Abstract
Objective
Comparison of cultured skin substitutes (CSS) and split-thickness skin autograft (AG) was performed to assess whether donor-site harvesting can be reduced quantitatively and whether functional and cosmetic outcome is similar qualitatively in the treatment of patients with massive cutaneous burns.
Summary Background Data
Cultured skin substitutes consisting of collagen-glycosaminoglycan substrates populated with autologous fibroblasts and keratinocytes have been shown to close full-thickness skin wounds in preclinical and clinical studies with acceptable functional and cosmetic results.
Methods
Qualitative outcome was compared between CSS and AG in 45 patients on an ordinal scale (0, worst; 10, best) with primary analyses at postoperative day 28 and after about 1 year for erythema, pigmentation, pliability, raised scar, epithelial blistering, and surface texture. In the latest 12 of the 45 patients, tracings were performed of donor skin biopsies and wounds treated with CSS at postoperative days 14 and 28 to calculate percentage engraftment, the ratio of closed wound:donor skin areas, and the percentage of total body surface area closed with CSS.
Results
Measures of qualitative outcome of CSS or AG were not different statistically at 1 year after grafting. Engraftment at postoperative day 14 exceeded 75% in the 12 patients evaluated. The ratio of closed wound:donor skin areas for CSS at postoperative day 28 was significantly greater than for conventional 4:1 meshed autografts. The percentage of total body surface area closed with CSS at postoperative day 28 was significantly less than with AG.
Conclusions
The requirement for harvesting of donor skin for CSS was less than for conventional skin autografts. These results suggest that acute-phase recovery of patients with extensive burns is facilitated and that complications are reduced by the use of CSS together with conventional skin grafting.
Recovery from massive cutaneous burn injuries requires the critical and integrated care of the burn team. Among the many patient needs are fluid resuscitation, airway management and respiratory support, nutritional support, wound and microbial management, psychosocial management, and physical and occupational therapy. 1 Although comprehensive and complex care is provided by experienced teams at specialized care centers, the predominant cause of death from massive burns remains sepsis, 2 which is associated with open wounds. Therefore, rapid and permanent wound closure remains a limiting factor for reducing risk and increasing the probability of survival from extensive burn injuries.
Accomplishment of wound closure after full-thickness burns depends on three fundamental steps: removing the burned skin, covering the wound temporarily to reduce fluid loss and microbial infection, and grafting the wound with autologous epithelium to restore the barrier properties of the skin. Early excision of burn wounds decreases the release of toxic metabolites from the eschar and removes a rich medium for microbial outgrowth. 3 Temporary covers include biologic materials (e.g., cadaveric skin allograft), synthetic dressings (e.g., BioBrane [Bertek Pharmaceuticals; Morgantown, WV] or medicated gauze), or medical devices (e.g., Integra Artificial Skin [Integra Lifesciences; Plainsboro, NJ] or TransCyte [Advanced Tissue Sciences; La Jolla, CA]). 4,5 Each of these materials provides certain medical advantages to patients, but none provides the permanent repair of full-thickness skin that is required for indefinite restoration of systemic homeostasis. Permanent wound closure currently requires grafting of autologous epithelium to restore the epidermal barrier that forms in the stratum corneum of the skin. Split-thickness skin autograft 6,7 (AG) remains the treatment of choice for permanent closure of excised burns, but it has limited availability in large burns, and it generates a donor site that is painful during healing and that may scar to become a cause of long-term morbidity. 8 In response to these limitations, alternatives to AG have been developed to replace either the epidermal 9,10 or dermal 11,12 components of skin in separate surgical procedures, or to replace both components in a single procedure. 13,14 Combinations of materials have also been used to replace the anatomic structure and physiologic function of both the epidermis and dermis of the skin. 15,16 Assessments of outcome have included ordinal scoring by the Vancouver Scar Scale, 17 “percentage final take,”18 histopathology, 19 and percentage of total body surface area (TBSA) closed. 20 General benefits of alternatives to AG include, among others, reduced harvesting of donor skin for permanent wound closure, fewer surgical procedures to complete skin grafting, a shorter length of hospital stay, and fewer problems with cutaneous scars.
Previous studies from this laboratory have reported a cultured skin substitute (CSS) with anatomic and physiologic similarity to AG 21,22 but with greater availability. Preclinical studies have shown regeneration of epidermal barrier in vitro, restoration of pigmentation by addition of melanocytes to CSS, expression of growth factors that promote wound healing, 23–26 and genetic modification of CSS to guide and accelerate wound closure. 27,28 Clinical studies have reported development of histiotypic cutaneous markers after grafting, 29 comparable qualitative outcome between CSS and AG, 30 and successful combination of CSS with the dermal substitute Integra to replace both autograft and allograft for complete and permanent wound closure. 31 The present study was performed to evaluate whether CSS reduces the amount of donor skin required to complete wound closure, and to assess whether the qualitative outcome after grafting of CSS to excised, full-thickness burns is similar to treatment with meshed, split-thickness AG.
METHODS
Experimental Design
Comparative assessment of treatment of excised, full-thickness burns with CSS or AG was performed in a prospective, randomized, nonblinded design. The study of CSS for quantitative closure of burn wounds was performed with two groups of ordinate measures. First, assessment of qualitative outcome was performed to compare directly the treatment of paired sites of excised, full-thickness burns with either CSS or AG. Two comparative sites, approximately 150 cm2, were selected and designated A (right-most, upper-most, or front-most) and B (left-most, lower-most, or rear-most). Treatment with either CSS or AG was randomized before performance of the study to assign site A or B to each graft type according to patient enrollment number. Wherever possible, contralateral sites were used. Comparative sites did not include joints, hands, or faces. Sites were assessed by ordinal scoring (0, worst; 10, normal) at serial time points beginning at postoperative day (POD) 14 through 1 year or longer for erythema, pliability, raised scar, epithelial blistering, and surface texture. Pigmentation was also scored on an ordinal scale of 0 = hyperpigmented, 5 = normal pigmentation, and 10 = hypopigmented.
