI] [PubMed] [Google Scholar]
- 5.Ivarsson ML, Holmdahl L, Franzen G, et al. Cost of bowel obstruction resulting from adhesions. Eur J Surg 1997; 163: 679–684. [PubMed] [Google Scholar]
- 6.Ahlberg G, Bergdahl S, Rutqvist J, et al. Mechanical small-bowel obstruction after conventional appendectomy in children. Eur J Pediatr Surg 1997; 7: 13–15. [DOI] [PubMed] [Google Scholar]
- 7.Zbar RI, Crede WB, McKhann CF, et al. The postoperative incidence of small bowel obstruction following standard, open appendectomy and cholecystectomy: a six-year retrospective cohort study at Yale-New Haven Hospital. Conn Med 1993; 57: 123–127. [PubMed] [Google Scholar]
- 8.Nieuwenhuijzen M, Reijnen MM, Kuijpers JH, et al. Small bowel obstruction after total or subtotal colectomy: a 10-year retrospective review. Br J Surg 1998; 85: 1242–1245. [DOI] [PubMed] [Google Scholar]
- 9.Beck DE, Opelka FG, Bailey HR, et al. Incidence of small-bowel obstruction and adhesiolysis after open colorectal and general surgery [published erratum appears in Dis Colon Rectum 1999 May;42(5): 578]. Dis Colon Rectum 1999; 42: 241–248. [DOI] [PubMed] [Google Scholar]
- 10.Monk BJ, Berman ML, Montz FJ. Adhesions after extensive gynecologic surgery: clinical significance, etiology, and prevention. Am J Obstet Gynecol 1994; 170: 1396–1403. [DOI] [PubMed] [Google Scholar]
- 11.Al Took S, Platt R, Tulandi T. Adhesion-related small-bowel obstruction after gynecologic operations. Am J Obstet Gynecol 1999; 180: 313–315. [DOI] [PubMed] [Google Scholar]
- 12.Montz FJ, Holschneider CH, Solh S, et al. Small bowel obstruction following radical hysterectomy: risk factors, incidence, and operative findings. Gynecol Oncol 1994; 53: 114–120. [DOI] [PubMed] [Google Scholar]
- 13.Poppen B, Svenberg T, Bark T, et al. Colectomy-proctomucosectomy with S-pouch: operative procedures, complications, and functional outcome in 69 consecutive patients. Dis Colon Rectum 1992; 35: 40–47. [DOI] [PubMed] [Google Scholar]
- 14.McMullen K, Hicks TC, Ray JE, et al. Complications associated with ileal pouch-anal anastomosis. World J Surg 1991; 15: 763–766. [DOI] [PubMed] [Google Scholar]
- 15.Skarsgard ED, Atkinson KG, Bell GA, et al. Function and quality of life results after ileal pouch surgery for chronic ulcerative colitis and familial polyposis. Am J Surg 1989; 157: 467–471. [DOI] [PubMed] [Google Scholar]
- 16.Becker JM, Dayton MT, Fazio VW, et al. Prevention of postoperative abdominal adhesions by a sodium hyaluronate-based bioresorbable membrane: a prospective, randomized, double-blind multicenter study. J Am Coll Surg 1996; 183: 297–306. [PubMed] [Google Scholar]
- 17.Oresland T, Fasth S, Nordgren S, et al. The clinical and functional outcome after restorative proctocolectomy. A prospective study in 100 patients. Int J Colorectal Dis 1989; 4: 50–56. [DOI] [PubMed] [Google Scholar]
- 18.Young CJ, Solomon MJ, Eyers AA, et al. Evolution of the pelvic pouch procedure at one institution: the first 100 cases. Aust NZ J Surg 1999; 69: 438–442. [DOI] [PubMed] [Google Scholar]
- 19.Vasilevsky CA, Rothenberger DA, Goldberg SM. The S ileal pouch-anal anastomosis. World J Surg 1987; 11: 742–750. [DOI] [PubMed] [Google Scholar]
- 20.Nicholls RJ, Holt SD, Lubowski DZ. Restorative proctocolectomy with ileal reservoir. Comparison of two-stage vs. three-stage procedures and analysis of factors that might affect outcome. Dis Colon Rectum 1989; 32: 323–326. [DOI] [PubMed] [Google Scholar]
