Abstract
Background
Appendicitis frequently presents in an atypical fashion leading to misdiagnosis or a delay in diagnosis. This is particularly true in early cases where the patient may be erroneously discharged from an emergency department and will invariably return with perforated appendicitis. The standard of care is hospital admission for observation or early operation. Adjunctive imaging tests have been used with mixed results in this equivocal patient population. The authors studied a promising new monoclonal antibody, 99mTc-labeled anti-CD 15 (LeuTech; Palatin Technologies, Inc., Princeton, NJ), which specifically targets neutrophils and may be used for imaging appendicitis. This prospective, multicenter, open-label study evaluated the diagnostic efficacy and clinical impact of LeuTech scintigraphy for detecting appendicitis in patients with an equivocal presentation.
Methods
A total of 200 patients (121 females, 79 males; age range 5–86 years; mean age 30.5 ± 16.5 years) completed the study. Management plan was formulated before and reassessed following LeuTech imaging to determine impact on management. Following intravenous injection of LeuTech, the abdomen was imaged with a standard gamma camera for 30 to 90 minutes.
Results
Fifty-nine patients had a histopathologic diagnosis of acute appendicitis. LeuTech identified 53 of 59 patients with appendicitis (90% sensitivity) and was negative in 122 of 141 patients without appendicitis (87% specificity). Accuracy, positive predictive value, and negative predictive value were 88%, 74%, and 95%, respectively. Diagnostic efficacy was unchanged in a subgroup of 48 pediatric patients (5–17 years). Diagnostic images for appendicitis were achieved within 8 minutes postinjection in 50% of patients and within 47 minutes in 90% of patients. Significant shifts in patient management decisions were evident following LeuTech results. LeuTech was well tolerated with no serious adverse events reported.
Conclusion
LeuTech is a convenient, safe, rapid, and sensitive imaging test for diagnosis of appendicitis and favorably impacts patient management in adult and pediatric patients with equivocal signs and symptoms.
Approximately 250,000 cases of acute appendicitis are diagnosed each year in the United States and many more patients with abdominal pain masquerading as appendicitis are evaluated in emergency departments and doctors offices. Appendicitis is the most common cause of acute abdominal pain requiring surgical intervention, and carries an associated mortality of 0.8% to 8%. 1-5 The incidence is highest in the second and third decades of life, but appendicitis may occur at any age. 4 In the presence of classic signs and symptoms of acute appendicitis, a quick and accurate diagnosis can be made based on history and physical examination alone. 5-7 However, as many as 50% of patients do not exhibit characteristic signs and symptoms. 4,6 In these equivocal patients, the diagnosis of appendicitis is difficult, delayed, and frequently inaccurate. 4
Complications including perforation, peritonitis, and death may occur with delayed diagnosis, particularly in the very young and in the elderly. 6 As many as 45% of cases are misdiagnosed, resulting in unnecessary hospitalizations and sometimes unnecessary surgery. 6 Surgical exploration has been reported to be negative in 10% to 30% of patients with a preoperative diagnosis of appendicitis. 8,9 More accurate diagnosis and reduction in unnecessary hospitalization or surgery in patients with an equivocal presentation would result in improved clinical outcomes and conservation of hospital resources.
The currently available radioisotope infection imaging technique using 99mTc-HMPAO labeled white blood cells (WBC) is very sensitive for detecting appendicitis. 10-13 The primary disadvantages of radiolabeled WBC imaging include a lengthy preparation time of 2 hours and sometimes longer if an off site radiopharmacy is used, the requirement of ex vivo white blood cell radiolabeling, the potential for external blood contamination and misadministration, personnel exposure to blood borne infection such as hepatitis and HIV, and the relatively high cost and technical demand of the procedure. Because of these disadvantages, very few medical centers are capable of using radiolabeled WBC imaging for the diagnosis of appendicitis. Monoclonal antibodies specific for surface antigens on neutrophils address many of these disadvantages and may be useful infection imaging agents when labeled with a radionuclide. 14
99mTc-labeled anti-CD 15 monoclonal antibody (LeuTech; Palatin Technologies, Inc., Princeton, NJ); also known as 99mTc-anti-stage-specific embryonic antigen [SSEA]-1 monoclonal antibody) binds avidly to surface CD 15 antigens that are expressed on human neutrophils in large numbers. 15-17 As a result of the in vivo systemic administration of radiolabeled LeuTech, radioactivity becomes concentrated in areas of infection or inflammation; thus, the need to withdraw blood from a patient to label WBC’s ex vivo is eliminated and the time required to perform the test is reduced significantly. In a prospective pilot study, we performed LeuTech scans on 49 patients with equivocal presentation of appendicitis. 18 The results were encouraging with sensitivity and negative predictive values of 100%. A multicenter clinical trial was undertaken to confirm these preliminary results.