Second, the absolute values in cm2 of the TBSA and of partial-thickness and full-thickness burns for each patient were obtained from the medical record. Also, the absolute values in cm2 of donor skin for CSS, CSS grafted, and wounds treated with CSS on POD 14 and 28 were determined by direct tracing and computerized planimetry. Closed and open areas of wounds treated with CSS or AG were traced on POD 14 and 28 to determine the percentage of engraftment, the ratio of closed wound:donor skin areas, and the percentage of TBSA closed with CSS or AG. The objective of this group of ordinate measures was to determine whether treatment with CSS provided a statistically significant increase in the ratio of closed wound:donor skin areas compared with meshed, split-thickness AG, and with no statistical difference in the percentage of engraftment between the two graft types.
Patient Population
All patients in this study were enrolled by informed consent into a human subjects protocol that was approved by the Institutional Review Board of the University of Cincinnati, or the University of California Davis. Enrollment criteria included patients with greater than 50% TBSA full-thickness cutaneous burns. Forty-five patients were enrolled and evaluated for qualitative outcome between April 1990 and July 1999. These patients had (mean ± SEM) %TBSA full-thickness burns of 64.6% ± 2.0% and were age 10.6 ± 1.6 years; there were 34 males and 11 females. The latest 12 of the 45 patients were enrolled between April 1998 and July 1999 and were evaluated for quantitative ordinate measures in addition to qualitative outcome. These 12 patients had %TBSA full-thickness burns of 76.9% ± 2.76% and were age 8.0 ± 1.5 years; there were 8 males and 4 females.
One additional patient, a 7-year-old girl with 92% TBSA full-thickness burns, was enrolled by emergency permission and treated at the Shriners Hospital for Children in Northern California. She was treated with autologous CSS prepared in Cincinnati. This anecdotal case is not included in the quantitative analysis of data sets, but results are summarized below.
Preparation of Cultured Skin Substitutes
Biopsy samples of split-thickness skin were collected as early as possible after injury, usually during the first week of the hospital stay. The absolute areas (cm2) to be treated with CSS and for CSS biopsy for each patient were estimated using the formulas:
1a. %TBSA eligible for CSS = (%TBSA of full-thickness burn) - (40% TBSA treated with AG).
1b. Estimated TBSA (cm2) to be treated with CSS = %TBSA eligible for CSS * TBSA (cm2).
2. Estimated cm2 of CSS biopsy = Estimated TBSA (cm2) to be treated with CSS × 0.01.
This estimation is based on the absolute value (cm2) of TBSA full-thickness burn from the medical record, and on the assumption that full-thickness burn covering 40% TBSA can be closed with autograft during the time that CSS are being prepared.
Split-thickness skin for preparation of CSS was collected from each patient as early as possible after injury and transferred to the laboratory for cell culture. Keratinocytes were isolated from epidermis and fibroblasts were isolated from dermis of each biopsy sample and placed into selective cell cultures as described previously 32,33 in a 5% CO2/95% air atmosphere with saturated humidity at 37°C. After sufficient populations of each cell type were available, fibroblasts were harvested and inoculated at an approximate density of 5 × 105 cells/cm2 onto collagen-glycosaminoglycan substrates 34 and incubated at least 18 hours to allow cell attachment. Next, keratinocytes were harvested and inoculated at an approximate density of 1 × 106 cells/cm2, which was defined as incubation day 0 for CSS. CSS were incubated submerged in culture medium for 2 days. On incubation day 3, CSS were lifted to the air–liquid interface to stimulate formation of epidermal barrier. 23 CSS were usually scheduled for surgical application on incubation day 10 to 14, subject to patient condition. In preparation for grafting, CSS (approximately 40 cm2 each) were meshed at a ratio of 1.5:1, not expanded, placed in petri dishes with sufficient medium to avoid desiccation, and transported to the operating room. For one patient who was treated in Sacramento, CSS were packaged in sealed jars, immobilized with sterile gauze packing that was kept moist with irrigation solution, and sent by express delivery for surgical application the next day.
Wound Treatment, Surgery, and Postoperative Care
Burn eschar was excised as early as possible and sites planned for treatment with CSS were covered with cadaveric allograft or the dermal substitute Integra. 4,11 For excised burns covered with allograft, it was usually removed 1 day before grafting of CSS and AG and irrigated at alternating 2-hour intervals with a 5% (wt/vol) solution of mafenide acetate in water and a solution of 40 μg/mL neomycin and 200 U/mL polymyxin B in saline delivered through perforated red rubber catheters into bulky gauze. The next day, dressings were removed, hemostasis was obtained, and prepared wounds were irrigated with a solution of nutrients and antimicrobials. For excised burns covered with the dermal substitute Integra, the outer Silastic layer was removed to expose the vascularized wound bed, and prepared wounds were irrigated as above. The irrigation solution consisted of a modified formulation of nutrient medium MCDB 153 supplemented with 5 μg/mL human recombinant insulin, 0.5 μg/mL hydrocortisone, 40 μg/mL neomycin, 700 U/mL polymyxin B, 20 μg/mL mupirocin, 20 μg/mL ciprofloxacin, and 1 μg/mL amphotericin B. 35,36 After irrigation of prepared wounds, CSS were grafted using a backing of N-terface dressing (Winfield Laboratories, Richardson, TX) and stapled in place. Split-thickness AG, meshed at ratios between 1:1.5 and 1:4, was expanded and stapled to wounds. CSS and AG were dressed with fine mesh gauze and covered with bulky gauze containing perforated red rubber catheters that were secured with Spandex that was stretched to apply gentle pressure and to immobilize the grafted sites. After surgery, CSS were irrigated with the solution of nutrients and antimicrobials described above at a dosage of 30 mL per CSS graft three times per day for 5 to 7 days. Dressing changes for CSS were routinely performed on POD 2, 4, 5, and daily thereafter until POD 7 to 9. This routine was modified in selected cases to meet patients’ medical needs. Staples and N-terface were removed on POD 5 to 7, after which CSS were treated with an ointment consisting of equal parts Neosporin, Bactroban, and Nystatin (NBN) and covered with dry bulky gauze. Dry, keratinized areas were treated with Curel lotion, and wet areas were treated with NBN ointment on Adaptic until healing was complete. If healing was not complete by POD 22, routine wound care for AG was performed on CSS sites. AG was routinely irrigated for 5 days with alternating solutions of 5% (wt/vol) mafenide acetate in water and a solution of 40 μg/mL neomycin and 700 U/mL polymyxin B in saline. Dressing changes were routinely performed for AG on POD 2 and 5, at which time staples were removed, irrigations were discontinued, and dry dressings were applied. Dry dressings for AG routinely consisted of Adaptic coated with an ointment consisting of either 3 parts silver sulfadiazine and 1 part bacitracin if no fungal organisms were cultured from the grafts, or equal parts silver sulfadiazine, bacitracin, and nystatin if fungi were cultured.
