Abstract
Objective
To analyze the association between pre- and perioperative factors and pouch-related septic complications (PRSC) in ulcerative colitis (UC) and in familial adenomatous polyposis (FAP) after ileal pouch–anal anastomosis (IPAA).
Summary Background Data
For patients with UC and FAP, IPAA is the surgical therapy of choice, but in some patients the outcome is compromised by PRSC.
Methods
A total of 706 consecutive patients (494 UC, 212 FAP) were assessed in a study aimed at identifying subgroups of patients who were at high risk for PRSC. The rate of PRSC was analyzed as a time-dependent function (Kaplan-Meier estimation). Patients with UC and FAP were stratified separately according to associated factors (age, sex, surgeon’s experience, temporary ileostomy, colectomy before IPAA, anastomotic tension, and several factors specific for UC).
Results
In all, 131 (19.2%) patients had PRSC (23.4% UC, 9.4% FAP). In patients with UC, the estimated 1-year PRSC rate was 15.6% and the estimated 3-year PRSC rate was 24.2%. In patients with FAP, the estimated 1-year and 3-year PRSC rates were 9.2%. The difference between the estimated rates of PRSC was significant (P < .001). In the univariate analysis, patients with UC younger than 50 years, with severe proctitis, with preoperative hemoglobin levels less than 10 g/L, or receiving corticoid medication had a significantly higher risk for PRSC (P = .039, P = .037, P = .047, P = .003, respectively). Multivariate analysis showed that patients with UC receiving a systemic prednisolone-equivalent corticoid medication of more than 40 mg/day had a significantly greater risk of developing pouch-related complications than patients with UC receiving 1 to 40 mg/day and patients with UC who were not receiving corticoid medication (RR: 3.78, 2.25, 1, respectively, P < .001). Patients with FAP proved to have a significantly higher risk for PRSC in the univariate and multivariate analyses if anastomotic tension had occurred (RR 3.60, P = .0086).
Conclusions
Pouch-related septic complications occur as late complications and should therefore be considered in regular, specific long-term follow-up examinations. The authors identified significant risk factors for PRSC specific to patients with UC and FAP; these must be considered for each individual surgical strategy.
Total colectomy with ileal pouch–anal anastomosis (IPAA) was first described in 1978 1 and has become the surgical therapy of choice for patients with ulcerative colitis (UC) and familial adenomatous polyposis coli (FAP). 2–6 Although the long-term functional results are satisfactory, 7,8 the outcome of this procedure may be compromised by a number of specific complications. 9–12 These may be related to pouch function, presenting as pouchitis, pouch outlet obstruction, or pouch-related septic complications (PRSC). The latter comprise anastomotic leaks, parapouchal abscesses, and pouch–anal fistulas. The prevalence of PRSC varies between 6%13 and 37%. 14,15 The implications of these complications may be severe: in the worst case, permanent defunctioning or excision of the pouch may be needed. 10,16 Identification of pre- and perioperative risk factors for PRSC may help to refine prevention strategies. Most recommendations found in the literature are based on empiric reports: no multivariate analysis of pre- or perioperative risk factors for PRSC is available. Univariate analyses were performed in several studies, 2,4,8,14,17–23 but there is great disparity between the findings of these studies, possibly because of small numbers of patients, a nonstandardized surgical procedure, restriction to a small number of influencing factors, and different durations and modes of follow-up.
The present study had three objectives: to evaluate the effectiveness of the proposed strategies for follow-up after IPAA, to assess the rate of PRSC after IPAA, and to identify potential pre- and perioperative risk factors for the development of these complications in univariate and multivariate analyses.
Knowledge of risk factors may help to prevent PRSC by establishing optimal indications and timing of IPAA, choosing the individual surgical procedure, and using specific follow-up strategies.
PATIENTS AND METHODS
The IPAA procedure was introduced at the Department of Surgery, University of Heidelberg, in January 1982. Based on our experience with IPAA in the first 6 years up to January 1988, we designed a program for long-term follow-up after IPAA, focusing on the occurrence of PRSC. A total of 706 consecutive patients (494 UC, 212 FAP) underwent IPAA between January 1988 and June 1999 at our hospital and were included in this retrospective study. In all, 16 patients with UC and 9 patients with FAP were lost to follow-up within the first 3 months after IPAA and were not included in the risk factor analysis.
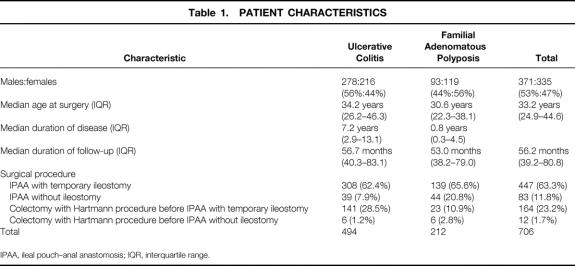
Data were entered into a clinical registry at the time of surgery and at each follow-up and were documented prospectively. The registry was maintained and statistical analysis of the data was performed by a biostatistician. Data regarding the clinical course of UC before surgery were obtained directly from the patient and his or her gastroenterologist. Patients were enrolled in a long-term follow-up program with standard follow-up intervals after IPAA of 3, 6, and 12 months in the first year, once yearly for another 4 years, and then once every 2 years, with the aim of detecting PRSC. In addition, patients were asked to present at the outpatient department of our hospital if any pouch-related symptoms developed, independently of the scheduled appointments. Patient characteristics are listed in Table 1.
Table 1. PATIENT CHARACTERISTICS
IPAA, ileal pouch–anal anastomosis; IQR, interquartile range.
We defined PRSC as the presence of fistulas or abscesses near the pouch (in the small pelvis), in the upper, middle, or lower part of the pouch, in the area of the rectal cuff, in the area of the IPAA, or in the area of the anal sphincter.