This paper reports the results from this prospective, multicenter trial. The aim of this study was to further evaluate and confirm the efficacy and safety of LeuTech scintigraphy for diagnosis of acute appendicitis in patients presenting with equivocal signs and symptoms at multiple centers and in a larger population. In addition, the potential impact of LeuTech imaging on intended patient management was assessed.
MATERIALS AND METHODS
Patient Selection
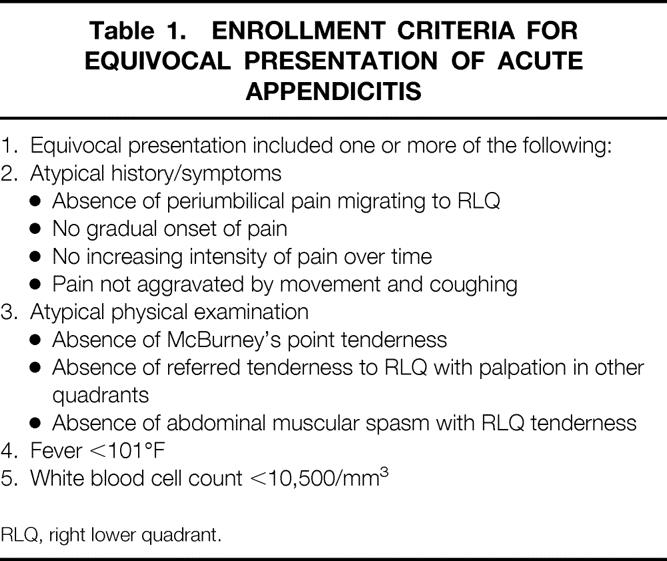
Two-hundred and three patients with right lower quadrant (RLQ) abdominal pain and equivocal presentation of appendicitis were enrolled in 10 centers from September 1998 to March 1999. Eight of the 10 centers had no prior experience with radioisotope imaging for appendicitis and two centers had prior experience with Tc-99m HMPAO labeled WBC imaging for appendicitis. Only one center (TCMC) had prior experience with LeuTech imaging for appendicitis. The attending surgeon evaluated each patient prior to enrollment and established that the diagnosis of appendicitis was equivocal according to the criteria outlined in Table 1. Female patients were excluded if they were pregnant, nursing, or diagnosed with pelvic inflammatory disease (PID). Females of childbearing potential were excluded unless pregnancy was ruled out by urine or serum pregnancy testing. The study protocol was approved by the FDA and by the Institutional Review Board or Ethics Committee at each site. Written informed consent was obtained from each patient, parent, or legal guardian prior to enrollment.
Table 1. ENROLLMENT CRITERIA FOR EQUIVOCAL PRESENTATION OF ACUTE APPENDICITIS
RLQ, right lower quadrant.
LeuTech Preparation and Administration
LeuTech was provided by Palatin Technologies, Inc., Princeton, New Jersey, as a lyophylized kit containing 250 micrograms of nonradioactive anti-CD-15 IgM antibody and the reagents required for reconstitution and radiolabeling.
The preparation and labeling of LeuTech was optimized during the phase 2 clinical trials and applied for use in this multicenter clinical trial. 18 Each vial was reconstituted with 0.25 ml saline containing 20 to 40 mCi 99m sodium pertechnetate. LeuTech was prepared by incubation with Tc-99m for 30 minutes in a 37 °C water bath. Following incubation, 0.75 ml Ascorbic Acid (250mg/ml) was added for stability and to increase the final volume to 1.0 ml. Quality control testing of the final preparation was performed to detect free Tc-99m Sodium pertechnetate using thin layer chromatography. The administered intravenous dose was 0.3 to 0.5 ml (75 to 125 micrograms of antibody) containing 10 to 20 mCi Tc-99m LeuTech. The dose was scaled downward for children to 0.21 mCi per kg of body weight up to a maximal dose of 20 mCi.
LeuTech Imaging
Immediately following injection, the patient was comfortably positioned lying supine under a large field-of-view gamma camera. A dynamic sequence of 10–4 minutes of anterior images were acquired for the first 40 minutes and played back as an endless loop cine. Following ambulation and voiding, static images of the abdomen and pelvis were acquired in the anterior, right anterior oblique, left anterior oblique, posterior, and anterior standing positions. A second dynamic imaging sequence of 8–4 minute frames was then acquired for the next 32 minutes. Imaging could be terminated at any time following the first 40 minutes of dynamic imaging sequence if the scan was determined to be unequivocally positive by the nuclear medicine physician. Completion of the entire 90 minutes of imaging protocol was required before a scan could be read as negative. Patients imaged for less than 30 minutes were considered not evaluable for efficacy analysis. All patients were observed for adverse events and vital signs were monitored for 60 minutes following injection.
