Abstract
Objective
To show that residual pancreatitis delays gastric emptying, the authors used surgical specimens and studied gastric stasis after pylorus-preserving pancreaticoduodenectomy (PPPD).
Summary Background Data
Delayed gastric emptying is a leading cause of complications after PPPD, occurring in 30% of patients. The pathogenesis of delayed gastric emptying remains unclear.
Methods
Surgical specimens of the pancreas from 25 patients undergoing PPPD and pancreaticogastrostomy were collected and examined by microscopy according to progressive pancreatic fibrosis and divided into three groups: no fibrosis, periductal fibrosis, and intralobular fibrosis. The authors then measured gastric output from the nasogastric tube, pancreatic output from the pancreatic tube, and the time until patients tolerated a solid diet.
Results
Pancreatic juice output was significantly related to the degree of pathologic findings, and gastric output was inversely related to them. A significant prolongation of postoperative solid diet tolerance correlated with increased pancreatic fibrosis and gastric fluid production.
Conclusions
Pancreatic fibrosis and increased gastric fluid production correlate with delayed gastric emptying after PPPD with pancreaticogastrostomy.
Since the time of the surgical pioneers in the field, Traverso and Longmire, 1 pylorus-preserving pancreaticoduodenectomy (PPPD) has been considered a good alternative to the Whipple procedure 2 for the resection of malignant or benign disease in the pancreaticoduodenal region. However, delayed gastric emptying (DGE) has been reported as a frequent complication of PPPD, with an incidence of 19% to 70% (average 30%). 3–9 This complication is not life-threatening, but it results in a prolonged length of stay and contributes to increased medical costs.
The pathogenesis of DGE after PPPD remains unclear, but several factors have been suggested. 10–13 Some European and American surgeons do not blame pylorus preservation but rather their own pancreatic anasto-motic technique. 5–7 In our series, we found DGE in about 10% of our patients without pancreatic leakage, and the gastric output was inversely related to pancreatic juice output.
We hypothesized that residual pancreatic fibrosis secondary to chronic pancreatic duct obstruction would lead to DGE. To investigate this possibility, we studied the pathologic findings of surgical specimens of resected pancreas, measured gastric output and pancreatic juice output, and determined time until a solid diet was tolerated.
PATIENTS AND METHODS
Patients
Between 1994 and 1996, 8 patients underwent our procedure at Aichi Prefectural Hospital, and between 1996 and July 2000, 24 patients underwent the same operation at Kainan Hospital. Four patients refused to permit a nasogastric tube for 5 postoperative days, two patients received extended pancreaticoduodenectomy because of cancerous involvement of the peripheral pancreas, and one patient drank water without permission within 5 postoperative days. Therefore, 25 patients qualified for our study. The diagnosis was carcinoma of the pancreatic head in 16 patients, carcinoma of the ampulla of Vater in 3, carcinoma of the lower bile duct in 2, intraductal papillary adenocarcinoma of the pancreas in 1, carcinoma of the duodenum in 1, malignant gastrointestinal stromal tumor of the duodenum in 1, and chronic pancreatitis in 1. There were 17 male and 8 female patients, ranging in age from 43 to 80 years (median 65). After receiving a full explanation of the purpose, procedure, and risks of the operation, all patients signed an informed consent statement.
Surgical Procedure
In 25 patients the pancreas was transected at the neck, preserving about a 2-mm length of the pancreatic duct from the pancreatic incision. After the resection, we performed our method of reconstruction (Fig. 1). Our modification of PPPD involved pancreatogastrostomy and a unique route of pancreatic duct exclusion. 14 The pancreatic duct was intubated with a polyethylene tube (S.B. Medical Co. Ltd., Tokyo, Japan) and secured tightly by a 4-0 absorbable monofilament suture. Then pancreatogastrostomy was performed as follows. The seromuscular layer of the posterior gastric wall was incised for about 4 cm in length. A row of interrupted 3-0 silk sutures was placed between the pancreatic edge and the seromuscular layer of the stomach. The tube was 65 cm long, 5F to 7.5F in diameter, similar to Rodney Smith type of tubes, and exteriorized through the stomach, jejunum, hepatic duct, liver, and the anterior abdominal wall to prevent spontaneous removal of the tube. Thus, pancreatic juice was completely discharged extracorporally through the pancreatic tube for 3 weeks.