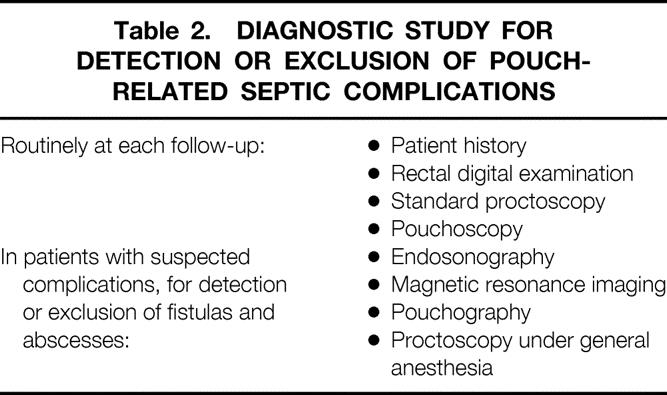
Diagnostic study for the detection of PRSC routinely involved obtaining the patient’s history and performing rectal digital and proctoscopic examination and pouchoscopy at each follow-up. Digital examination included palpation of the complete circumference of the rectal cuff, the pouch–anal anastomosis, and the anal canal. This examination has a high sensitivity in detecting PRSC because of the localized pain that is provoked. If PRSC was suspected, an extensive examination was performed to detect and localize or exclude PRSC (Table 2).
Table 2. DIAGNOSTIC STUDY FOR DETECTION OR EXCLUSION OF POUCH-RELATED SEPTIC COMPLICATIONS
To avoid misdiagnosis of Crohn’s disease, all biopsy samples were reviewed before surgery by an experienced pathologist skilled in the evaluation of inflammatory bowel diseases, and all candidates for IPAA were examined endoscopically by the surgeon. If Crohn’s disease was suspected, we performed a Hartmann procedure until the final results of the pathologic examination were available. The effectiveness of this strategy was proven by the fact that in the entire series of patients who underwent IPAA, no case of previously undetected Crohn’s disease was identified by histologic examination of the resected specimen or of the biopsy samples at follow-up. As a routine procedure, the histologic specimens from the resected colon were reviewed by the pathologist in patients with fistulas. Again, no case of Crohn’s disease was diagnosed.
The surgical procedure consisted of abdominal proctocolectomy, complete transanal mucosectomy leaving a rectum cuff with a length of 3 to 4 cm, construction of a 15-cm ileal J pouch using two 90-mm linear staplers (GIA 90 Premium, US Surgical Corp., Norwalk, CT), and a hand-sewn IPAA using 14 to 18 stitches. In the first years of the period investigated, a defunctioning loop ileostomy was invariably used. Since 1996, however, the protective ileostomy has been omitted in several patients who were in good condition, in whom the anastomosis could be completed without any technical difficulty, and who were receiving low-dose or no corticoid medication.
During surgery, each of the surgeons explicitly documented the state of the IPAA and noted whether there was any tension.
A surgeon’s experience has been shown to have an effect on the outcome of IPAA 8,24,25 and was therefore analyzed as a potential factor for PRSC in our study. We defined operations as being performed by an experienced surgeon if the surgeon had done at least 30 IPAAs.
With respect to the extent of the disease, we distinguished between pancolitis with backwash ileitis (BWI), pancolitis without BWI, and left-sided colitis with inflammatory manifestations only distal to the hepatic flexure. We diagnosed BWI when inflammation of the terminal ileal mucosa was present over at least 5 cm.
The disease activity of UC was measured using the Colitis Activity Index introduced by Rachmilewitz. 26 We distinguished between three grades of severity (scores 0–7, 8–15, 16–31). The grade of proctitis was measured according to the histologic classification developed by Truelove and Richards. 27
Systemic corticoid medication was calculated as the prednisolone equivalent dose in milligrams.
Statistical Analysis
We used SAS software (Release 8.00, SAS Institute, Inc., Cary, NC) for statistical analysis. The distribution of age at IPAA and duration of disease were presented as the median with an interquartile range. In many patients with UC, PRSC occurred not only during the first year but after a long period of follow-up as well. The rate of PRSC was therefore regarded as a time-dependent function and was estimated by the Kaplan-Meier method. The Kaplan-Meier estimation considered the first occurrence of a PRSC and was presented as a PRSC rate curve. Patients alive without PRSC were censored, as were 13 patients who died without having developed PRSC.
Although the surgical procedure of IPAA and the follow-up procedure are identical for patients with UC and those with FAP, some risk factors for PRSC are specific for UC. Risk factors for PRSC were therefore analyzed separately for the two groups of patients. The univariate associations between influencing factors and the complication rate were examined by the log-rank test. Factors independently associated with the complication rate were identified by proportional hazards regression (Cox model). Stepdown regression methods were used to develop a parsimonious statistical model for associating the individual factors with the complication rates among patients for whom data were complete. A factor was kept in the model if the value of the log likelihood statistic changed significantly. 28 Probability values were considered as statistically significant at the 0.05 level. All tests were two-sided.
RESULTS
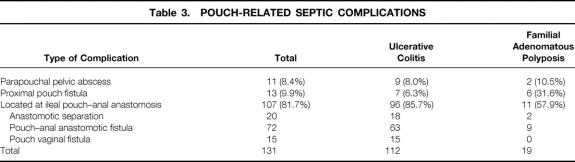
The frequency of PRSC was 19.2% in all patients with IPAA (23.4% in patients with UC, 9.4% in patients with FAP). The frequencies of the different forms of PRSC in patients with UC and those with FAP are shown in Table 3. Fistulas or leaks of the J pouch-anal anastomosis were the predominant PRSC in both groups of patients.
Table 3. POUCH-RELATED SEPTIC COMPLICATIONS
In patients with UC, the estimated 1-year PRSC rate was 15.6% and the 3-year PRSC rate was 24.2% (Table 4). In patients with FAP, the 1-year and 3-year PRSC rates were 9.2% (Table 5). As shown by the Kaplan-Meier curve (Fig. 1), the risk of PRSC was significantly greater in patients with UC than in patients with FAP (P < .001). In patients with FAP, 90% of the PRSCs (17/19) occurred within the first 12 months of follow-up, whereas in patients with UC only 63% of the PRSCs (71/112) occurred within this period.