Each LeuTech scan was interpreted by the principle investigator (the attending nuclear radiologist) as positive or negative for infection. Equivocal or indeterminate readings were not permitted. Positive scans were further classified as acute appendicitis or other infection based on the location of abnormal uptake. Criteria for a positive scan for appendicitis was abnormal persistent LeuTech accumulation within the right lower abdominal quadrant (Figure 1). Diffuse or multifocal abdominal activity extending into the right lower quadrant was considered positive for appendicitis as well, because of the possibility of perforation (Figure 2). Negative scans did not demonstrate any abnormal activity in the abdomen or pelvis (Figure 3). For positive cases, the time the scan first became diagnostic was recorded.
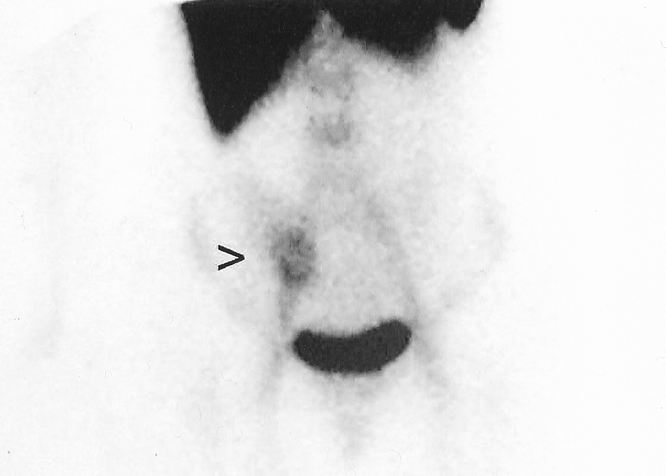
FIG. 1. LeuTech scan performed in a 43-year-old woman presenting with right lower quadrant abdominal pain, atypical history and equivocal physical examination. Initial management plan was to admit and observe. This anterior LeuTech image performed 40 minutes following injection demonstrates high intensity focal accumulation in the right lower quadrant typical for acute appendicitis. Following the positive scan interpretation, emergency laparotomy was performed. Surgical and histopathological findings indicated acute appendicitis.
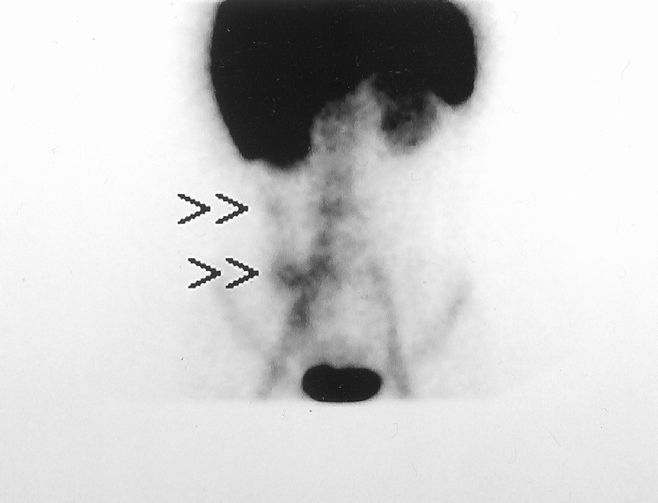
FIG. 2. LeuTech scan performed in a 17-year-old woman presenting with right lower quadrant abdominal pain and equivocal history and physical examination. Initial management plan was to admit and observe. This anterior LeuTech image performed 12 minutes following injection demonstrates abnormal accumulation in the right lower quadrant with additional activity tracking along the right colon/paracolic gutter area suggesting perforated appendicitis. Following the positive scan interpretation, emergency laparotomy was performed. Surgical findings indicated perforated appendicitis.
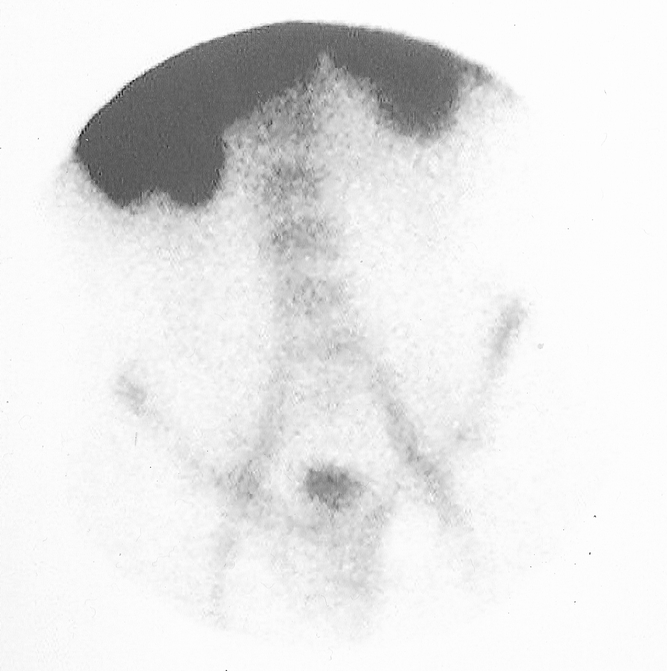
FIG. 3. LeuTech scan performed in a 26-year-old woman with atypical clinical presentation of appendicitis but with right lower quadrant tenderness and elevated WBC count of 15.5. Initial management plan was to operate for probable appendicitis. This anterior LeuTech image performed 60 minutes following injection was negative for appendicitis. Following the negative scan interpretation, the management plan was altered and the patient was discharged from the Emergency Department and followed as an outpatient. The patient did not have appendicitis during a 2 week follow up period.
