Abstract
Objective
To determine the efficacy of portal thrombendvenectomy in cases of portal vein thrombosis at the time of orthotopic liver transplantation.
Summary Background Data
Portal vein thrombosis (PVT) has been reported to have an incidence of 2% to 39% in end-stage liver disease. Multiple techniques have been suggested to treat this finding. Several reports have suggested suboptimal results after liver transplantation in recipients with PVT.
Methods
The authors prospectively collected data on 1,546 patients who underwent an initial orthotopic liver transplant at the authors’ institution between December 1984 and October 1999. There were 820 male patients and 726 female patients. All recipients received either cyclosporine or tacrolimus immunosuppression. Intraoperative flows of the portal vein and hepatic artery were routinely measured. Duplex sonography was routinely performed on the first postoperative day and routinely 1, 2, 5, and 10 years after transplantation. Eighty-five patients underwent thrombendvenectomy for organized thrombus partially or completely occluding the portal vein. Postoperative treatment included low-molecular-weight dextran for 48 hours and daily aspirin for 3 months. There were 53 male patients and 32 female patients. The PVT group was compared with a control group consisting of transplant recipients without PVT.
Results
When compared with the control group, PVT patients were older at the time of transplantation and had a higher incidence of liver disease secondary to cryptogenic cirrhosis and Laennec’s cirrhosis. There were no significant differences among both groups for 1-, 3-, and 6-year patient and graft survival rates.
Conclusions
Thrombendvenectomy provides a rapid resolution of an otherwise complex problem. It is the authors’ procedure of choice in cases of organized PVT at the time of transplantation. Operative time and length of stay in the intensive care unit are not prolonged, and patient and graft survival rates are not compromised.
Portal vein thrombosis (PVT) constitutes a technical impediment initially thought to be an absolute contraindication to orthotopic liver transplantation (OLTx). It is a well-recognized complication of end-stage liver disease, and its incidence is estimated to range from 2% to 19%, reaching as high as 39% in certain patient populations. 1–8 Refinements in technique have allowed recipients with PVT to undergo transplantation. Despite these advances, several centers have reported suboptimal outcomes under these circumstances. To thrombectomize an acutely thrombosed vein is an immediate solution for any surgeon. However, the presence of an organized thrombus was for many years an indication for a venous conduit between the superior mesenteric vein, or some other mesenteric vein of significant size, and the portal vein. 3,9–11 At Baylor University Medical Center we began to remove an organized thrombus by a technique mostly similar to thromboendarterectomy, where the intima is also removed. Thus, the thrombendvenectomy technique was developed. In this report we review our 15-year experience performing portal vein thrombendvenectomy at the time of liver transplantation in order to achieve adequate venous inflow to the hepatic allograft. We further evaluated the results of this series and compare them with other reports and with the outcome observed in recipients without PVT undergoing transplants at our institution.
METHODS
We prospectively collected data on 1,546 patients who underwent primary OLTx at Baylor University Medical Center between December 1984 and October 1999. There were 820 male patients and 726 female patients. All recipients were treated either with cyclosporine or tacrolimus-based immunosuppression. Intraoperative flows of the portal vein and hepatic artery were measured electromagnetically on a routine basis once hemostatic and thermal stability was reached. Duplex (real-time and Doppler) sonography was performed on the first postoperative day and during routine follow-up evaluations at 1, 2, 5, and 10 years after transplantation. Duplex sonography was repeated in all patients with postoperative liver dysfunction not explainable by liver biopsy. Eighty-five patients (5.5%) underwent thrombendvenectomy for organized thrombus within the portal vein and its tributaries. Ten (12%) of these patients had surgical portosystemic shunts constructed before transplantation.