- 21.Fonkalsrud EW, Stelzner M, McDonald N. Experience with the endorectal ileal pullthrough with lateral reservoir for ulcerative colitis and polyposis. Arch Surg 1988; 123: 1053–1058. [DOI] [PubMed] [Google Scholar]
- 22.Nyam DC, Brillant PT, Dozois RR, et al. Ileal pouch-anal canal anastomosis for familial adenomatous polyposis: early and late results. Ann Surg 1997; 226: 514–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marcello PW, Roberts PL, Schoetz DJJr, et al. Obstruction after ileal pouch-anal anastomosis: a preventable complication? Dis Colon Rectum 1993; 36: 1105–1111. [DOI] [PubMed] [Google Scholar]
- 24.Francois Y, Dozois RR, Kelly KA, et al. Small intestinal obstruction complicating ileal pouch-anal anastomosis. Ann Surg 1989; 209: 46–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galandiuk S, Pemberton JH, Tsao J, et al. Delayed ileal pouch-anal anastomosis. Complications and functional results. Dis Colon Rectum 1991; 34: 755–758. [DOI] [PubMed] [Google Scholar]
- 26.Fazio VW, Ziv Y, Church JM, et al. Ileal pouch-anal anastomoses complications and function in 1005 patients. Ann Surg 1995; 222: 120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalbfleish J, Prentice R. The statistical analysis of failure time data. New York: John Wiley & Sons; 1980.
- 28.Cox D. Regression models and life-tables (with discussion). J R Stat Soc B 1972; 34: 187–220. [Google Scholar]
- 29.Lifshitz S, Johnson R, Roberts JA, et al. Intestinal fistula and obstruction following pelvic exenteration. Surg Gynecol Obstet 1981; 152: 630–632. [PubMed] [Google Scholar]
- 30.Orr JW Jr, Shingleton HM, Hatch KD, et al. Gastrointestinal complications associated with pelvic exenteration. Am J Obstet Gynecol 1983; 145: 325–332. [DOI] [PubMed] [Google Scholar]
- 31.Miller JS, Ferguson CM, Amerson JR, et al. Ileal pouch-anal anastomosis. The Emory University experience. Am Surg 1991; 57: 89–95. [PubMed] [Google Scholar]
- 32.Barkan H, Webster S, Ozeran S. Factors predicting the recurrence of adhesive small-bowel obstruction. Am J Surg 1995; 170: 361–365. [DOI] [PubMed] [Google Scholar]
- 33.Replogle RL, Johnson R, Gross RE. Prevention of postoperative intestinal adhesions with combined promethazine and dexamethasone therapy: experimental and clinical studies. Ann Surg 1966; 163: 580–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adhesion Study Group. Reduction of postoperative pelvic adhesions with intraperitoneal 32% dextran 70: a prospective, randomized clinical trial. Fertil Steril 1983; 40: 612–619. [DOI] [PubMed] [Google Scholar]
- 35.Jansen RP. Failure of intraperitoneal adjuncts to improve the outcome of pelvic operations in young women. Am J Obstet Gynecol 1985; 153: 363–371. [DOI] [PubMed] [Google Scholar]
- 36.Borten M, Seibert CP, Taymor ML. Recurrent anaphylactic reaction to intraperitoneal dextran 75 used for prevention of postsurgical adhesions. Obstet Gynecol 1983; 61: 755–757. [PubMed] [Google Scholar]
- 37.Yemini M, Shoham Z, Katz Z, et al. Effectiveness of silicone sheeting in preventing the formation of pelvic adhesions. Int J Fertil 1989; 34: 71–73. [PubMed] [Google Scholar]
- 38.Badawy SZ, Baggish MS, El Bakry MM, et al. Evaluation of tissue healing and adhesion formation after an intraabdominal amniotic membrane graft in the rat. J Reprod Med 1989; 34: 198–202. [PubMed] [Google Scholar]
- 39.Galan N, Leader A, Malkinson T, et al. Adhesion prophylaxis in rabbits with Surgicel and two absorbable microsurgical sutures. J Reprod Med 1983; 28: 662–664. [PubMed] [Google Scholar]