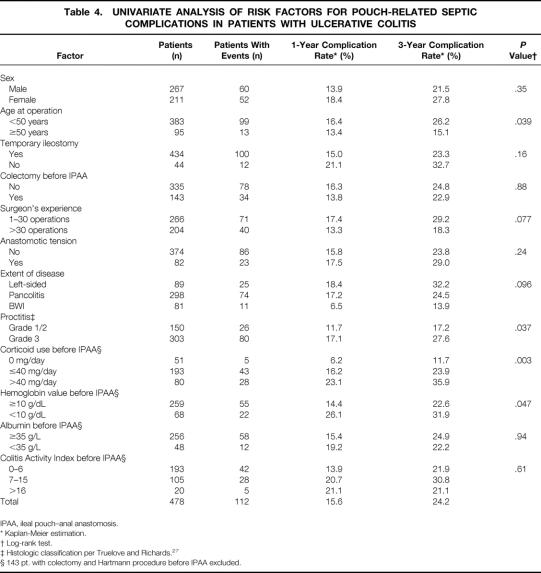
Table 4. UNIVARIATE ANALYSIS OF RISK FACTORS FOR POUCH-RELATED SEPTIC COMPLICATIONS IN PATIENTS WITH ULCERATIVE COLITIS
IPAA, ileal pouch–anal anastomosis.
* Kaplan-Meier estimation.
† Log-rank test.
‡ Histologic classification per Truelove and Richards. 27
§ 143 pt. with colectomy and Hartmann procedure before IPAA excluded.
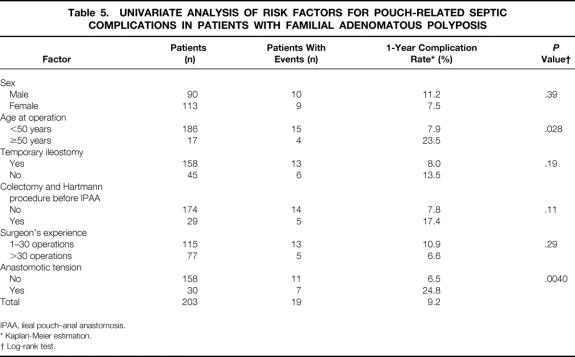
Table 5. UNIVARIATE ANALYSIS OF RISK FACTORS FOR POUCH-RELATED SEPTIC COMPLICATIONS IN PATIENTS WITH FAMILIAL ADENOMATOUS POLYPOSIS
IPAA, ileal pouch–anal anastomosis.
* Kaplan-Meier estimation.
† Log-rank test.
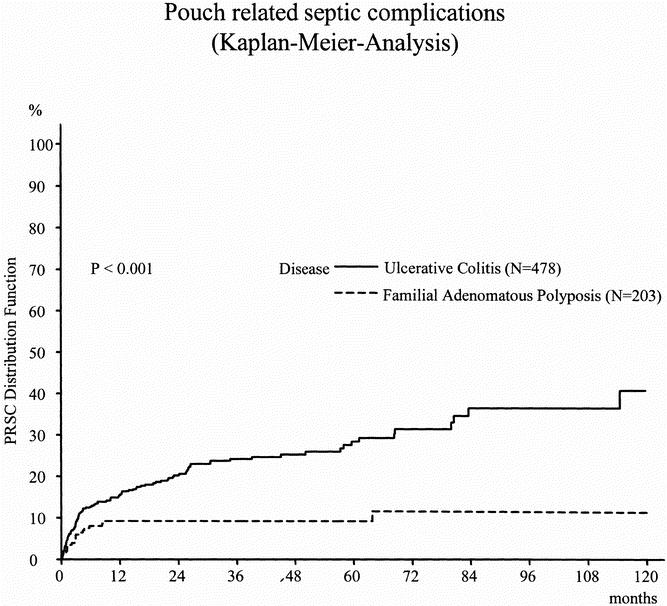
Figure 1. Distribution function of pouch-related septic complications in patients with ulcerative colitis versus familial adenomatous polyposis.
Patients With Ulcerative Colitis
Results of the univariate analysis of potential risk factors for PRSC in patients with UC are listed in Table 4. In patients with UC, the risk for PRSC was significantly greater when patients were receiving systemic corticoid medication before surgery, were younger than 50 years, had proctitis (grade 3), or showed preoperative hemoglobin levels of less than 10 g/L (P = .003, P = .039, P = .037, P = .047, respectively) (Fig. 2). Patients with BWI showed a tendency toward a lower risk of PRSC (P = .096). A surgeon’s experience had a nonsignificant influence on the rate of PRSC (P = .077).
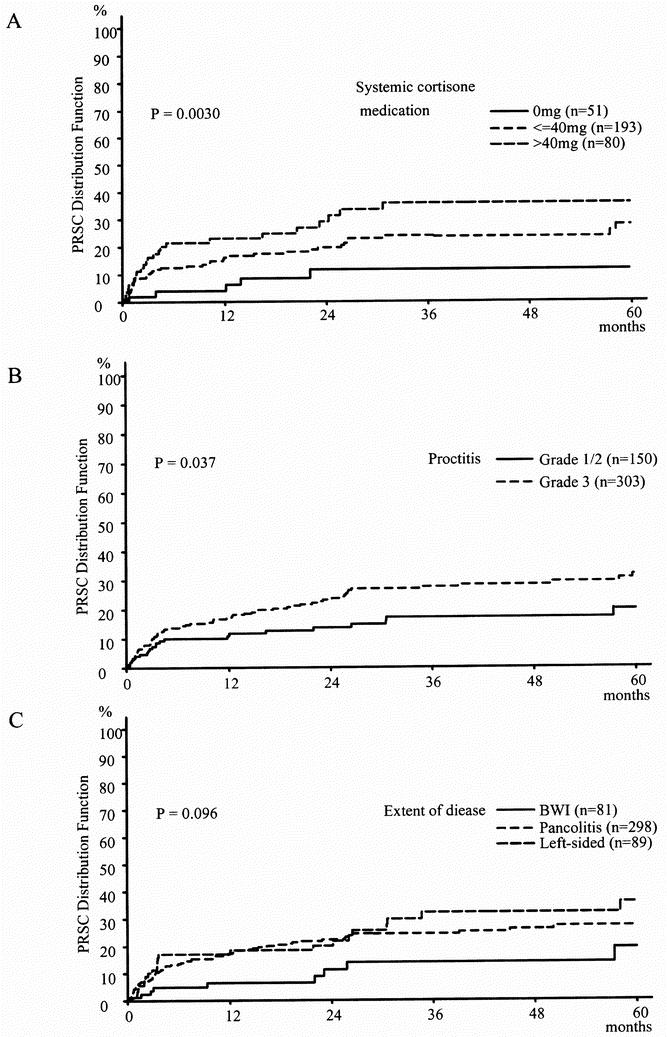
Figure 2. Distribution function of pouch-related septic complications in ulcerative colitis (Kaplan-Meier estimation).