Study Measurements and Data Collection
Study sites were assessed for parameters of quantitative coverage and qualitative outcome. Quantitative coverage was determined by planimetry of direct tracings at POD 14 and 28 of open and closed areas treated with CSS and AG, and of the biopsy sample collected for preparation of CSS. From these tracings, five ordinate measures were quantified: the percentage of treated area closed at POD 14 and 28; %TBSA closed at POD 14 and 28; the ratio of closed wound:donor skin areas at POD 28; correlation of the percentage of CSS area closed at POD 14 with %TBSA full-thickness burn; and correlation of %TBSA closed with CSS at POD 28 with %TBSA full-thickness burn.
Comparative sites were assessed by visual examination at POD 7 and 14 for color, keratinization, percentage of site covered, and percentage of sites with exudate. All of each treated field was scored. Color and keratinization were scored on an ordinal scale of 0 to 10 (0, worst result; 10, best result). Values for color and keratinization in heterogeneous sites were averaged from estimated percentages of each wound site multiplied by the ordinal score (e.g., [25% × 4] + [75% × 8] = 7).
Qualitative outcome was assessed by ordinal scoring of closed wounds for six ordinal measures: erythema, pigmentation, pliability, raised scar, epidermal blisters, and surface texture. Only healed areas of treated fields were scored. Scoring was performed for all ordinate measures, except pigmentation, on a scale of 0 to 10 (0, worst result; 10, best result). Scales ranged for erythema from purple to none, for pliability from tight to normal, for raised scar from 5 mm to flat, for epidermal blisters from many to none, and for surface texture from rough to smooth. Pigmentation was scored ranging from 0 for hyperpigmentation, to 5 for normal pigmentation, to 10 for hypopigmentation. Fractional values for qualitative outcome in heterogeneous sites were calculated as described above for site assessment of color and keratinization. Incidence of regrafting for graft failure was scored as an absolute event for each patient. Qualitative outcomes of CSS and AG sites were scored beginning on POD 14 and incrementally thereafter until approximately 1 year after grafting. Primary analyses were performed at POD 28 and about 1 year after grafting.
Calculations and Biostatistics
Quantitative measures were calculated by the formulas:
3. Percentage engraftment at POD 14 and 28 = (Area (cm2) closed with dry epithelium/total area treated (cm2)) × 100
4. Closed wound:donor skin areas at POD 28 = Area (cm2) closed with dry epithelium/Area (cm2) of donor biopsy
5. %TBSA closed at POD 14 and 28 = (Area (cm2) closed with dry epithelium/TBSA (cm2)) × 100
Means ± SEM were calculated for these measures. The percentage of engraftment at POD 14 was considered acceptable if greater than 80. The ratio of closed wound:donor skin areas was considered significant if it was greater than 4 by single-value t test.
After POD 14, data for qualitative outcome were collected from each paired site on each patient during five observation periods: 0.5 to 1 month, 2 months, 3 to 6 months, 7 to 12 months, and 13 months and later. Data were compiled in spreadsheets, analyzed, and subjected to statistical analyses using SigmaStat (Jandel Scientific, San Rafael, CA) and Statistical Analysis Software (SAS Institute, Cary, NC).
Primary analyses of data were performed on POD 28 for quantitative and qualitative end points, and at 1 year ± 1 month for qualitative outcome. Qualitative data sets were analyzed for overall significance by the Kruskal-Wallis test. If overall significance was found, then differences were subjected to the Wilcoxon rank-sum test. These ordinate measures included erythema, pigmentation, pliability, raised scar, epidermal blistering, and surface texture. Data from positive/negative scoring of site regrafting was subjected to the Fisher exact test.
RESULTS
Figure 1 shows the macroscopic and microscopic anatomy of CSS in vitro. By 10 to 14 days of incubation in vitro, CSS had sufficient mechanical strength to be handled easily with a backing material such as N-terface. Typical CSS were 35 to 40 cm2 in area and about 300 μm thick. The epithelium was well keratinized and attached biologically to the dermal substitute that was populated heavily with dermal fibroblasts. The collagen-glycosaminoglycan substrate in the dermal substitute was partially degraded. Development of the epidermal barrier in vitro provided immediate biologic protection to wounds at the time of grafting.
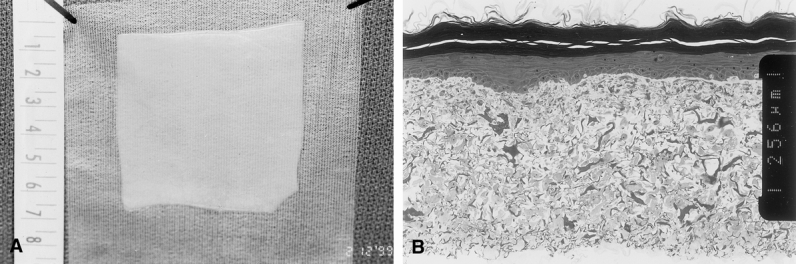
Figure 1. Composition of autologous cultured skin substitutes (CSS) in vitro. CSS were (A) approximately 40 cm2 in area and (B) approximately 0.3 mm thick. They consisted of a well-stratified and cornified epithelium attached biologically to a reticulated sponge of bovine skin collagen and chondroitin-sulfate populated with dermal fibroblasts. Scale = 0.256 mm between inset notches.
After grafting of CSS and AG to wounds, the clinical outcome in a patient with 90% TBSA burns at POD 73 is shown in Figure 2 A. Adjoining edges of CSS were detectable as thin linear scars with complete epithelial healing across the surfaces of the CSS. AG on the left abdomen was also epithelialized completely and hyperpigmented compared with the uninjured skin in the right axilla. The back of this patient was also treated and closed completely with CSS (see Fig. 2 B). Healed skin was soft, smooth, and strong with irregular pigmentation. In this patient, more than 25% TBSA was closed permanently with CSS. Irregularity of pigmentation is shown in this patient, who was treated with multiple preparations of CSS in serial applications. The lower chest and abdomen (see Fig. 2 C) were treated with CSS prepared earlier in the hospital course and were mostly pigmented. The back (see Fig. 2 D) was treated with CSS prepared later in the hospital course and was spotted with pigment within hypopigmented skin.