- 40.Linsky CB, Diamond MP, Cunningham T, et al. Adhesion reduction in the rabbit uterine horn model using an absorbable barrier, TC-7. J Reprod Med 1987; 32: 17–20. [PubMed] [Google Scholar]
- 41.Diamond MP, Linsky CB, Cunningham T, et al. Synergistic effects of Interceed (TC7) and heparin in reducing adhesion formation in the rabbit uterine horn model. Fertil Steril 1991; 55: 389–394. [PubMed] [Google Scholar]
- 42.Linsky CB, Diamond MP, Cunningham T, et al. Adhesion reduction in the rabbit uterine horn model using an absorbable barrier, TC-7. J Reprod Med 1987; 32: 17–20. [PubMed] [Google Scholar]
- 43.Diamond MP, Linsky CB, Cunningham T, et al. A model for sidewall adhesions in the rabbit: reduction by an absorbable barrier. Microsurgery 1987; 8: 197–200. [DOI] [PubMed] [Google Scholar]
- 44.Adhesion Study Group. Postoperative adhesion development after operative laparoscopy: evaluation at early second-look procedures. Operative Laparoscopy Study Group. Fertil Steril 1991; 55: 700–704. [PubMed] [Google Scholar]
- 45.Diamond MP. Reduction of adhesions after uterine myomectomy by Seprafilm membrane (HAL-F): a blinded, prospective, randomized, multicenter clinical study. Seprafilm Adhesion Study Group. Fertil Steril 1996; 66: 904–910. [PubMed] [Google Scholar]
- 46.Siegler AM, Kontopoulos V, Wang CF. Prevention of postoperative adhesions in rabbits with ibuprofen, a nonsteroidal anti-inflammatory agent. Fertil Steril 1980; 34: 46–49. [PubMed] [Google Scholar]
- 47.Holtz G. Failure of a nonsteroidal anti-inflammatory agent (ibuprofen) to inhibit peritoneal adhesion reformation after lysis. Fertil Steril 1982; 37: 582–583. [DOI] [PubMed] [Google Scholar]
- 48.Steinleitner A, Lambert H, Kazensky C, et al. Pentoxifylline, a methylxanthine derivative, prevents postsurgical adhesion reformation in rabbits. Obstet Gynecol 1990; 75: 926–928. [PubMed] [Google Scholar]
- 49.Steinleitner A, Kazensky C, Lambert H. Calcium channel blockade prevents postsurgical reformation of adnexal adhesions in rabbits. Obstet Gynecol 1989; 74: 796–798. [PubMed] [Google Scholar]
- 50.Jansen RP. Failure of peritoneal irrigation with heparin during pelvic operations upon young women to reduce adhesions. Surg Gynecol Obstet 1988; 166: 154–160. [PubMed] [Google Scholar]
- 51.Gervin AS, Puckett CL, Silver D. Serosal hypofibrinolysis. A cause of postoperative adhesions. Am J Surg 1973; 125: 80–88. [DOI] [PubMed] [Google Scholar]
- 52.Goldberg EP, Sheets JW, Habal MB. Peritoneal adhesions: prevention with the use of hydrophilic polymer coatings. Arch Surg 1980; 115: 776–780. [DOI] [PubMed] [Google Scholar]
- 53.Fowler JM, Lacy SM, Montz FJ. The inability of Gore-Tex surgical membrane to inhibit post-radical pelvic surgery adhesions in the dog model. Gynecol Oncol 1991; 43: 141–144. [DOI] [PubMed] [Google Scholar]
- 54.Montz FJ, Fowler JM, Wolff AJ, et al. The ability of recombinant tissue plasminogen activator to inhibit post-radical pelvic surgery adhesions in the dog model. Am J Obstet Gynecol 1991; 165: 1539–1542. [DOI] [PubMed] [Google Scholar]
- 55.Menzies D, Ellis H. Intra-abdominal adhesions and their prevention by topical tissue plasminogen activator. J R Soc Med 1989; 82: 534–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Doody KJ, Dunn RC, Buttram VC Jr. Recombinant tissue plasminogen activator reduces adhesion formation in a rabbit uterine horn model. Fertil Steril 1989; 51: 509–512. [DOI] [PubMed] [Google Scholar]