A total of 273 patients with UC were receiving systemic corticoid medication for a period of more than 30 days before IPAA. Corticoid medication required in patients with UC at the time of IPAA was confirmed as an independent risk factor for PRSC in the multivariate analysis of patients who had not undergone colectomy and Hartmann procedure before IPAA. Patients with a systemic prednisolone-equivalent corticoid medication of more than 40 mg/day had a significantly greater risk of developing PRSC than patients with UC receiving 1 to 40 mg/day and patients with UC who were not receiving corticoid medication (relative risk [RR]: 3.78, 2.25, 1, respectively;P < .001;Table 6). The tendency toward a lower risk for PRSC in patients with BWI was confirmed as being significant in the multivariate analysis (RR: 0.42;P = .042). No other factor could be identified in the multivariate analysis as having an influence on the risk for PRSC.
Table 6. MULTIVARIATE ANALYSIS (FINAL COX MODEL) OF RISK FACTORS FOR POUCH-RELATED SEPTIC COMPLICATIONS IN ULCERATIVE COLITIS
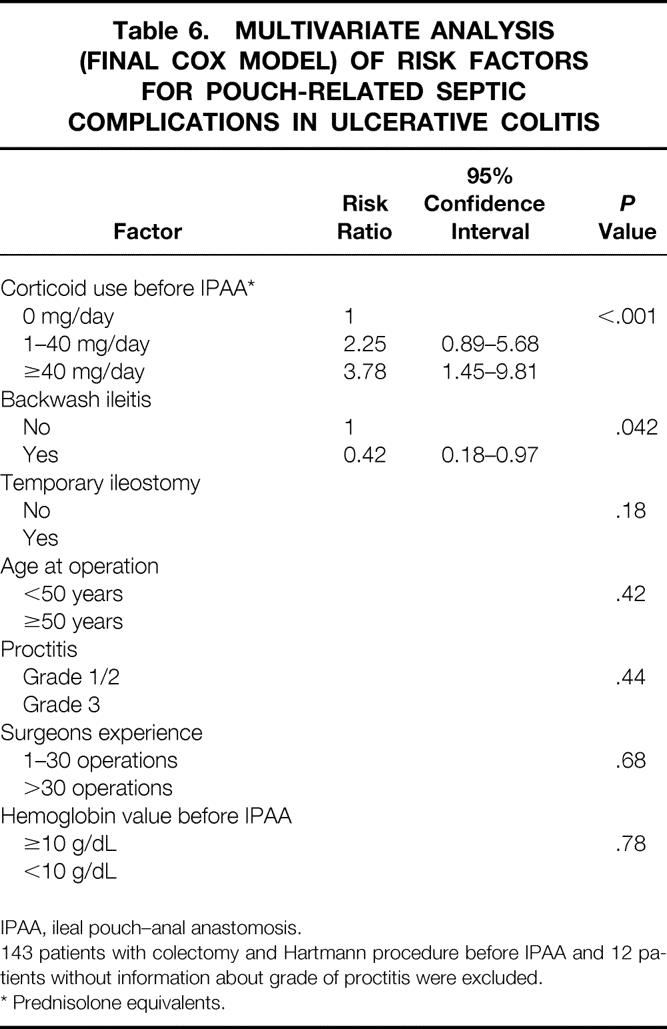
IPAA, ileal pouch–anal anastomosis.
143 patients with colectomy and Hartmann procedure before IPAA and 12 patients without information about grade of proctitis were excluded.
* Prednisolone equivalents.
Patients With Familial Adenomatous Polyposis
Results of the univariate analysis showed a significantly greater risk for PRSC in patients with FAP in whom anastomotic tension was present and who were older than 50 years (P = .004, P = .028, respectively;Fig. 3, Table 5).
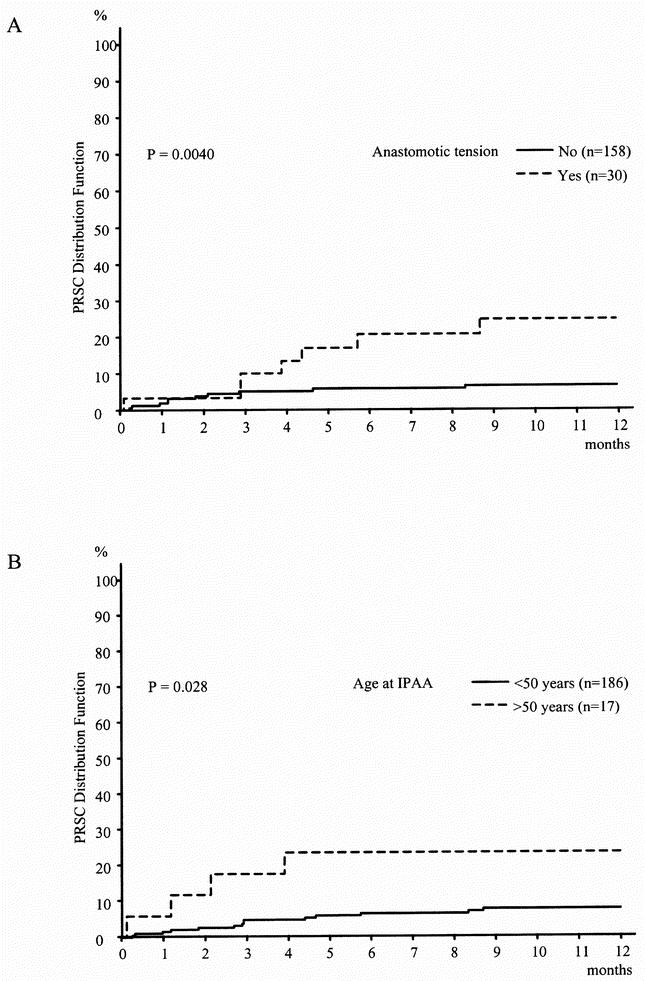
Figure 3. Distribution function of pouch-related septic complications in familial adenomatous polyposis (Kaplan-Meier estimation).