Patient Management
Following the initial history, physical examination, and complete blood count, the attending surgeon completed a prescan questionnaire selecting one of three management tracks; immediate surgery, hospital admission for observation, or discharge home. Immediately following completion of the scan, the attending surgeon was asked to incorporate the scan results into a new management plan and complete the same management questionnaire (postscan). A case was not evaluable for impact on management if the same surgeon did not complete the prescan and postscan questionnaires.
Final Diagnosis
Each patient was determined to be positive or negative for appendicitis based on the final institutional diagnosis. Positive cases required a histopathology report indicating appendicitis using the strict criteria reported previously;18 negative cases were required to have a histopathology report negative for appendicitis, or a minimum of 2-week clinical follow-up without appendicitis. The pathologist was blinded to the results of the LeuTech scan. The results of other diagnostic studies were also used to determine final diagnosis in patients not undergoing surgery. A diagnosis of nonspecific abdominal pain of unknown etiology was assigned to those patients whose symptoms resolved without receiving specific treatment and in whom a specific cause of abdominal pain was not identified.
Outcome Measures
Efficacy
Diagnostic efficacy of LeuTech scintigraphy was assessed by comparing scan results to final diagnosis. Efficacy parameters (sensitivity, specificity, accuracy, NPV, and PPV) were evaluated for the entire evaluable population and for specific demographic subgroups including children, elderly, females, and males.
Patient management
The impact of LeuTech imaging on intended patient management was evaluated by comparing the prescan to the postscan management questionnaires. Shifts in management were then compared to optimal patient management based upon final diagnosis and outcome.
RESULTS
Demographics
Of the 203 patients enrolled, 200 (121 females, 79 males) were evaluable. There were two patients with negative scans who were subsequently lost to follow-up and one patient was not evaluable because the attending surgeon operated on the patient prior to completion of 30 minutes of imaging. Review of these images found them to be diagnostic, but the patient was excluded due to the predefined protocol criterion. Overall, 152 (76%) of evaluable patients were adults greater than or equal to 18 years and 48 (24%) were children from 5 to 17 years. Baseline demographics and frequency distributions of signs and symptoms of equivocal presentation are summarized in Table 2.
Table 2. BASELINE DEMOGRAPHICS
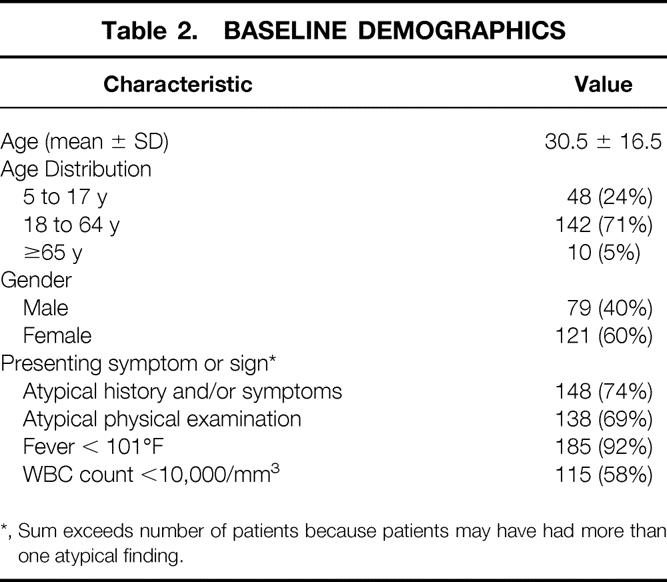
*, Sum exceeds number of patients because patients may have had more than one atypical finding.
Surgical Results
A total of 74 patients underwent surgery with a preoperative diagnosis of acute appendicitis. Fifty-nine patients had a final diagnosis of acute appendicitis confirmed by histopathology. Thirteen of the 59 (22%) patients with acute appendicitis had a perforated appendix. Seven patients had surgical findings of other pathology requiring surgical intervention, including two patients with periappendicitis and one each with a ruptured bladder, peritonitis, Crohn’s ileitis, perforated gallbladder, and perforated colon cancer. Eight patients underwent exploratory laparotomy, for which there were no findings to support surgical intervention. The negative laparotomy rate was 11% (8 of 74).