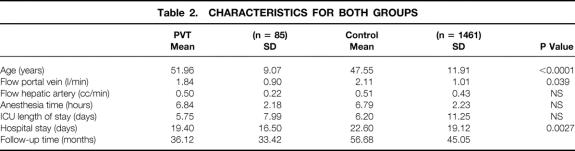
Partial or complete PVT may be detected before or during surgery. In either case, our technique is the same. The approach does not vary initially. The hilum is addressed first, and the hepatic artery and bile duct are tied and transected. This allows for an intimate exposure and dissection of the portal vein in its entirety. The portal vein is manually and visually examined to determine the extent of involvement by the thrombus. Systemic venovenous bypass is started at this point. Once this is achieved, we proceed to tie (whenever possible) the right and left branches of the vein (Fig. 1). We then manually occlude the portal vein and transect it as close to the liver as possible. Two or three tonsil clamps are used to maintain the portal vein open by holding the edges apart. This allows the surgeon to separate the clot and the attached intimal layer from the media of the vein. With the aid of either a lifter or a tonsil clamp, the clot is dislodged circumferentially from the media of the vein (Fig. 2). This maneuver is extended as far as necessary, frequently entering the splenic and superior mesenteric veins. After having achieved separation of the clot from the surrounding vessel wall, the free edge of the clot is clamped with a tonsil or ring clamp and rotated circumferentially (Fig. 3 A). This maneuver is performed first clockwise and then counterclockwise (or vice versa) while simultaneously the clot is pushed toward the origin of the portal vein or into the superior mesenteric or splenic vein if the clot extends into these veins. After the clot is dislodged by means of this circular and inward maneuver, it is pulled out (see Fig. 3 B). By pushing the clot into the vein, the tearing strain is placed only at the most distal end of the clot. Trying to pull the clot in an outward fashion without dislodging it from its circumferential attachments may result in rupture of the portal, superior mesenteric, or splenic vein. Our technique allows for removal of as much clot material as possible, with enhancement of venous flow. For the most part, the entire clot is removed with its smoothly tapering distal (mesenteric and/or splenic) end. In all cases in which the portal vein is thrombosed, we use only systemic venovenous bypass. Subsequently, at the time of the portal anastomosis, we perform it with as much length of donor portal vein as possible. Before completing the anastomosis, we flush the recipient portal vein with blood. This not only removes any clots left behind from the thrombendvenectomy but also eliminates any newly formed ones. After surgery, recipients are administered an intravenous drip of low-molecular-weight dextran. When oral intake is adequate, the dextran is discontinued and replaced with a daily aspirin, which is continued for 3 months.
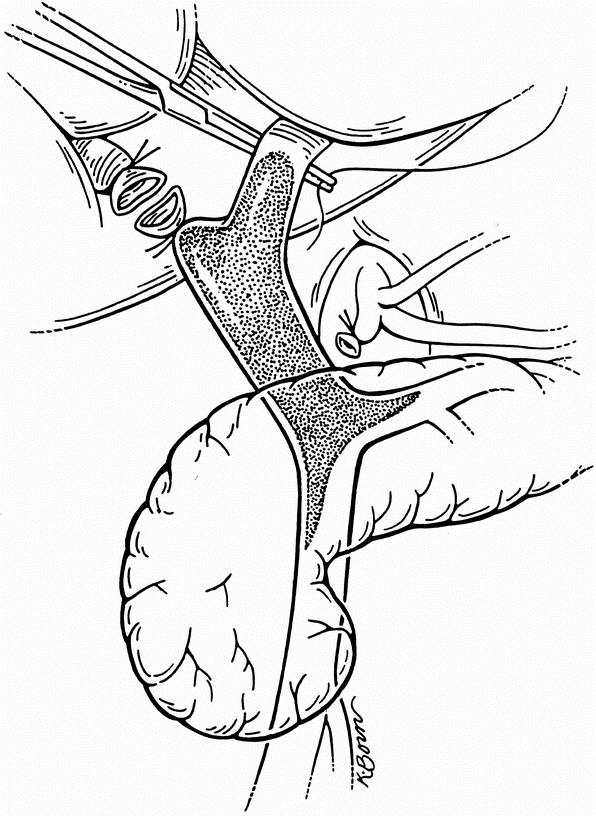
Figure 1. The portal vein has been dissected and fully exposed. After the extent of the thrombus is manually determined, the right and left branches are tied.
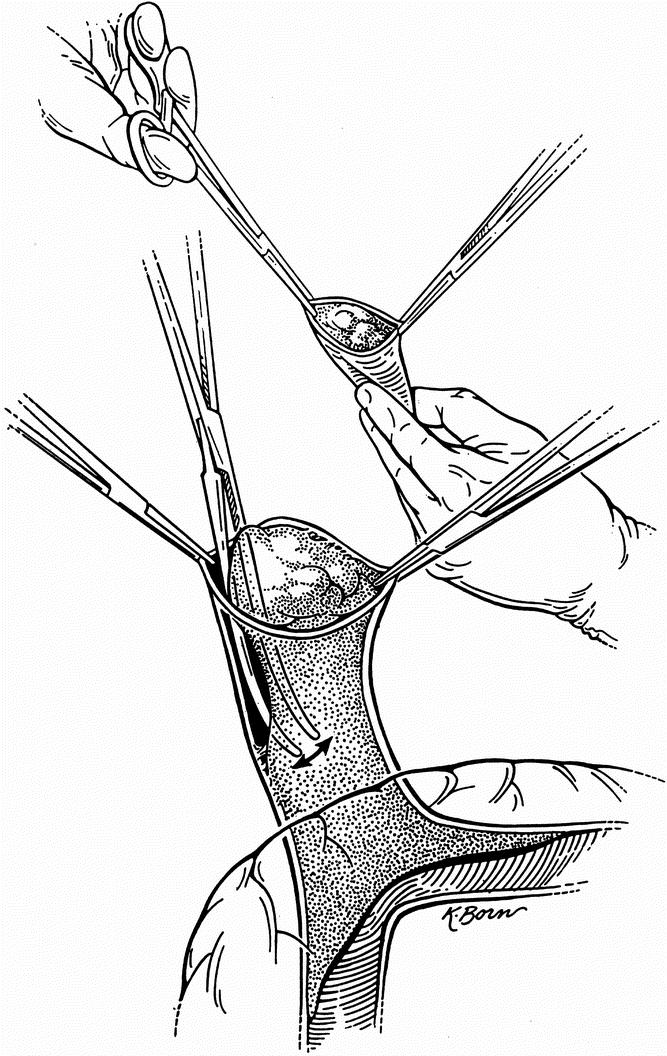
Figure 2. Two or three tonsil clamps are used to maintain the portal vein open by holding the edges apart. The clot and the attached intimal layer of the vein are subsequently dislodged.