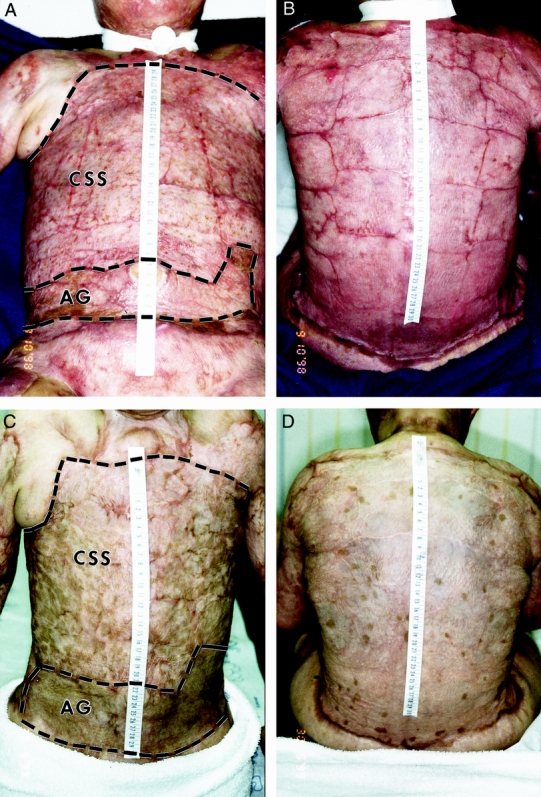
Figure 2. Clinical photos after grafting of cultured skin substitutes (CSS) and meshed, split-thickness skin autograft (AG) on a patient with 90% total body surface area burns. Anterior torso (A, C) includes comparative sites. Posterior torso (B, D) was treated entirely with CSS. (A, B) Postoperative days 73 and 59 respectively; (C, D) postoperative days 337 and 323, respectively. Grafted areas are within dashed lines. Scale in centimeters.
A higher incidence of regrafting was recorded for CSS (35.6%) than for AG (2.2%) in the 45 patients evaluated for qualitative outcome. These differences were statistically different at P < .05. The frequency of regrafting for CSS decreased to 16.6% (2/12) in the latest 12 patients, and that regrafting was partial, not total. No regrafting of AG was needed in these 12 patients.
Percentages of treated areas closed at POD 14 and 28 in the latest 12 patients are shown in Figure 3. Engraftment at POD 14 was 89.2% ± 2.5% for CSS and 94.9% ± 3.6% for AG; at POD 28 it was 95.4% ± 1.8% for CSS and 99.0% ± 0.8% for AG. Percentages of treated areas closed in these patients were not significantly different between groups at each time point. %TBSA closed at POD 14 was 15.4% ± 2.2% for CSS and 60.0% ± 1.6% for AG; at POD 28 it was 16.7% ± 2.6% for CSS and 58.7% ± 1.8% for AG. These data show that rates of engraftment for CSS and AG are comparable, but that most of the wound area in these patients was closed with AG.
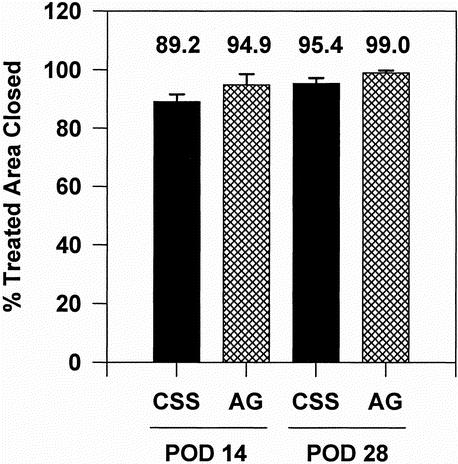
Figure 3. Percentage engraftment of cultured skin substitutes (CSS) and meshed, split-thickness skin autograft (AG) at postoperative days 14 and 28 (n = 12). No statistical differences were found between CSS and AG by repeated measures analysis of variance.
Figure 4 shows the ratio of closed wound:donor skin areas for CSS and AG at POD 28 in 12 patients. Wounds closed with CSS covered 66.8 ± 16.3 times the area of the donor biopsy. Values for AG were not calculated from tracings but compared with the conventional standard of 1:4 expansion of meshed autograft. These values were significantly different (P < .01) by a one-sample t test and demonstrate the conservation of donor skin by grafting of CSS in place of AG.
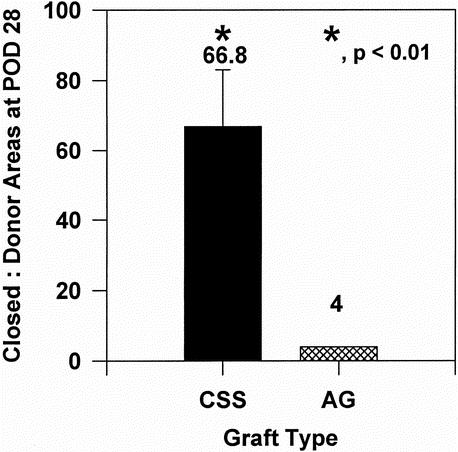
Figure 4. Donor-site utilization at postoperative day 28 (n = 12). Ratios of closed wound:donor skin areas for cultured skin substitutes (66.8 ± 16.3) and autograft (4.0 ± 0.0) were significant at the P < .01 level of confidence by one-sample t test.
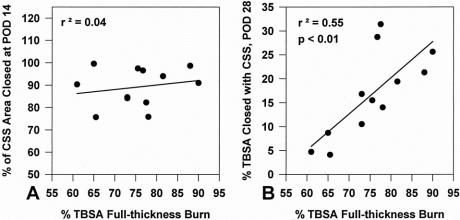
Figure 5 shows correlation of %TBSA full-thickness burn with the percentage of CSS area closed at POD 14 and %TBSA closed with CSS at POD 28 in 12 patients. No correlation (r2 = 0.04) was found between the percentage of CSS area closed at POD 14 and %TBSA full-thickness burn. A positive and significant (P < .05) correlation was found between %TBSA closed with CSS at POD 28 and %TBSA full-thickness burn. These results demonstrate that engraftment of CSS is independent of burn area and that the impact of CSS on wound closure increases proportionately with the magnitude of the wound area.