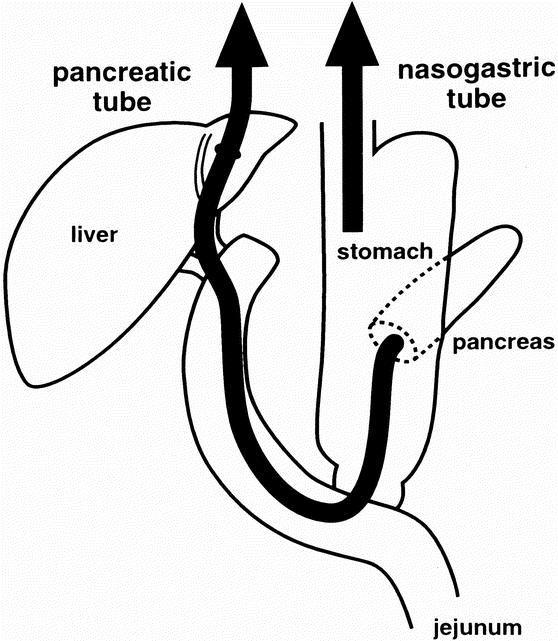
Figure 1. Our gastrointestinal reconstruction after pylorus-preserving pancreaticoduodenectomy. Nasogastric tube drainage was needed for as little as 5 postoperative days, and transhepatic catheters were used to drain pancreatic juice for 21 postoperative days.
A nasogastric tube (16F in diameter; Create Medic Co. Ltd., Yokohama, Japan) was inserted during the operation and removed as early as postoperative day 5. The amounts of discharged pancreatic juice and gastric juice were measured every day.
Pancreatic Morphology
Serial cross-sections of the neck of the pancreas were fixed in 20% neutral phosphate-buffered formalin for light microscopy. Paraffin sections were stained with hematoxylin and eosin. The patients were divided into three groups (no fibrosis, periductal fibrosis, or intralobular fibrosis) according to the progression of the pancreatic fibrosis of the resected specimens determined by two morphologists familiar with pancreatic histopathology. They were unaware of the clinical outcome and agreed on the classification. 15
The no fibrosis group (n = 8) was characterized by pancreatic edema and slight inflammatory cell infiltration into the parenchyma of the pancreas (Fig. 2). The periductal fibrosis group (n = 9) was characterized by periductal fibrosis and severe inflammatory cell infiltration. The intralobular fibrosis group (n = 8) was characterized by acinar necrosis, intralobular fibrosis, and inflammatory cell infiltration.
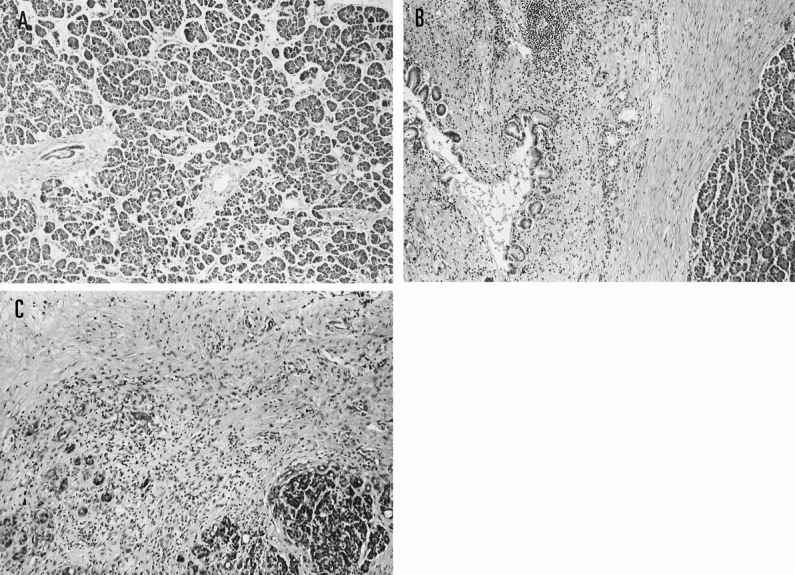
Figure 2. Surgical specimens of the pancreas in the three groups (hematoxylin and eosin, ×40). (A) No fibrosis group. Pancreatic edema and a few inflammatory cell infiltrations into the parenchyma of the pancreas are shown. (B) Periductal fibrosis group. Periductal fibrosis and many inflammatory cell infiltrations are seen. (C) Intralobular fibrosis group. Acinar necrosis, lobular fibrosis, and inflammatory cell infiltrations are seen.