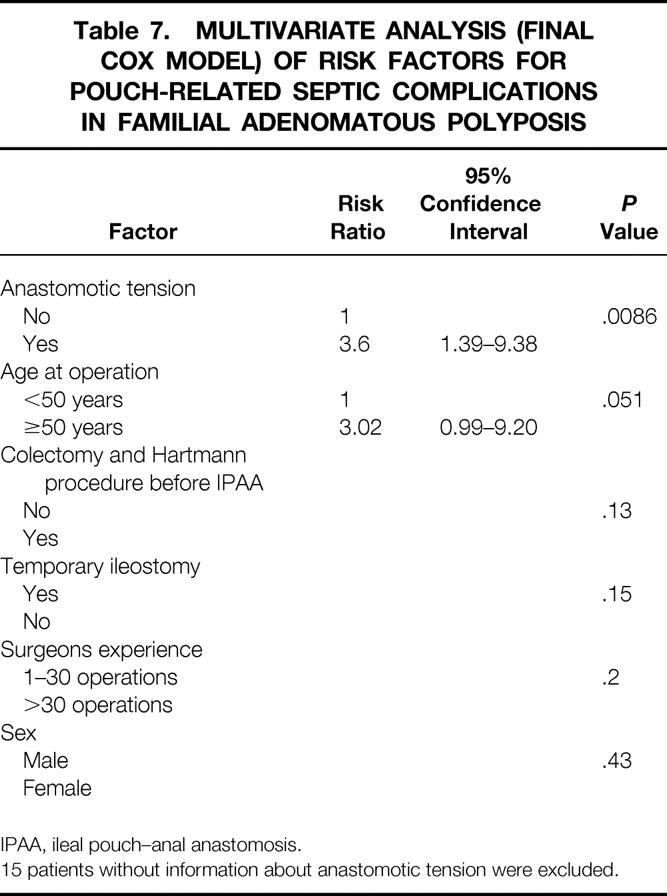
The multivariate analysis confirmed anastomotic tension as an independent risk factor for PRSC, but age older than 50 years just missed being statistically significant as a risk factor (RR = 3.6, P = .0086; RR = 3.02, P = .051, respectively;Table 7). No other factor was identified in the multivariate analysis as having an influence on the risk for PRSC.
Table 7. MULTIVARIATE ANALYSIS (FINAL COX MODEL) OF RISK FACTORS FOR POUCH-RELATED SEPTIC COMPLICATIONS IN FAMILIAL ADENOMATOUS POLYPOSIS
IPAA, ileal pouch–anal anastomosis.
15 patients without information about anastomotic tension were excluded.
DISCUSSION
The pelvic pouch procedure has proved to be an effective and safe surgical therapy for patients with UC and FAP and thus has been established as the surgical treatment of choice for these patients since the early 1980s. 2–6 Patient acceptance is high, the death rate is low, and functional results are satisfactory. 7,8 Nevertheless, PRSC may compromise the outcome of the procedure in some patients, as shown in earlier investigations. 9–12 In large patient series, the rates of PRSC ranged from 4%29 to 37.4%, 15 but variations in methods of investigation, surgical technique, and mode of follow-up, as well as lack of differentiation between patients with UC and patients with FAP, limit comparisons between the results of different centers. Varying surgical techniques for construction of the ileal pouch have been used during the past 20 years in terms of the pouch design (S, W, and J pouch), the procedure of mucosectomy, and the suturing technique of the pouch–anal anastomosis. 9,16,20 Various studies have even shown these variations to be influencing factors for PRSC. 12,30,31
Our study design provided several advantages. Data about the preclinical status and data obtained from direct contact with the patients at each of the follow-up consultations were entered into a clinical registry and documented prospectively. Follow-up was of long median duration and standardized with a focus on PRSC, among other complications. The extensive diagnostic study safely detected or excluded PRSC, so that for the analysis of risk factors, patients who had PRSC could be precisely distinguished from those who did not. The surgical procedure for IPAA was uniform over the whole period of investigation. The rate of PRSC was regarded as a time-dependent function and was therefore estimated by the Kaplan-Meier method, and potential influencing factors were identified in a multivariate analysis. Initial misdiagnosis of Crohn’s disease as UC is described as a major risk factor for PRSC in the literature. 20,32–34 Because histologic examination of the resected specimen identified no case of Crohn’s disease among the patients examined in our study, we can exclude any bias caused by undetected Crohn’s disease.
The rate of PRSC showed a great disparity between patients with UC and patients with FAP. The estimated cumulative risk of PRSC was markedly greater in patients with UC than in patients with FAP, indicating the predominant role of the underlying disease for the development of PRSC. This is in line with earlier studies that also showed a markedly greater risk of PRSC in patients with UC than in patients with FAP. 4,10,11,13,18,20,23,32,34–37
The analysis of PRSC with the Kaplan-Meier method as a time-dependent event was not performed in earlier investigations. It is remarkable that PRSCs in patients with UC still occur after a long follow-up, whereas in patients with FAP nearly all PRSCs occur within the first 9 months of follow-up. This emphasizes the importance of standardized long-term surveillance, including, in particular, consideration of PRSC for patients with UC.
The effect of steroid medication on the risk of PRSC has been discussed in the literature, but this has not been clarified. Steroid medication was examined in two univariate analyses. Cohen et al 18 showed that steroid medication is a risk factor for anastomotic leaks, whereas Ziv et al 38 showed that systemic steroid therapy before IPAA was not associated with an increased rate of PRSC within 30 days after surgery. All other speculations on the role of corticoids as risk factors for PRSC are based merely on empiric findings. 20,32,39 Long-term steroid use was examined for the first time as an independent risk factor for PRSC by multivariate analysis in our study. A total of 143 patients with UC had been treated with colectomy and a Hartmann procedure before IPAA. Most of these patients were receiving steroids before colectomy but not at the time of IPAA. To avoid bias, these patients were excluded from the analysis of steroid use as a risk factor for PRSC.