LeuTech Scan Results
LeuTech imaging identified 53 of 59 cases of appendicitis for sensitivity of 91% and was negative in 122 of 141 patients without appendicitis for specificity of 87%(Table 3). The overall accuracy of LeuTech imaging for acute appendicitis was 88%, with PPV and NPV of 74% and 95%, respectively.
Table 3. DIAGNOSTIC EFFICACY OF LEUTECH IMAGING FOR ACUTE APPENDICITIS
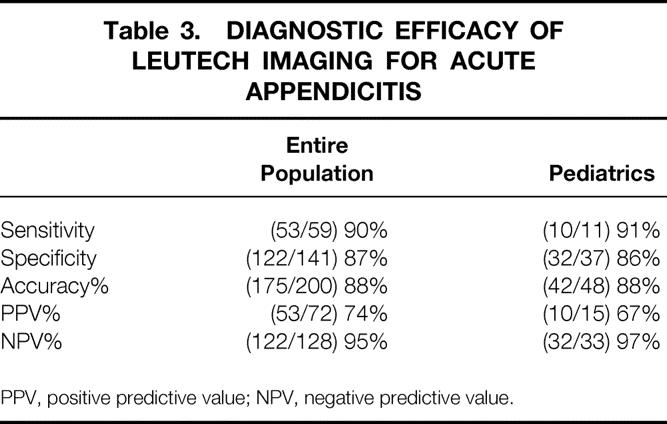
PPV, positive predictive value; NPV, negative predictive value.
The diagnostic efficacy of LeuTech imaging in the pediatric subpopulation is presented in Table 3. In the subset of 48 pediatric patients, 11 had appendicitis and 37 did not have appendicitis. Sensitivity for LeuTech imaging was 91% and specificity was 86%. Although only 10% of patients were older than 65 years of age, the sensitivity and specificity values in this age group were similar to those compared to the whole population, but the numbers are too small for meaningful statistical analysis. There were no differences in diagnostic efficacy of LeuTech based on gender.
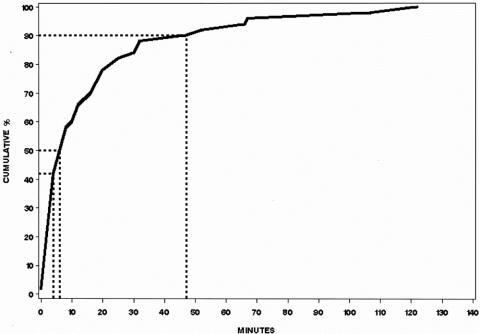
A diagnosis of appendicitis was made within 90 minutes of the LeuTech injection in all patients with a true positive scan. Moreover, positive scans were diagnostic for appendicitis within 8 minutes post-injection in 50% of patients and within 47 minutes in 90% of patients (Figure 4).
FIG. 4. Time from LeuTech injection to scintigraphic diagnosis of acute appendicitis in patients with appendicitis and positive scans. 50% of scans were read as positive for appendicitis within the first 8 minutes post-injection and 90% by 47 minutes.
Impact on Patient Management
Intended patient management decisions were examined in those patients who either had appendicitis or who did not have appendicitis or other right lower quadrant inflammatory conditions. The management was significantly (P < .001) impacted following the LeuTech scan results in 168 evaluable cases where the same surgeon completed the prescan and postscan questionnaire (Figure 5). Prior to the scan only 35% (19) of the patients ultimately found to have appendicitis would have been operated upon had the scan not been available. Following the scan 89% (47) immediately underwent surgery; the remainder were observed in the hospital and ultimately underwent appendectomy. There were five patients with appendicitis who would have been sent home from the emergency department according to the prescan questionnaire. LeuTech was positive for appendicitis in four of these five patients, eliminating potential delay in treatment and probable perforation.
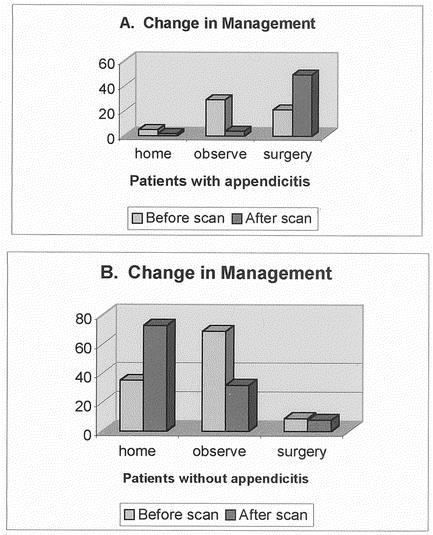
FIG. 5. Impact of LeuTech imaging on patient management. 168 patients were included in this analysis; 10 patients with pre- and post questionnaires completed by different surgeons, 1 patient with a pre-scan questionnaire completed after imaging and 12 patients with other right lower quadrant inflammatory or infectious conditions were excluded. Figure 5a illustrates the shift in patient management occurring after a LeuTech scan in patients with appendicitis. Most pronounced shift was a dramatic shift in patients who would have been admitted for observation to immediate surgery. Figure 5b illustrates the shift in patient management occurring after a LeuTech scan in patients without appendicitis or other right lower quadrant inflammatory disease. There was a dramatic shift from hospital admission for observation to discharge and follow up as an outpatient.