Figure 5. Correlation of the percentage total body surface area (%TBSA) full-thickness burn (n = 12) with (A) the percentage of site closed with cultured skin substitutes (CSS) at postoperative day 14 showed no statistical correlation and (B) %TBSA closed with CSS at postoperative day 28 showed a positive correlation that was statistically significant (P < .01).
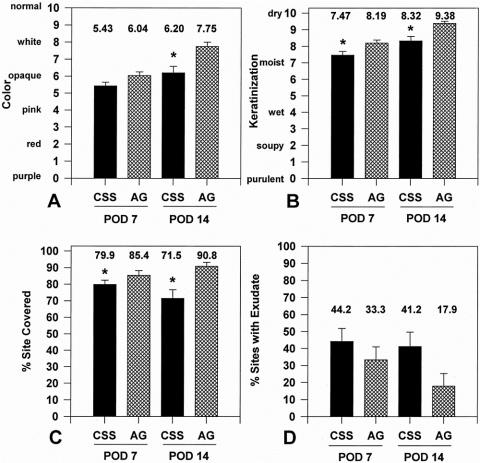
Figure 6 presents site assessments during the first 14 days after grafting from 45 patients. Both CSS and AG became less pink and whiter as keratinized epidermis formed between POD 7 and 14. CSS were pinker than AG at both POD 7 and 14. Keratinization of CSS was slower than AG in general, with CSS values lower than AG at both time points. Coverage of grafted sites for CSS decreased from 79.9% to 71.5%, indicating graft loss between POD 7 and 14, whereas coverage with AG increased from 85.4% to 90.8% in the same interval. Sites with exudate were more frequent for CSS than AG at both time points but were not significantly different among groups.
Figure 6. Site assessments at postoperative days 7 and 14 (n = 45). (A) Color, (B) keratinization, (C) percentage site covered, (D) percentage of sites with exudate. Significant differences (*P < .05) between graft types at marked intervals are indicated.
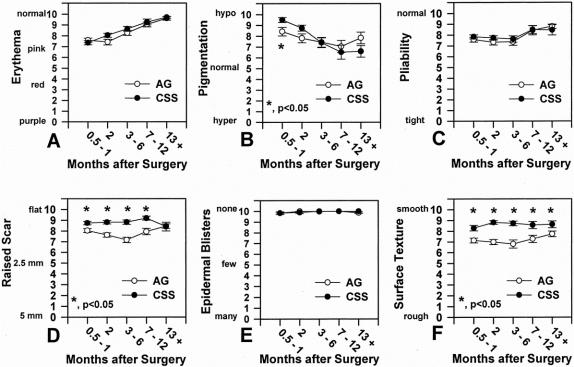
Qualitative outcome after grafting with CSS and AG is presented in Figure 7. Erythema resolved progressively for both graft types during the first year after grafting, with no significant differences between groups. Conversely, pigmentation increased progressively for both graft types during the first year, with a significant difference (P < .05) between groups at 0.5 to 1 month after grafting. Pliability was not significantly different between graft types at each time interval and gradually increased during the first year. After 1 year, both graft types had pliability similar to normal skin. Raised scar was significantly less for CSS than AG up to 1 year after grafting; after 1 year there was no difference between the graft types. Virtually no epidermal blisters were observed in either graft type during the first year after grafting. This result with CSS shows the rapid and strong attachment of cultured epithelium to wounds. Surface texture of CSS was significantly smoother (P < .02) than AG during each interval. Application of CSS as sheet grafts and AG as expanded mesh grafts may contribute to this result.
Figure 7. Qualitative outcome versus months after surgery (n = 45). (A) Erythema, (B) pigmentation, (C) pliability, (D) raised scar, (E) epidermal blisters, (F) surface texture. Significant differences (*P < .05) between graft types at marked intervals are indicated.
The histologic anatomy of CSS and AG at more than 1 year after grafting is shown in Figure 8. CSS develop an interdigitated dermal–epidermal junction similar to the rete ridges of uninjured skin. AG also develops an undulating dermal–epidermal junction and orthogonal distribution of collagen, resembling normal skin. These results account for the pliability of both graft types observed by clinical examination.
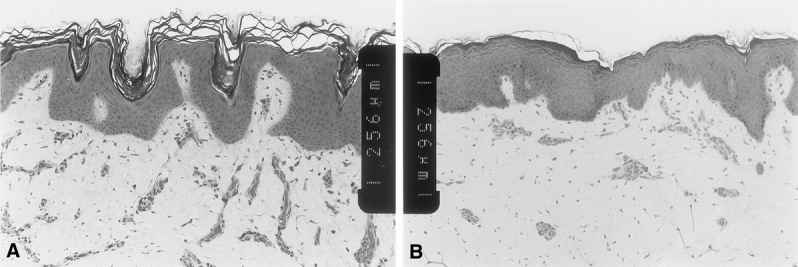
Figure 8. Histology of healed cultured skin substitutes (CSS) and meshed, split-thickness skin autograft (AG). (A) CSS at >1 year; (B) AG at >1 year. Scale bars = 0.256 mm between inset notches.
Not included in the data above are results from the treatment of one patient at the Shriners Hospital for Children in Sacramento. This patient received two treatments with 12 CSS for a total of about 1,000 cm2, applied to the chest and abdomen. Engraftment at POD 14 was about 85%, the ratio of closed to donor area at POD 28 was 77, and coverage with CSS was about 7% of her TBSA. These results agree well with the other data presented here.
DISCUSSION
The data presented in this report show that autologous CSS reduce requirements for harvesting of skin autograft. Reduction in the harvesting of donor skin reduces short-term complications from wounds at donor sites and long-term problems from development of scar, pruritus, and chronic wounds. Also, the increased availability of skin grafts for permanent wound closure of excised burns may shorten the hospital stay. Although length of stay was not evaluated in this study, multiple patients in this population with greater than 85% TBSA full-thickness burns had their wounds closed within 2 months of injury. Therefore, it would be expected that use of autologous CSS as described here will decrease the length of hospital stay from the standard of 1 to 1.5 hospital days per %TBSA full-thickness burn. 37
Autologous CSS require about 4 weeks to prepare after collection of the biopsy for cell culture. Data here show that CSS can be expanded by about 67 times the area of donor skin compared with a value of 4 for meshed AG. Therefore, if the capacity of preparation were not limiting, a biopsy of less than 2% of the TBSA would be sufficient to resurface the entire body in about 1 month. The current study was constrained by limited capacity to prepare CSS, but burns of greater than 85% TBSA were still closed in less than 2 months. Removal of the constraint of preparation will further shorten the time to final wound closure for massive burns. Further, although time for preparation of autologous CSS limits availability for emergent treatment of acute wounds, such as full-thickness burns, preparation time is not limiting in the treatment of elective procedures, such as reconstruction of burn scar or congenital skin lesions. Both burn scars and giant nevi have been treated successfully using autologous CSS. 38 Therefore, the medical benefits from the reduction of requirements for harvesting of donor skin may be extended from emergent treatment of burns to elective treatment of other cutaneous lesions that require skin grafting.