Our results showed that the risk of PRSC in patients with UC was significantly dependent on the presence and dose of long-term systemic corticoid medication at the time of IPAA. The estimated rate of PRSC with respect to the dose of corticoid medication at the time of IPAA in patients with UC is shown in the Kaplan-Meier analysis (see Fig. 2). A marked difference can be seen between the curves of the estimated rates of PRSC in patients not receiving preoperative corticoid medication, those receiving long-term corticoid medication of up to 40 mg/day, and those receiving long-term corticoid medication of more than 40 mg/day. This difference is caused by a higher increase in the rate of PRSC in patients receiving corticoid medication during the first 4 months of follow-up. After that period, the curves of the estimated rates of PRSC run in parallel so that no difference can be observed in the increase of the curves. From this, we conclude that the use of systemic corticoid medication before surgery affects the rate of PRSC in the first 4 months after IPAA, but not after that. The effect of preoperative systemic steroids on the rate of PRSC during the first 4 months after IPAA could be caused by the fact that steroids impair the healing of bowel anastomosis, increase susceptibility to infection, and have various endocrinologic and metabolic side effects. 40–43
Independent of any possible pathophysiologic effects of steroids, the dose of corticoid medication might reflect the severity of UC better than the other parameters of disease activity. It is conceivable that treatment of severe colitis with high doses of corticoids resulted in milder symptoms and fewer findings of UC. In these patients, the parameters of colitis disease activity would be low, whereas actually the severity of UC was high, as reflected by the dose of corticoid medication.
As a consequence, the presence and dose of systemic steroids should be taken into consideration in planning the surgical therapy to help prevent PRSC in patients with UC. IPAA should be performed during intervals when the use of corticoid medication is low or, if possible, during corticoid-free intervals. In patients who require high doses of corticoid medication, the surgical therapy should consist of colectomy with Hartmann closure of the rectum as the first step and subsequent IPAA when corticoid medication is no longer necessary.
Earlier studies discussed whether the indication for IPAA depends on the patient’s age, pointing out the reservations of different centers in recommending this surgical therapy for elderly patients. 13,15,44–47 The results of our univariate and multivariate analyses showed that the risk of PRSC is not higher in elderly patients with UC. Hence, we see no reason to restrict the use of IPAA in elderly patients with UC. Patients with FAP who were older than 50 showed a tendency toward a higher risk of PRSC. This association was significant in the univariate analysis, but age older than 50 did not reach significance in the multivariate analysis.
The severity of proctitis in patients with UC was a significant risk factor for PRSC in the univariate analysis. Even if this risk is not confirmed as an independent factor in the multivariate analysis, this finding suggests that successful local medical treatment of proctitis (e.g., with cortisone or mesalamine enemas) before IPAA in patients with UC might help minimize the risk of PRSC.
The surgical procedure has been discussed as having an influence on the outcome of IPAA in several earlier studies. 16,23,48,49 Disparities concerning the surgical techniques used in the series of patients may have introduced some errors into earlier studies. In our study, the surgical procedure for IPAA was largely uniform throughout the entire series in terms of design and size of the ileal pouch, technique for construction of the ileal J pouch–anal anastomosis, length of the rectum cuff, and mucosectomy. This allowed us to enter the surgeon’s experience and anastomotic tension as aspects of the surgical technique into the analysis in our study, minimizing possible sources of bias from other technical variables. Our results showed a trend toward a higher rate of PRSC in patients who were operated on by a surgeon who had performed fewer than 30 IPAA. This trend was stronger in patients with UC than in patients with FAP but was not significant in either group. This finding may be explained by the fact that performing IPAA, complete mucosectomy in particular, is technically more demanding in patients with UC than in patients with FAP.
Our investigation identified anastomotic tension as a significant risk factor for PRSC in patients with FAP by both univariate and multivariate analyses. As a consequence, we recommend avoiding any tension on the J pouch–anal anastomosis. Attempts should be made to improve the mobility of the J pouch by dividing all tissues connecting the mesentery of the J pouch to the retroperitoneum, scoring the peritoneum in multiple locations, and dividing the ileocolic artery and all mesenteric tissue between the divided ileocolic artery and the next major branch. In all patients in whom these measures are not sufficient to avoid anastomotic tension, a diverting ileostomy is mandatory.
The terminal ileum is used for construction of the ileoanal pouch. BWI is defined as inflammation of the terminal ileum before proctocolectomy and has therefore been suggested as predisposing to PRSC. 50 The frequency of BWI was 18.2% among patients with UC in an earlier study from our hospital 51 and 16.9% in the present study. The results of our investigation show that the risk of PRSC is not increased in patients with UC and BWI. Therefore, the presence of BWI is not a contraindication for IPAA in patients with UC and does not require resection of the inflamed terminal ileum before construction of the ileoanal pouch.
Regarding the surgical technique, investigations during the past two decades considered the advantages and disadvantages of hand-sewn and stapled pouch–anal anastomoses. 12,30,31,38,52–57 PRSC was among the main endpoints of these investigations. Nevertheless, the question of which of the two techniques provides superior results concerning PRSC has not been satisfactorily answered. We showed that the occurrence of PRSC is time-dependent in hand-sewn J ileal pouches with complete mucosectomy. This should be taken into consideration for further studies comparing the two anastomotic techniques.
CONCLUSIONS
The risk of PRSC after restorative proctocolectomy is markedly different in patients with UC and patients with FAP. In patients with UC, PRSCs occur as late complications in many patients, and the estimated cumulative risk is significantly higher than in patients with FAP. This emphasizes the importance of regular long-term follow-up regarding PRSC, particularly in patients with UC. Corticoid medication use in patients with UC was identified as an independent significant risk factor for PRSC. For future studies of risk factors for PRSC, the strong time dependence of the occurrence of these complications should be taken into consideration.
Acknowledgment
The authors thank Mr. Clark Jones for help with data collection and support in the survey of current literature, which he performed in the course of his medical degree.
Footnotes
Correspondence: Udo Heuschen, MD, Department of Surgery, University of Heidelberg, Kirschnerstrasse 1, D-69120 Heidelberg, Germany.
E-mail: Udo_Heuschen@med.uni-heidelberg.de
Accepted for publication June 8, 2001.