In patients without appendicitis, a similar shift in management occurred following the scan results. Only 31% (35) of the patients without appendicitis would have been sent home had the scan not been available. Following a negative scan, 64% (73) were sent home. Nine patients without appendicitis would have gone to surgery for appendicitis based on the prescan questionnaire. The postscan questionnaire results indicated that five of these patients were shifted to discharge and two to hospital admission for observation. There was no shift in management in four of these patients despite the negative scan, with the result that these patients underwent negative laparotomy for appendicitis.
Patient management was adversely impacted following LeuTech imaging in only 4 out of 200 patients. As a result of false positive scans, three underwent laparotomy. No surgical pathology was found, and as a result of a false negative scan, one patient was sent home with appendicitis. This patient returned and eventually underwent appendectomy without evidence of perforation or extended hospital stay.
Adverse Events
No serious or severe adverse events were reported. Of the 203 patients injected with LeuTech, 17 (8%) reported 20 mild and 4 moderate adverse events. None of these events were reported as definitely drug related. The most commonly reported adverse events were vasodilation (n = 8), dyspnea (n = 3), syncope (n = 2), headache (n = 2), and dizziness (n = 2). Nine (4.4%) patients experienced significant changes in vital signs; however, none was assessed as drug related.
DISCUSSION
The current management of the patient with equivocal signs and symptoms of appendicitis typically involves hospital admission for observation until the diagnosis is clarified, or for patients at particularly high risk, early operation. Because of their low negative predictive value, most adjunctive imaging tests are helpful in this clinical setting only if they are positive. Ultrasonography is one such test with a negative predictive value insufficient to permit patients with a negative study to be discharged from the Emergency Department and followed as outpatients. It has been reported that preoperative ultrasound or barium enema in a series of patients did not reduce the number of negative laparotomies or lower the perforation rate. 6,19 These types of imaging studies may be useful in individual cases, but have not been proven to affect overall patient outcome. In contrast, helical CT has demonstrated high sensitivity and specificity in certain institutions. 19,20 This imaging technique, however, requires the use of oral and rectal contrast media, transforming a noninvasive test into a somewhat invasive test. Waiting for contrast to reach the appendix in patients with an ileus potentially delays results. Additionally, the instillation of contrast into the rectum is inconvenient and uncomfortable. 99mTc-HMPAO labeled WBC imaging has also been evaluated for diagnosis of equivocal appendicitis. 10, 11 This test was found to have high sensitivity (98%) and negative predictive value (98%), though a positive scan was not highly specific for appendicitis. Nonetheless, surgeons who received a positive scan result generally had a heightened degree of suspicion and treated the patient accordingly. The unique value of this test is that a negative result permits the surgeon to rule out appendicitis and other intraabdominal inflammatory conditions.
Despite these advantages, 99mTc-HMPAO labeled WBC imaging for appendicitis has not gained widespread use because of inherent disadvantages. Radiolabeled WBC’s are prepared by time-consuming, labor-intensive techniques generally requiring special equipment and well-trained personnel. In many centers the blood must be sent to an offsite radiopharmacy for cell separation and radiolabeling, increasing the cost of the study, potentially placing the cells in adverse environmental conditions and prolonging the time to make a diagnosis. In many circumstances, there may be a delay of up to 3 or 4 hours for preparation of radiolabeled WBC’s added on to the 2 hours of imaging that this agent requires. The major risk attributed to handling blood during these ex vivo cell labeling procedures is exposure of the nuclear medicine technologists and radiopharmacists to the hazards of blood-borne infections such as hepatitis and HIV. Additionally, reinjection of radiolabeled WBC’s carries a known risk of misadministration into the wrong patient, prompting some centers and radiopharmacies to limit the number of WBC labeling procedures to one every 2 hours.
The agent under investigation in this study, LeuTech, addresses many of these disadvantages by eliminating the risks and inefficiency associated with 99mTc-HMPAO labeled WBC imaging. In preclinical studies, LeuTech had a high safety profile and did not elicit an elevation in HAMA levels, frequently found in other monoclonal antibodies. 15-17 Furthermore, in Phase 2 clinical studies at this center LeuTech demonstrated sensitivity of 100%, specificity of 82% and accuracy of 93% for detection of appendicitis. 18 Following LeuTech imaging 79% of patient management decisions were correct compared with 21% without the scan, resulting in significantly reduced unnecessary hospital admissions and unnecessary surgery.