The results of this study distinguish this model of dermal–epidermal skin substitute from keratinocyte sheets 9,18 used for the treatment of burns. Previous reports 20,39 have shown a negative correlation between engraftment of keratinocyte sheets and %TBSA full-thickness burn that is associated with increased requirements for regrafting and extended time to definitive wound closure. In the latest 12 patients, there was no significant correlation between the percentage of engraftment of CSS at POD 14 and %TBSA full-thickness burn, and virtually no regrafting of autologous CSS. Therefore, medical factors in patients with massive burns, such as microbial contamination, hypermetabolism, and immune deficiency, did not reduce the efficacy of CSS. Conversely, there was a strong and significantly positive correlation between %TBSA closed at POD 28 and %TBSA full-thickness burn after treatment with CSS. This result shows that the clinical impact of autologous CSS increases as a function of the magnitude of injury. Therefore, CSS can provide the greatest medical benefit to patients who have the greatest medical need.
High rates of engraftment and rapid wound closure after grafting of CSS result from formation of functional epidermis and biologic attachment of stratified epithelium to a dermal substitute in vitro 13 and the use of broad-spectrum, noncytotoxic topical antimicrobial agents. 36,40 These properties of the anatomy and physiology of CSS contribute to the rapid formation of stable skin tissue. Use of a wet dressing technique 41 for delivery of antimicrobial agents as solutions during the first week after grafting provides an efficient and uniform delivery of agents to control microbial destruction of grafts. Reduced regrafting of CSS in the latest 12 of the 45 patients is attributed to increased engraftment of CSS from improvements in their anatomy and physiology, and use of an irrigation solution composed of noncytotoxic antimicrobial agents delivered in a modified cell culture medium. 35 This suggests that the use of noncytotoxic antimicrobials controls contamination without injury to transplanted cells, and nutrients support the nutritional requirements of cells until ingrowth of blood supply restores systemic nutrition and immunity. In selected patients, wounds were treated with 0.5% (wt/vol) silver nitrate solution beginning at about 1 week after grafting of CSS. Although the outer layers of healing CSS developed the characteristic black color of oxidized silver, the discolored surface was later shed with desquamating epidermis to reveal stable, healed skin beneath. This increased tolerance of healed CSS to strong topical antimicrobial agents is believed to result from the superior anatomy and physiology of dermal–epidermal CSS compared with separate applications of dermal and epidermal materials.
The qualitative outcome of CSS compared favorably to that of meshed, split-thickness AG. Rapid epithelial closure of wounds by high rates of CSS engraftment suppresses the formation of granulation tissue and scar. Epithelial closure results in part from application of CSS as sheets rather than expanded meshed autograft. Therefore, the characteristic mesh pattern is not observed in healed CSS, and secondary healing of mesh interstices by epithelial migration is not required. Consequently, ordinal scoring of qualitative outcome shows that healed CSS are smoother and have less raised scar than healed autograft. In the present study, excised burns were most often treated with cadaveric allograft skin before grafting of CSS. Although these results are favorable, the application of autologous CSS over Integra Artificial Skin has been even more favorable. 31 In that study, the texture and pliability of healed skin were not distinguished from uninjured skin. Together, the results from both studies suggest that restoration of connective tissue in the wound bed is a dependent factor for a favorable qualitative outcome after grafting of full-thickness burns. Attention to this factor may be expected to improve function and cosmesis after grafting and reduce long-term complications that lead to problems or additional surgical procedures for reconstruction. This expectation is consistent with the clinical observations from this study that wounds closed with CSS continue to increase in area as children grow (data not shown).
The anecdotal treatment at the Shriners Hospital for Children in Sacramento of a patient with 92% full-thickness burns shows the utility of this medical device outside the site of fabrication. Two CSS grafting procedures were performed, one of which followed commercial express delivery without attendance of the CSS laboratory staff. After minimal training of the medical and nursing staffs, written protocols were sufficient to obtain successful wound closure with this device. This experience suggests that CSS grafts are stable biologically for 24 to 48 hours in appropriate packaging, and that training for successful use of this device will not be excessive. Both of these factors are essential to the introduction of this device as a routine therapy for grafting full-thickness cutaneous wounds.
Despite the multiple benefits of autologous CSS shown here, two important deficiencies remain: nonuniform pigmentation and slower vascularization compared with skin autograft. Although the average degree of pigmentation for CSS was not different from autograft after 1 month, both were relatively hypopigmented, and the distribution of pigment in CSS was organized in distinct foci. Histologically, these foci consist of melanocytes distributed at a relatively normal density (not shown). However, the frequency of pigmented foci is generally low, and each reaches a diameter of 1 to 2 cm by 1 year after grafting. This distribution of pigmentation is explained by “passenger melanocytes” that are present in the keratinocyte cultures used to prepare CSS. It is well known that the growth rate of keratinocytes is more rapid than melanocytes from the same individual. 42–44 Therefore, as keratinocyte populations expand exponentially in culture, melanocytes become diluted to very small proportions. Also, the expression of pigment occurs predominantly after grafting, so the distribution of melanocytes in CSS in vitro is difficult to determine. Preclinical studies have shown that the addition of melanocytes to CSS has restored complete pigmentation of healed skin. 22,24 Also, cultured autologous melanocytes have been transplanted clinically for the treatment of pigment disorders such as vitiligo. 45 Therefore, it is expected that establishment of selective cultures of melanocytes from patients followed by addition of autologous melanocytes to CSS will result in uniform pigmentation of healed skin.