References
- 1.Parks AG, Nicholls RJ. Proctocolectomy without ileostomy for ulcerative colitis. Br Med J 1978; 2: 85–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dozois RR, Kelly KA, Welling DR, et al. Ileal pouch-anal anastomosis: Comparison of results in familial adenomatous polyposis and chronic ulcerative colitis. Ann Surg 1989; 210: 268–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams NS, Marzouk DEMM, Hallan RI, et al. Function after ileal pouch and stapled pouch-anal anastomosis for ulcerative colitis. Br J Surg 1989; 76: 1168–1171. [DOI] [PubMed] [Google Scholar]
- 4.Marcello PW, Roberts PL, Schoetz DJ, et al. Long-term results of the ileoanal pouch procedure. Arch Surg 1993; 128: 500–504. [DOI] [PubMed] [Google Scholar]
- 5.Järvinen HJ, Luukkonen P. Experience with restorative proctocolectomy in 201 patients. Ann Chir Gynaecol 1993; 82: 159–164. [PubMed] [Google Scholar]
- 6.Kartheuser AH, Parc R, Penna CP, et al. Ileal pouch-anal anastomosis as the first choice operation in patients with familial adenomatous polyposis: a ten-year experience. Surgery 1996; 119: 615–623. [DOI] [PubMed] [Google Scholar]
- 7.Romanos J, Samarasekera DN, Stebbing JF, et al. Outcome of 200 restorative proctocolectomy operations: the John Radcliffe Hospital experience. Br J Surg 1997; 84: 814–818. [PubMed] [Google Scholar]
- 8.Meagher AP, Farouk R, Dozois RR, et al. J ileal pouch-anal anastomosis for chronic ulcerative colitis: Complications and long-term outcome in 1310 patients. Br J Surg 1998; 85: 800–803. [DOI] [PubMed] [Google Scholar]
- 9.Fazio VW, Ziv Y, Church JM, et al. Ileal pouch-anal anastomoses: Complications and function in 1005 patients. Ann Surg 1995; 222: 120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Körsgen S, Keighley MRB. Causes of failure and life expectancy of the ileoanal pouch. Int J Colorectal Dis 1997; 12: 4–8. [DOI] [PubMed] [Google Scholar]
- 11.Seidel SA, Newman M, Sharp KW. Ileoanal pouch versus ileostomy: Is there a difference in quality of life? Am Surg 2000; 66: 540–547. [PubMed] [Google Scholar]
- 12.Scotte M, Del Gallo G, Steinmetz L, et al. Ileoanal anastomosis for ulcerative colitis: Results of an evolutionary surgical procedure. Hepato-Gastroenterology 1998; 45: 2123–2125. [PubMed] [Google Scholar]
- 13.Reissman P, Teoh TA, Weiss EG, et al. Functional outcome of the double stapled ileoanal reservoir in patients more than 60 years of age. Am Surg 1996; 62: 178–183. [PubMed] [Google Scholar]
- 14.Mikkola K, Luukkonen P, Järvinen HJ. Long-term results of restorative proctocolectomy for ulcerative colitis. Int J Colorectal Dis 1995; 10: 10–14. [DOI] [PubMed] [Google Scholar]
- 15.Tan HAT, Connolly AB, Morton D, et al. Results of restorative proctocolectomy in the elderly. Int J Colorectal Dis 1997; 12: 319–322. [DOI] [PubMed] [Google Scholar]
- 16.McRae HM, McLeod RS, Cohen Z, et al. Risk factors for pelvic pouch failure. Dis Colon Rectum 1997; 40: 257–262. [DOI] [PubMed] [Google Scholar]
- 17.Richard CS, Cohen Z, Stern HS, et al. Outcome of the pelvic pouch procedure in patients with prior perianal disease. Dis Colon Rectum 1997; 40: 647–652. [DOI] [PubMed] [Google Scholar]
- 18.Cohen Z, McLeod RS, Stephen W, et al. Continuing evolution of the pelvic pouch procedure. Ann Surg 1992; 216: 506–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wettergren A, Gyrtrup HJ, Grosmann E, et al. Complications after J-pouch ileoanal anastomosis: Stapled compared with hand-sewn anastomosis. Eur J Surg 1993; 159: 121–124. [PubMed] [Google Scholar]
- 20.Nicholls RJ, Holt SDH, Lubowski DZ. Restorative proctocolectomy with ileal reservoir. Comparison of two-stage vs. three-stage procedures and analysis of factors that might affect outcome. Dis Colon Rectum 1989; 32: 323–326. [DOI] [PubMed] [Google Scholar]
- 21.Lee PY, Fazio VW, Church JM, et al. Vaginal fistula following restorative proctocolectomy. Dis Colon Rectum 1997; 40: 752–759. [DOI] [PubMed] [Google Scholar]
- 22.Galandiuk S, Wolff BG, Dozois RR, et al. Ileal pouch-anal anastomosis without ileostomy. Dis Colon Rectum 1991; 34: 870–873. [DOI] [PubMed] [Google Scholar]
- 23.Groom JS, Nicholls RJ, Hawley PR, et al. Pouch-vaginal fistula. Br J Surg 1993; 80: 936–940. [DOI] [PubMed] [Google Scholar]
- 24.Gemlo BT, Wong WD, Rothenberger DA, Goldberg SM. Ileal pouch-anal anastomosis: Patterns of failure. Arch Surg 1992; 127: 784–787. [DOI] [PubMed] [Google Scholar]
- 25.McMullen K, Hicks TC, Ray JE, et al. Complications associated with ileal pouch-anal anastomosis. World J Surg 1991; 15: 763–767. [DOI] [PubMed] [Google Scholar]
- 26.Rachmilewitz D. Coated mesalazine (5-aminosalicylic acid) versus sulphasalazine for the treatment of active ulcerative colitis: A randomized trial. Br Med J 1989; 298: 82–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Truelove SC, Richards WCD. Biopsy studies in ulcerative colitis. Br Med J 1956; 9: 1315–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colettt D. Modelling survival data in medical research. London: Chapman & Hall; 1994.