Delayed Gastric Emptying
Gastric emptying is considered delayed when postoperative nasogastric suction is required for more than 7 days or the patient cannot tolerate a solid diet on or before postoperative day 14. 7
Nasogastric Intubation and Solid Diet
All patients had been fasting as early as postoperative day 5 because a nasogastric tube was inserted. It was removed when the gastric output had decreased to less than 300 mL/24 hours. When gastric emptying was delayed and the nasogastric tube had to be left in for more than 5 days, we chose the day of maximum output among the 5 consecutive days for analysis. Patients could take a liquid diet on the first day after the nasogastric tube was removed and, barring complaints, a solid diet on the second day.
Statistical Analysis
A one-way analysis of variance was performed for comparison among the groups using the StatView J 4.5 statistical package (Abacus Concepts, Berkeley, CA) on an iMac computer. All data are reported as mean ± standard deviation. Statistical significance was achieved at P < .05.
RESULTS
A total of 32 patients underwent the same operation at Aichi Prefectural Hospital and Kainan Hospital. The death rate was 0% and the complication rate was 22%. There were no pancreatic leakages, no biliary leakages, and no pancreatitis in the postoperative period. DGE was observed in four patients, cholangitis in two, and wound abscess in two. Three of the four patients with DGE were in the intralobular fibrosis group and one was from the periductal fibrosis group.
Twenty-five patients participated in our study. Their preoperative characteristics, intraoperative parameters, and histologic findings are summarized in Table 1. There were no significant differences among the three groups except for the diameter of the pancreatic duct, which is significantly related to the histologic progression of pancreatic fibrosis. There was no secondary pancreatitis caused by preoperative biliary drainage in our series.
Table 1. BASELINE CHARACTERISTICS OF PATIENTS
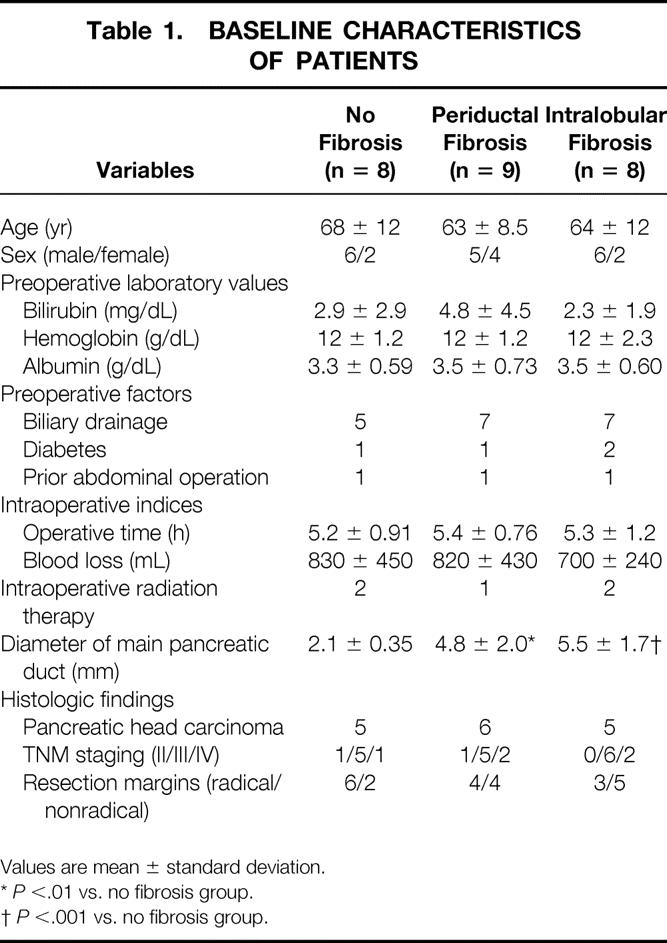
Values are mean ± standard deviation.