This paper reports the results from a larger prospective multicenter clinical trial evaluating LeuTech imaging for equivocal appendicitis in a similar patient population. The results reported here demonstrate that LeuTech was convenient, sensitive, and well tolerated for detecting appendicitis in patients with equivocal signs and symptoms, corroborating our previous study. Importantly, patient management decisions based on comparisons of pre- and post-LeuTech imaging management plans significantly improved diagnostic accuracy and clinical management in adult and pediatric patients with an equivocal presentation.
Time to diagnosis is an important consideration for managing patients with suspected acute appendicitis. LeuTech images provided rapid diagnostic results, with 50% of the images for true-positive cases becoming evident within 8 minutes of injection and 90% within 47 minutes. Investigators’ evaluations of LeuTech scans relative to final institutional diagnosis demonstrated high sensitivity (90%) and specificity (86%). These results also indicate consistency of performance among investigators with varying levels of expertise with labeled white blood cell scintigraphy across multiple clinical sites. Most (8 out of 10) clinical trial sites had been using helical CT or ultrasound to diagnose appendicitis and were unfamiliar with radiolabeled WBC imaging for appendicitis. Only one site had previous experience with LeuTech imaging prior to starting this study.
In this study, LeuTech imaging again demonstrated a remarkably high negative predictive value (96%). This is particularly important if a study is to be used as a screening test. A high negative predictive value is often useful to surgeons because it provides for increased confidence in the intended patient management by avoiding either unnecessary time in the hospital for observation or, in some cases, inappropriate surgery. Overall, the diagnostic efficacy data in this multicenter clinical trial were consistent with the data from our earlier pilot study.
The impact of LeuTech imaging on intended patient management was clinically important. The referring surgeons’ intended patient management decisions made before and after completion of the LeuTech scan demonstrated a significant shift (P < .001) toward improved management. This was true in both patients with and without appendicitis, in adults and children, and in males and females. The negative laparotomy rate was quite low in this trial (11%; n = 8), comparing favorably to widely reported negative laparotomy rates between 15% and 30%, although some reports place this figure to be as high as 45%. 6 A negative laparotomy rate as low as 11% would be impressive even in a typical population, and is particularly impressive considering this was an equivocal patient population.
LeuTech performed equally well in pediatric and adult patients. Pediatric patients often have higher rates of negative laparotomy, perforation, and morbidity than adult patients with equivocal signs and symptoms of appendicitis. 21 The diagnostic efficacy of LeuTech imaging reported in the pediatric patients is promising, as this population historically is more difficult to diagnose with appendicitis than are patients in other age groups. These difficulties may be minimized with early utilization of LeuTech imaging.
Overall, LeuTech was well tolerated in this trial and was shown to be safe with no serious adverse events reported in 203 patients. LeuTech has definite advantages over currently available diagnostic agents for acute appendicitis. LeuTech is a neutrophil-specific agent with a high affinity for sequestered and circulating neutrophils. 14 This agent can be used with standard nuclear medicine equipment, is readily available in community hospitals and does not require special training or skills on the part of the nuclear medicine technologist or physician. The LeuTech kit allows for easy preparation and eliminates the need for handling of patient’s blood. Images produced with this agent are highly sensitive, with rapid diagnostic uptake and no requirements for SPECT imaging. It is proposed that emergency department physicians and surgeons will find the use of LeuTech prior to hospital admission to be beneficial in patients with an equivocal presentation of appendicitis in order to facilitate immediate and more cost-effective patient management.
CONCLUSION
LeuTech scintigraphic imaging is convenient, rapid, and sensitive for the detection of acute appendicitis in patients presenting with equivocal signs and symptoms. LeuTech imaging is safe. Patient management was substantially improved with the use of this novel diagnostic agent in both adults and children with suspected appendicitis.
ACKNOWLEDGMENT
This study was performed as part of a phase III clinical trial of 99mTc-labeled anti-CD 15 (LeuTech). The authors thank Palatin Technologies, Inc., Princeton, New Jersey for supplying the LeuTech.
Footnotes
This study was supported by a clinical research grant from Palatin Technologies, Inc., Princeton, NJ.
Correspondence: Samuel L. Kipper, MD, Assistant Clinical Professor of Radiology, University of California, San Diego, Department of Nuclear Medicine, Tri-City Medical Center, 4002 Vista Way, Oceanside, California 92056.
E-mail: samsalmon@aol.com
Accepted for publication July 6, 2001.