Vascularization of CSS requires 4 to 5 days compared with 2 to 3 days for AG. This delay in restoration of blood supply results in part from the absence of a vascular plexus in CSS that requires regeneration of blood supply de novo from the wound. The additional time required for vascularization of CSS provides an opportunity for cell death from malnutrition and microbial contamination. Although this deficiency is managed by irrigation with nutrients and antimicrobials, more rapid vascularization would simplify clinical use of CSS. One approach to solving this deficiency is genetic modification of cells in CSS to produce angiogenic factors. Candidate factors include basic fibroblast growth factor, transforming growth factor β-1, and vascular endothelial cell growth factor. Recent studies from this laboratory have shown that genetic modification of keratinocytes with CSS results in more rapid vascularization in vivo. 27 Although regulation of gene expression is a critical question to transplantation of genetically modified cells, it is expected that with appropriate regulation this approach may provide a mechanism for selective expression of angiogenic factors in wounds grafted with CSS. Because it is understood that allogeneic keratinocytes do not persist in wounds, addition of genetically modified cells from an allogeneic donor may limit expression of transfected genes. The addition of one or more gene products to healing wounds may compensate for the remaining anatomic and physiologic deficiencies of CSS.
The data presented here show that CSS provide new medical benefits for the closure of burn wounds greater than 50% TBSA. This technology may be introduced for advancement of burn care and for elective treatment of chronic wounds and cutaneous lesions that require reconstructive surgery.
Acknowledgments
The authors thank Andrew Supp, Karen Hart, Jodi Hesselbach, Todd Schuermann, Viki Swope, Gail Macke, Chad Robinson, Ben Anderson, Kiersten Thompson, Lynn Caudill, and Laura Fowler for assistance in performance of this study.
Footnotes
Supported by the US Public Health Service (FDA grant FD-R-000672, and NIH grant GM50509) and the Shriners Hospitals for Children (grants #8670 and #8450).
Correspondence: Steven Boyce, PhD, Shriners Burns Hospital, 3229 Burnet Avenue, Cincinnati, OH 45229.
E-mail: boycest@email.uc.edu
Accepted for publication June 4, 2001.
References
- 1.Herndon DN, Muller MJ, Blakeney PE. Teamwork for total burn care: achievements, directions and hopes. In Herndon DN, ed. Total burn care. Philadelphia: WB Saunders; 1996: 1–4.
- 2.Hansbrough JF, Dominic W, Gadd M, et al. Burns: critical decisions. Prob Crit Care 1987; 1: 558–610. [Google Scholar]
- 3.Burke JF, Quinby WC, Bondoc CC. Early excision and prompt wound closure supplemented with immunosuppression. Surg Clin North Am 1978; 58: 1141–1150. [DOI] [PubMed] [Google Scholar]
- 4.Heimbach D, Luterman A, Burke JF, et al. Artificial dermis for major burns; a multi-center randomized clinical trial. Ann Surg 1988; 208: 313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hansbrough JF, Mozingo DW, Kealey GP, et al. Clinical trials of a biosynthetic temporary skin replacement, Dermagraft-TC compared to cryopreserved human cadaver skin for temporary coverage of excised burn wounds. J Burn Care Rehabil 1997; 18: 43–51. [DOI] [PubMed] [Google Scholar]
- 6.Tanner JC, Vandeput J, Olley JF. The mesh skin autograft. Plast Reconstr Surg 1964; 34: 287–292. [PubMed] [Google Scholar]
- 7.Hansbrough JF. Wound coverage with biologic dressings and cultured skin substitutes. Austin: RG Landes; 1992.
- 8.Robson MC, Barnett RA, Leitch IOW, et al. Prevention and treatment of postburn scars and contracture. World J Surg 1992; 16: 87–96. [DOI] [PubMed] [Google Scholar]
- 9.Gallico GG,III O’Connor NE, Compton CC, Kehinde O, Green H. Permanent coverage of large burn wounds with autologous cultured human epithelium. N Engl J Med 1984; 311: 448–451. [DOI] [PubMed] [Google Scholar]
- 10.Heck EL, Bergstresser PR, Baxtor CR. Composite skin graft: Frozen dermal allografts support the engraftment and expansion of autologous epidermis. J Trauma 1985; 25: 106–112. [PubMed] [Google Scholar]
- 11.Burke JF, Yannas IV, Quinby WC, et al. Successful use of a physiologically acceptable skin in the treatment of extensive burn injury. Ann Surg 1981; 194: 413–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wainwright D, Madden M, Luterman A, et al. Clinical evaluation of an acellular allograft dermal matrix in full-thickness burns. J Burn Care Rehabil 1996; 17: 124–136. [DOI] [PubMed] [Google Scholar]
- 13.Boyce ST, Hansbrough JF. Biologic attachment, growth, and differentiation of cultured human epidermal keratinocytes on a graftable collagen and chondroitin-6-sulfate substrate. Surgery 1988; 103: 421–431. [PubMed] [Google Scholar]
- 14.Bell E, Sher S, Hull B, et al. The reconstitution of living skin. J Invest Dermatol 1983; 81: 2S–10S. [DOI] [PubMed] [Google Scholar]
- 15.Cuono C, Langdon R, Birchall N, et al. Composite autologous-allogeneic skin replacement: development and clinical application. Plast Reconstr Surg 1987; 80: 626–635. [DOI] [PubMed] [Google Scholar]
- 16.Compton CC, Hickerson W, Nadire K, et al. Acceleration of skin regeneration from cultured epithelial autografts by transplantation to homograft dermis. J Burn Care Rehabil 1993; 14: 653–662. [DOI] [PubMed] [Google Scholar]
- 17.Sullivan T, Smith H, Kermode J, et al. Rating the burn scar. J Burn Care Rehabil 1990; 11: 256–260. [DOI] [PubMed] [Google Scholar]
- 18.Odessey R. Addendum. Multicenter experience with cultured epithelial autografts for treatment of burns. J Burn Care Rehabil 1992; 13: 174–180. [PubMed] [Google Scholar]