*P <.01 vs. no fibrosis group.
†P <.001 vs. no fibrosis group.
The pancreatic outputs for 10 days and the gastric outputs for 5 days are shown in Figure 3. The progressive pancreatic fibrosis at the resected pancreas was significantly related to the exocrine output volume of the remnant pancreas: 2,400 ± 920 mL in the no fibrosis group, 1,200 ± 670 mL in the periductal fibrosis group, and 270 ± 330 mL in the intralobular fibrosis group (P < .01).
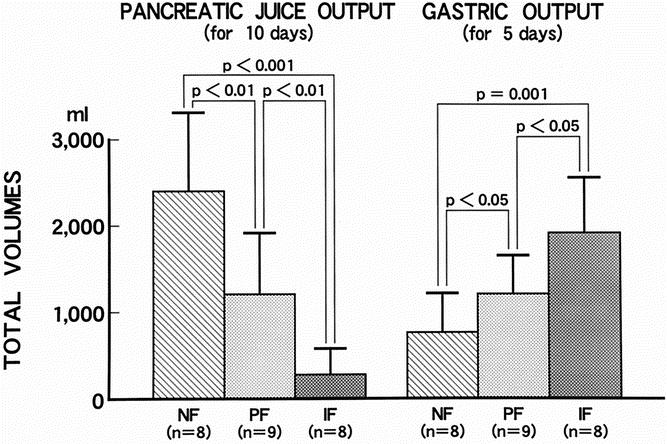
Figure 3. Pancreatic juice output and gastric output in the three groups. Progressive pancreatic fibrosis by histology was significantly associated with decreased pancreatic juice production and increased gastric output. NF, no fibrosis group; PF, periductal fibrosis group; IF, intralobular fibrosis group.
The gastric output was 760 ± 420 mL in the no fibrosis group, 1,200 ± 470 mL in the periductal fibrosis group, and 1,900 ± 650 mL in the intralobular fibrosis group. The gastric output was inversely related to the progressive pancreatic fibrosis with a significance of P < .05.
There was a significant prolongation of the days until a solid diet was tolerated orally in both the periductal and intralobular fibrosis groups versus the no fibrosis group (Fig. 4). The no fibrosis group spent 8.5 ± 1.3 days before a solid diet was tolerated, significantly shorter than the 12 ± 3.9 days in the periductal fibrosis group (P < .05) and the 16 ± 6.2 days in the intralobular fibrosis group (P < .01).
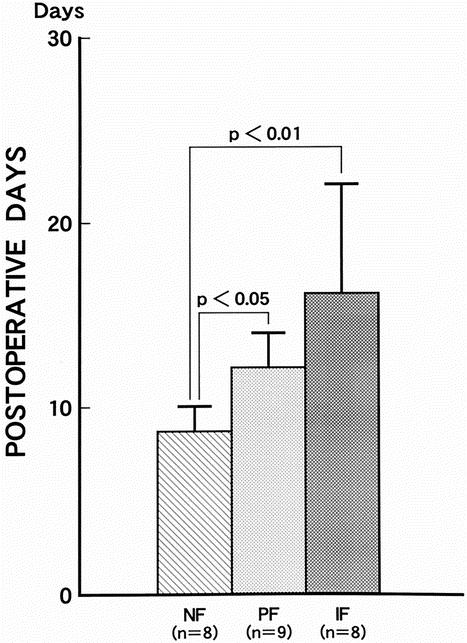
Figure 4. Time until a solid diet was tolerated in the three groups. There was a significant prolongation of postoperative solid diet toleration in both the periductal and intralobular fibrosis groups versus both the no fibrosis group. NF, no fibrosis group; PF, periductal fibrosis group; IF, intralobular fibrosis group.