References
- 1.Addiss DG, Shaffer N, Fowler BS, et al. The epidemiology of appendicitis and appendectomy in the United States. Am J Epidemiol 1990; 132: 910–925. [DOI] [PubMed] [Google Scholar]
- 2.Eriksson S. Acute appendicitis-ways to improve diagnostic accuracy. Eur J Surg 1996; 162: 435–442. [PubMed] [Google Scholar]
- 3.Farthmann EH, Schöffel U. Principles and limitations of operative management of intraabdominal infections. World J Surg 1990; 14: 210–217. [DOI] [PubMed] [Google Scholar]
- 4.Graffeo CS, Counselman FL. Appendicitis. Emerg Med Clin North Am 1996; 14: 653–671. [DOI] [PubMed] [Google Scholar]
- 5.Rusnak RA, Borer JM, Fastow JS. Misdiagnosis of acute appendicitis: common features discovered in cases after litigation. Am J Emerg Med 1994; 12: 397–402. [DOI] [PubMed] [Google Scholar]
- 6.Sarfati MR, Hunter GC, Witzke DB, et al. Impact of adjunctive testing on the diagnosis and clinical course of patients with acute appendicitis. Am J Surg 1993; 166: 660–665. [DOI] [PubMed] [Google Scholar]
- 7.Wilcox RT, Traverso LW. Have the evaluation and treatment of acute appendicitis changed with new technology? Surg Clin North Am 1997; 77: 1355–1370. [DOI] [PubMed] [Google Scholar]
- 8.Dolgin SE, Beck AR, Tartter PL. The risk of perforation when children with possible appendicitis are observed in the hospital. Surg Gynecol Obstet 1992; 175: 320–324. [PubMed] [Google Scholar]
- 9.Pearl RH, Hale DA, Molloy M, et al. Pediatric appendectomy. J Pediatr Surg 1995; 30: 173–181. [DOI] [PubMed] [Google Scholar]
- 10.Rypins B, Evans DG, Hinrichs W, et al. Tc-99m-HMPAO white blood cell scan for diagnosis of acute appendicitis in patients with equivocal clinical presentation. Ann Surg 1997; 226: 58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rypins EB, Kipper SL. Tc-99m-hexamethylpropyleneamine (Tc-WBC) scan for diagnosing acute appendicitis in children. Am Surg 1997; 63: 878–881. [PubMed] [Google Scholar]
- 12.Kao DH, Lin HT, et al. Tc-99m HMPAO-labeled WBC scans to detect appendicitis in women. Clin Nuc Med 1996; 221: 768–771. [DOI] [PubMed] [Google Scholar]
- 13.Evetts BK, Foley CR, Latimer RG, et al. Tc-99 hexamethylpropyleneamineoxide scanning for the detection of acute appendicitis. J Am Coll Surg 1994; 79: 197–201. [PubMed] [Google Scholar]
- 14.Thakur ML, Richard FW III. Monoclonal antibodies as agents for selective radiolabeling of human neutrophil. J Nucl Med 1988; 29: 1817–1825. [PubMed] [Google Scholar]
- 15.Gratz S, Behr T, Herrmann A, et al. Intraindividual comparison of 99mTC-labelled anti-SSEA-1 antigranulocyte antibody and 99mTc-HMPAO labeled white blood cells for the imaging of infection. Eur J Nucl Med 1998; 25: 386–393. [DOI] [PubMed] [Google Scholar]
- 16.Thakur ML, Marcus CS, Henneman P, et al. Imaging inflammatory diseases with neutrophil-specific technetium-99m-labeled monoclonal antibody Anti-SSEA-1. J Nucl Med 1996; 37: 1789–1795. [PubMed] [Google Scholar]
- 17.Mozley PD, Stubbs JB, Dresel SH, et al. Radiation dosimetry of a 99mTc-labeled IgM murine antibody to CD15 antigens on human granulocytes. J Nucl Med 1999; 40: 625–630. [PubMed] [Google Scholar]
- 18.Kipper SL, Rypins EB, Evans DG, et al. Neutrophil-specific Tc-99m-labeled anti-CD 15 monoclonal antibody imaging for diagnosis of equivocal appendicitis: clinical evaluation of safety, efficacy and time performance characteristics. J Nucl Med 2000; 41: 449–455. [PubMed] [Google Scholar]
- 19.Garcia Pena BM, Mandl KD, Kraus SJ, et al. Ultrasonography and limited computed tomography in the diagnosis and management of appendicitis in children. JAMA 1999; 15: 1041–1046. [DOI] [PubMed] [Google Scholar]
- 20.Rao PM, Rhea JT, Novelline RA, et al. Effect of computed tomography of the appendix on treatment of patients and use of hospital resources. N Engl J Med 1998; 338: 141–146. [DOI] [PubMed] [Google Scholar]
- 21.Rothrock SG, Skeoch G, Rush JJ, et al. Clinical features of misdiagnosed appendicitis in children. Ann Emerg Med 1991; 20: 45–50. [DOI] [PubMed] [Google Scholar]