- 29.McIntyre PB, Pemberton JH, Beart RW, et al. Double-stapled vs. hand-sewn ileal pouch-anal anastomosis in patients with chronic ulcerative colitis. Dis Colon Rectum 1994; 37: 430–433. [DOI] [PubMed] [Google Scholar]
- 30.Gullberg K, Liljeqvist L. Gains and losses with stapling an omission of loop ileostomy in pelvic pouch surgery: A matched control study. Int J Colorectal Dis 1999; 14: 255–260. [DOI] [PubMed] [Google Scholar]
- 31.Reilly WT, Pemberton JH, Wolff BG, et al. Randomized prospective trial comparing ileal pouch-anal anastomosis performed by excising the anal mucosa to ileal pouch-anal anastomosis performed by preserving the anal mucosa. Ann Surg 1997; 225: 666–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dayton MT, Larsen KP. Outcome of pouch-related complications after ileal pouch-anal anastomosis. Am J Surg 1997; 174: 728–732. [DOI] [PubMed] [Google Scholar]
- 33.Ozuner G, Hull Tracy, Lee P, Fazio VW. What happens to a pelvic pouch when a fistula develops? Dis Colon Rectum 1997; 40: 543–547. [DOI] [PubMed] [Google Scholar]
- 34.Schoetz DJ Jr, Coller JA, Veidenheimer MC. Can the pouch be saved? Dis Colon Rectum 1988; 31: 671–675. [DOI] [PubMed] [Google Scholar]
- 35.Grobler SP, Hosie KB, Affei E, et al. Outcome of restorative proctocolectomy when the diagnosis is suggestive of Crohn’s disease. Gut 1993; 34: 1384–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poppen B, Svenberg T, Bark T, et al. Colectomy-proctomucosectomy with S-pouch. Operative procedures, complications and functional outcome in 69 consecutive patients. Dis Colon Rectum 1992; 35: 40–47. [DOI] [PubMed] [Google Scholar]
- 37.Pricolo VE, Potenti FM, Luks FI. Selective preservation of the anal transition zone in ileoanal pouch procedures. Dis Colon Rectum 1996; 39: 871–877. [DOI] [PubMed] [Google Scholar]
- 38.Ziv Y, Church JM, Fazio VW, et al. Effect of systemic steroids on ileal pouch-anal anastomosis in patients with ulcerative colitis. Dis Colon Rectum 1996; 39: 504–508. [DOI] [PubMed] [Google Scholar]
- 39.Scott NA, Dozois RR, Beart RW, et al. Postoperative intra-abdominal and pelvic sepsis complicating ileal pouch-anal anastomosis. Int J Colorectal Dis 1988; 3: 149–152. [DOI] [PubMed] [Google Scholar]
- 40.Reding R, Michel LA, Donckier J, et al. Surgery in patients on long-term steroid therapy: A tentative model for risk assessment. Br J Surg 1990; 77: 1175–1178. [DOI] [PubMed] [Google Scholar]
- 41.Price LA. The effects of steroids on ileorectal anastomosis in ulcerative colitis. Br J Surg 1968; 55: 840–841. [DOI] [PubMed] [Google Scholar]
- 42.Aszodi A, Ponsky JL. Effects of corticosteroid on healing of bowel anastomosis. Am Surg 1984; 50: 546–548. [PubMed] [Google Scholar]
- 43.Furst MB, Stromberg BV, Blatchford GJ, et al. Colonic anastomosis: Bursting strength after corticosteroid treatment. Dis Colon Rectum 1994; 37: 12–15. [DOI] [PubMed] [Google Scholar]
- 44.Setti-Carraro P, Ritchie JK, Wilkinson KH, et al. The first 10 years’ experience of restorative proctocolectomy for ulcerative colitis. Gut 1994; 35: 1070–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Samarasekera DN, Stebbing FJ, Kettlewell MGW, et al. Outcome of restorative proctocolectomy with ileal reservoir for ulcerative colitis: Comparison of distal colitis with more proximal disease. Gut 1996; 38: 574–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takao Y, Gilliland R, Nogueras JJ, et al. Is age relevant to functional outcome after restorative proctocolectomy for ulcerative colitis? Ann Surg 1998; 227: 187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Farouk R, Pemberton JH, Wolff BG, et al. Functional outcomes after ileal pouch-anal anastomosis for chronic ulcerative colitis. Ann Surg 2000; 231: 919–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reissman P, Piccirillo M, Ulrich A, et al. Functional results of the double-stapled ileoanal reservoir. J Am Coll Surg 1995; 181: 444–450. [PubMed] [Google Scholar]
- 49.Wettergren A, Gyrtrup HJ, Grosmann E, et al. Complications after J-pouch ileoanal anastomosis: stapled compared with hand-sewn anastomosis. Eur J Surg 1993; 159: 121–124. [PubMed] [Google Scholar]
- 50.Gustavsson S, Weiland LH, Kelly KA. Relationship of backwash ileitis to ileal pouchitis after ileal pouch-anal anastomosis. Dis Colon Rectum 1987; 30: 25–28. [DOI] [PubMed] [Google Scholar]
- 51.Heuschen UA, Hinz U, Allemeyer EH, et al. Backwash ileitis is strongly associated with colorectal carcinoma in ulcerative colitis. Gastroenterology 2001; 120: 841–847. [DOI] [PubMed] [Google Scholar]
- 52.Gozzetti G, Poggioli G, Marchetti F, et al. Functional outcome in hand-sewn versus stapled ileal pouch-anal anastomosis. Am J Surg 1994; 168: 325–329. [DOI] [PubMed] [Google Scholar]
- 53.Gemlo BT, Belmonte C, Wiltz O, Madoff RD. Functional assessment of ileal pouch-anal anastomotic techniques. Am J Surg 1995; 169: 137–142. [DOI] [PubMed] [Google Scholar]
- 54.Tuckson WB, Lavery IC, Fazio VW, et al. Manometric and functional comparison of ileal pouch anal anastomosis with and without anal manipulation. Am J Surg 1991; 161: 90–96. [DOI] [PubMed] [Google Scholar]
- 55.Slors JFM, Ponson AE, Taat CW, Bosma A. Risk of residual rectal mucosa after proctocolectomy and ileal pouch-anal reconstruction with the double-stapling technique. Postoperative follow-up study. Dis Colon Rectum 1995; 38: 207–210. [DOI] [PubMed] [Google Scholar]
- 56.Seow-Choen F, Tsunoda A, Nicholls RJ. Prospective randomized trial comparing anal function after hand-sewn ileoanal anastomosis without mucosectomy in restorative proctocolectomy. Br J Surg 1991; 78: 430–434. [DOI] [PubMed] [Google Scholar]
- 57.Luukkonen P, Järvinen H. Stapled vs. hand-sutured ileoanal anastomosis in restorative proctocolectomy. A prospective, randomized study. Arch Surg 1993; 128: 437–440. [DOI] [PubMed] [Google Scholar]