DISCUSSION
When Warshaw and Torchiana 3 first reported DGE as a unique complication after PPPD in 1985, the incidence of DGE was 70%. In 1986, Braasch et al 4 reported that the incidence had been reduced to 45%. In 1990, Grace et al’s review 6 calculated the incidence as 27%. Although the most recent symposium reported by Yeo et al 9 in 1998 indicated an incidence of 19%, DGE remains a leading cause of complications. The reason for the decrease in the incidence rate was speculated to be due to the advances in surgical techniques that decreased the incidence of intraabdominal complications such as pancreatic fistula, anastomotic insufficiency, and intraabdominal abscess.
Several factors are thought to play a role in the pathophysiology of DGE: disruption of the gastroduodenal neural connection, 10 ischemic injury to the antropyloric muscle mechanism, 11 gastric atony in circulating levels of motilin in response to a reduction of the duodenum, 12 and gastric dysrhythmias secondary to an intraabdominal abscess. 13
As for neural disruption and ischemic injury, some authors have advocated preserving the right gastric artery because of its arterial supply to the pylorus and the proximal duodenum. 16,17 Others have suggested that ligation of the right gastric artery does not affect the antropyloric mechanism. 7,8 In our series, the right gastric artery was always cut, and the incidence of DGE was 13% (out of a total of 32 patients), no greater than in any other reports.
Resection of the duodenum, the primary production site of most gastrointestinal hormones, might play a role in the pathogenesis of this complication. Motilin, a gastrointestinal hormone, initiates interdigestive motility in the gastric antrum and small bowel and is produced primarily in the duodenum. 18,19 To date two clinical studies of motilin analogs have been performed on DGE after pancreatoduodenectomy. Yeo et al 20 reported that erythromycin reduced the incidence of DGE, and Matsunaga et al 21 reported that leucine 13-motilin decreased the gastric juice output. However, motilin analogs did not shorten the hospital stay or shorten the days until a solid diet was tolerated. At this time, the effectiveness of motilin analogs is not known.
Hocking et al 13 reported gastric dysrhythmias in a patient after PPPD and indicated that they may have been exacerbated by perigastric inflammation. We had hypothesized that these dysrhythmias might be caused by residual pancreatitis instead of an intraabdominal abscess. In our consecutive 32 patients, no pancreatic fistula occurred, and there was no abscess in the abdomen. Thus, we came to believe that perigastric inflammation in the remaining pancreas causes DGE.
When the main pancreatic duct was obstructed by malignant tumors at the proximal site of the pancreas, the pancreas upstream suffered obstructive pancreatitis. The obstruction of the main pancreatic duct was reported to damage the parenchymal cells of the pancreas and to induce inflammatory cell infiltration and promote pancreatic fibrosis upstream. 22–24 Banks et al, 25 who studied gastric acid secretion in patients with chronic pancreatitis, found that their basal acid output was high compared with that of controls. Further, Sato et al 26 found that the higher the degree of pancreatic fibrosis, the higher the acid output and serum gastrin levels tended to be. The increased gastric fluid production was related to the gastric emptying in patients with pancreatic fibrosis after PPPD.
A variety of methods for managing the pancreatic stump have been advocated, including ligation of the pancreatic stump, 27–29 pancreatic duct occlusion, 30 pancreaticojejunostomy, 31 and pancreaticogastrostomy. 32–34 The method of pancreatic duct ligation or occlusion is not recommended because it leads to a high incidence of fistula formation and has also provided a fertile field for DGE instead of avoiding the use of anastomosis. 35,36 Pancreatic drainage may be the best preventive method for DGE.