- 19.Compton CC, Gill JM, Bradford DA, et al. Skin regenerated from cultured epithelial autografts on full-thickness burn wounds from 6 days to 5 years after grafting. Lab Invest 1989; 60: 600–612. [PubMed] [Google Scholar]
- 20.Rue LW, Cioffi WG, McManus WF, et al. Wound closure and outcome in extensively burned patients treated with cultured autologous keratinocytes. J Trauma 1993; 34: 662–667. [PubMed] [Google Scholar]
- 21.Boyce ST, Williams ML. Lipid supplemented medium induces lamellar bodies and precursors of barrier lipids in cultured analogues of human skin. J Invest Dermatol 1993; 101: 180–184. [DOI] [PubMed] [Google Scholar]
- 22.Swope VB, Supp AP, Cornelius JR, et al. Regulation of pigmentation in cultured skin substitutes by cytometric sorting of melanocytes and keratinocytes. J Invest Dermatol 1997; 109: 289–295. [DOI] [PubMed] [Google Scholar]
- 23.Boyce ST, Supp AP, Harriger MD, et al. Surface electrical capacitance as a non-invasive index of epidermal barrier in cultured skin substitutes in athymic mice. J Invest Dermatol 1996; 107: 82–87. [DOI] [PubMed] [Google Scholar]
- 24.Boyce ST, Medrano EE, Abdel-Malek ZA, et al. Pigmentation and inhibition of wound contraction by cultured skin substitutes with adult melanocytes after transplantation to athymic mice. J Invest Dermatol 1993; 100: 360–365. [DOI] [PubMed] [Google Scholar]
- 25.LePoole IC, Boyce ST. Keratinocytes suppress transforming growth factor beta-1 expression by fibroblasts in cultured skin substitutes. Br J Dermatol 1999; 140: 409–416. [DOI] [PubMed] [Google Scholar]
- 26.Goretsky MJ, Harriger MD, Supp AP, et al. Expression of interleukin 1α, interleukin 6, and basic fibroblast growth factor by cultured skin substitutes before and after grafting to full-thickness wounds in athymic mice. J Trauma 1996; 40: 894–900. [DOI] [PubMed] [Google Scholar]
- 27.Supp DM, Supp AP, Bell S, et al. Enhanced vascularization of cultured skin substitutes genetically modified to overexpress vascular endothelial growth factor. J Invest Dermatol 2000; 114: 5–13. [DOI] [PubMed] [Google Scholar]
- 28.Supp DM, Supp AP, Morgan JR, et al. Genetic modification of cultured skin substitutes by transduction of human keratinocytes with PDGF-A. Wound Repair Regen 1998; 8: 26–35. [DOI] [PubMed] [Google Scholar]
- 29.Boyce ST, Greenhalgh DG, Kagan RJ, et al. Skin anatomy and antigen expression after burn wound closure with composite grafts of cultured skin cells and biopolymers. Plast Reconstr Surg 1993; 91: 632–641. [DOI] [PubMed] [Google Scholar]
- 30.Boyce ST, Goretsky MJ, Greenhalgh DG, et al. Comparative assessment of cultured skin substitutes and native skin autograft for treatment of full-thickness burns. Ann Surg 1995; 222: 743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boyce ST, Kagan RJ, Meyer NA, et al. The Clinical Research Award. Cultured skin substitutes combined with Integra to replace native skin autograft and allograft for closure of full-thickness burns. J Burn Care Rehabil 1999; 20: 453–461. [DOI] [PubMed] [Google Scholar]
- 32.Boyce ST, Ham RG. Cultivation, frozen storage, and clonal growth of normal human epidermal keratinocytes in serum-free media. J Tiss Cult Meth 1985; 9: 83–93. [Google Scholar]
- 33.Boyce ST. Methods for serum-free culture of keratinocytes and transplantation of collagen-GAG based composite grafts. In Morgan JR, Yarmush M, eds. Methods in tissue engineering. Totowa, NJ: Humana Press; 1998: 365–389.
- 34.Boyce ST, Christianson DJ, Hansbrough JF. Structure of a collagen-GAG dermal skin substitute optimized for cultured human epidermal keratinocytes. J Biomed Mater Res 1988; 22: 939–957. [DOI] [PubMed] [Google Scholar]
- 35.Boyce ST, Supp AP, Harriger MD, et al. Topical nutrients promote engraftment and inhibit wound contraction of cultured skin substitutes in athymic mice. J Invest Dermatol 1995; 104: 345–349. [DOI] [PubMed] [Google Scholar]
- 36.Boyce ST, Warden GD, Holder IA. Non-cytotoxic combinations of topical antimicrobial agents for use with cultured skin. Antimicrob Agents Chemother 1995; 39: 1324–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pruitt BA,Jr, Mason AD. Epidemiological, demographic and outcome characteristics of burn injury. In Herndon DN, ed. Total burn care. Philadelphia: WB Saunders; 1996: 5–15.
- 38.Boyce ST, Kagan RJ, Corcoran J, et al. Autologous cultured skin substitutes reduce donor site harvesting in grafting of extensive, full-thickness burns [abstract]. J Burn Care Rehabil 2000; 21: S222. [Google Scholar]
- 39.Williamson J, Snelling C, Clugston P, et al. Cultured epithelial autograft: Five years of clinical experience with twenty-eight patients. J Trauma 1995; 39: 309–319. [DOI] [PubMed] [Google Scholar]
- 40.Boyce ST, Warden GD, Holder IA. Cytotoxicity testing of topical antimicrobial agents on human keratinocytes and fibroblasts for cultured skin grafts. J Burn Care Rehabil 1995; 16: 97–103. [DOI] [PubMed] [Google Scholar]
- 41.Warden GD, Saffle JR, Kravitz M. A two-stage technique for excision and grafting of burn wounds. J Trauma 1982; 22: 98–103. [DOI] [PubMed] [Google Scholar]
- 42.Boyce ST, Ham RG. Calcium-regulated differentiation of normal human epidermal keratinocytes in chemically defined clonal culture and serum-free serial culture. J Invest Dermatol 1983; 81: 33S–40S. [DOI] [PubMed] [Google Scholar]
- 43.Pittelkow MR, Shipley GD. Serum-free culture of normal human melanocytes: growth kinetics and growth factor requirements. J Cell Physiol 1989; 140: 565–576. [DOI] [PubMed] [Google Scholar]
- 44.Swope VB, Medrano EE, Smalara D, et al. Long-term proliferation of human melanocytes is supported by the physiologic mitogens α-melanotropin, endothelin-1, and basic fibroblast growth factor. Exp Cell Res 1995; 217: 453–459. [DOI] [PubMed] [Google Scholar]
- 45.Lerner AB, Halaban R, Klaus SN, et al. Transplantation of human melanocytes. J Invest Dermatol 1987; 89: 219–224. [DOI] [PubMed] [Google Scholar]