We conclude that residual pancreatic fibrosis is the most important cause of DGE after PPPD without pancreatic fistula formation.
Footnotes
Correspondence: Hiroya Murakami, MD, Kainan Hospital, 396 Yatomicho Amagun, Aichi 498-8502, Japan.
Accepted for publication May 25, 2001.
References
- 1.Traverso LW, Longmire WP Jr. Preservation of the pylorus in pancreaticoduodenectomy. Surg Gynecol Obstet 1978; 146: 959–962. [PubMed] [Google Scholar]
- 2.Whipple AO. Pancreaticoduodenectomy for islet carcinoma: 5-year follow-up. Ann Surg 1945; 121: 847–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Warshaw AL, Torchiana DL. Delayed gastric emptying after pylorus-preserving pancreaticoduodenectomy. Surg Gynecol Obstet 1985; 160: 1–4. [PubMed] [Google Scholar]
- 4.Braasch JW, Rossi RL, Watkins EJr, et al. Pyloric and gastric preserving pancreatic resection: experience with 87 patients Ann Surg 1986; 204: 411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hunt DR, McLean R. Pylorus-preserving pancreatectomy: functional results. Br J Surg 1989; 76: 173–176. [DOI] [PubMed] [Google Scholar]
- 6.Grace PA, Pitt HA, Longmire WP. Pylorus-preserving pancreatoduodenectomy: an overview. Br J Surg 1990; 77: 968–974. [DOI] [PubMed] [Google Scholar]
- 7.van Berge Henegouwewn MI, van Gulik TM, De Wit LT, et al. Delayed gastric emptying after standard pancreaticoduodenectomy versus pylorus-preserving pancreaticoduodenectomy: an analysis of 200 consecutive patients. J Am Coll Surg 1997; 185: 373–379. [DOI] [PubMed] [Google Scholar]
- 8.Hishinuma S, Ogata Y, Matsui J, et al. Complications after pylorus-preserving pancreatoduodenectomy with gastrointestinal reconstruction by the Imanaga method. J Am Coll Surg 1998; 186: 10–16. [DOI] [PubMed] [Google Scholar]
- 9.Yeo CJ, Hruban RH, Conlon KC, et al. Pancreatic cancer symposium. J Am Coll Surg 1998; 187: 429–442. [PubMed] [Google Scholar]
- 10.Tanaka M, Sarr M. Total duodenectomy: effect on canine gastrointestinal motility. J Surg Res 1987; 42: 483–493. [DOI] [PubMed] [Google Scholar]
- 11.Liberski SM, Koch KL, Atnip RG, et al. Ischemic gastroparesis: resolution after revascularization. Gastroenterology 1990; 99: 252–257. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka M, Sarr M. Role of the duodenum in the control of canine gastrointestinal motility. Gastroenterology 1988; 94: 622–629. [DOI] [PubMed] [Google Scholar]
- 13.Hocking MP, Harrison WD, Sninsky CA. Gastric dysrhythmias following pancreatoduodenectomy. Dig Dis Sci 1990; 35: 1226–1230. [DOI] [PubMed] [Google Scholar]
- 14.Murakami H, Yasue M. A vertical stomach reconstruction following pylorus-preserving pancreaticoduodenectomy. Am J Surg 2001; 181: 149–152. [DOI] [PubMed] [Google Scholar]
- 15.Howard JM, Nedwich A. Correlation of the histologic observations and operative findings in patients with chronic pancreatitis. Surg Gynecol Obstet 1971; 132: 387–395. [PubMed] [Google Scholar]
- 16.Grace PA, Pitt HA, Longmire WP. Pancreatoduodenectomy with pylorus preservation for adenocarcinoma of the head of the pancreas. Br J Surg 1986; 73: 647–650. [DOI] [PubMed] [Google Scholar]
- 17.Itani KM, Coleman RE, Meyers WC, et al. Pylorus-preserving pancreatoduodenectomy. A clinical and physiologic appraisal. Ann Surg 1986; 204: 655–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fox JE, Daniel EE, Robothan H. The mechanism of motilin excitation of the canine small intestine. Life Sci 1984; 34: 1001–1006. [DOI] [PubMed] [Google Scholar]
- 19.Vantrappen G, Janssens J, Peeters TL, et al. Motilin and the interdigestive migrating motor complex in man. Dig Dis Sci 1979; 24: 497–500. [DOI] [PubMed] [Google Scholar]
- 20.Yeo CJ, Barry MK, Sauter PK, et al. Erythromycin accelerates gastric emptying after pancreaticoduodenectomy: a prospective, randomized, placebo-controlled trial. Ann Surg 1993; 218: 229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsunaga H, Tanaka M, Naritomi G, et al. Effect of leucine 13-motilin (KW5139) on early gastric stasis after pylorus-preserving pancreatoduodenectomy. Ann Surg 1998; 227: 507–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohshio G, Saluja A, Steer ML. Effects of short-term pancreatic duct obstruction in rats. Gastroenterology 1991; 100: 196–202. [DOI] [PubMed] [Google Scholar]
- 23.Lerch MM, Saluja AK, Runzi M, et al. Pancreatic duct obstruction triggers acute necrotizing pancreatitis in the opossum. Gastroenterology 1993; 104: 853–861. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka T, Miura Y, Matsugu Y, et al. Pancreatic duct obstruction is an aggravating factor in the canine model of chronic alcoholic pancreatitis. Gastroenterology 1998; 115: 1248–1253. [DOI] [PubMed] [Google Scholar]
- 25.Banks PA, Dyck WP, Dreiling MA, et al. Secretory capacity of stomach and pancreas in man. Gastroenterology 1967; 53: 575–578. [PubMed] [Google Scholar]
- 26.Sato T, Kameyama J, Sasaki I, et al. Gastric acid secretion and serum gastrin levels in chronic pancreatitis. Gastroenterol Jpn 1981; 16: 93–99. [DOI] [PubMed] [Google Scholar]
- 27.Dreyer BJ, Mararis JS. Pancreatoduodenectomy with ligation of pancreatic duct. Arch Surg 1962; 85: 465–469. [DOI] [PubMed] [Google Scholar]
- 28.Goldsmith HS, Ghosh BC, Huvos AG. Ligation versus implantation of the pancreatic duct after pancreaticoduodenectomy. Surg Gynecol Obstet 1971; 132: 87–92. [PubMed] [Google Scholar]
- 29.Powis SJA, Young HB. A modified pancreaticoduodenectomy. Surg Gynecol Obstet 1973; 137: 259–262. [PubMed] [Google Scholar]
- 30.Flautner L, Tihanyi T, Bock G, et al. A new approach, pylorus-preserving pancreatoduodenectomy, in the surgical treatment of diseases of the pancreas head. Orv Hetil 1982; 123: 1181–1183. [PubMed] [Google Scholar]
- 31.Howard JM. Pancreatojejunostomy: leakage is preventable complication of the Whipple resection. J Am Coll Surg 1997; 184: 454–457. [PubMed] [Google Scholar]
- 32.Mackie JA, Rhoads JE, Park CD. Pancreaticogastrostomy. A further evaluation. Ann Surg 1975; 181: 541–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Icard P, Dubois F. Pancreaticogastrostomy following pancreatoduodenectomy. Ann Surg 1988; 207: 253–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Delcore R, Thomas JH, Pierce GE, et al. Pancreatogastrostomy: a safe drainage procedure after pancreatoduodenectomy. Surgery 1990; 108: 641–647. [PubMed] [Google Scholar]
- 35.Aston SJ, Longmire WP. Pancreaticoduodenal resection. 20 years experience. Arch Surg 1988; 207: 253–256. [DOI] [PubMed] [Google Scholar]
- 36.Papachristou DN, D’Agostino H, Fortner JG. Ligation of the pancreatic duct in pancreatectomy. Br J Surg 1980; 67: 260–262. [DOI] [PubMed] [Google Scholar]