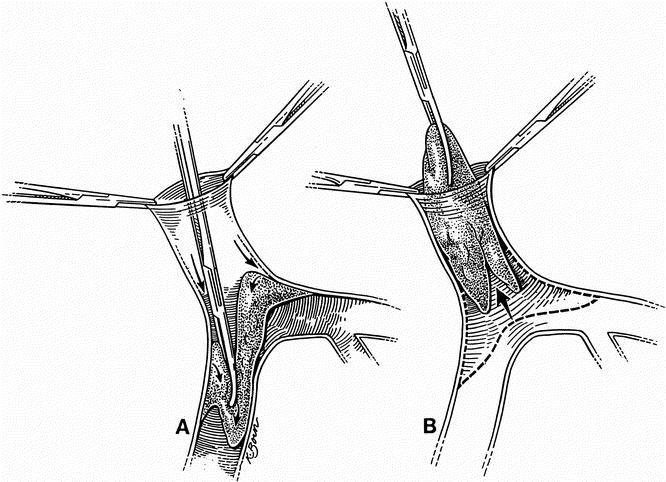
Figure 3. (A) The clot is dislodged by pushing it into the vein with a circular maneuver. (B) After the clot is dislodged by this circular and inward motion, it is pulled out.
RESULTS
We have performed this procedure in 85 recipients with PVT; this constitutes 5.5% of the overall transplant population. Diagnosis of complete or partial PVT was made during or before surgery. Results after thrombendvenectomy in either instance were identical. When the diagnosis was made before transplantation, the patency of the superior mesenteric and/or splenic vein was confirmed when necessary by angiography. There were 53 men (62%) and 32 women (34%) with a mean age at transplantation of 53.0 ± 9.1 years. Mean follow-up was 56.7 months (range 0–178). Ten patients (12%) had surgical portosystemic shunts before transplantation. Three of these patients had documented shunt thrombosis at transplantation. Four underwent splenectomy in the peritransplant period, one had the shunt taken down at the time of surgery, and two had patent shunts left in place. Graft survival rates were 80.0%, 78.3%, and 59.9% at 1, 3, and 6 years, respectively. Patient survival rates were 84.9%, 81.3%, and 62.4% at 1, 3, and 6 years. Mean hepatic artery flow was 500 mL/min ± 0.22 mL/min (range 150–1,100). Mean portal vein flow was 1.84 L/min (range 0.50–5.10). There was a 9.2% incidence of hepatic artery thrombosis). There were two instances (2.4% incidence) of portal vein rethrombosis, both in patients with patent shunts. The first case occurred in a 50-year-old woman with a history of cryptogenic cirrhosis and a portosystemic shunt. At transplantation, portal vein flow was 1.06 L/min. She presented 83 days after transplantation with abdominal pain. Doppler ultrasound examination and subsequently angiography were used to diagnose PVT associated with thrombosis of the superior mesenteric vein, splenic vein, splenorenal shunt, and right renal vein. She was successfully treated with urokinase administration and anticoagulation therapy and is currently alive and well. The second case was in a 40-year-old woman with Laennec’s cirrhosis, hepatitis C, and a portosystemic shunt. At transplantation, she had a patent portosystemic shunt. She presented 38 days after transplantation with abdominal pain. PVT was diagnosed using Doppler ultrasound and angiography. Anticoagulation was not possible given that this patient had had a hemorrhagic cerebrovascular accident before transplantation. She was treated symptomatically. Delayed angiographic examination showed PVT with decompression of the mesenteric system via a patent shunt. She is currently alive and well.
The portal vein thrombendvenectomy group was subsequently compared with a control group comprising all recipients without PVT at transplantation. Table 1 lists the etiology of liver failure in both groups. There was a significantly higher incidence of recipients with end-stage liver disease secondary to cryptogenic cirrhosis and Laennec’s cirrhosis among those with PVT at the time of OLTx. When the status of the recipients at transplantation was considered (data not shown), we observed no significant difference among the patients with PVT and the control group. Table 2 compares both groups with respect to age, intraoperative portal vein and hepatic artery flows, anesthesia time, postoperative length of intensive care unit stay, postoperative length of hospital stay, and follow-up time. There was a significantly higher flow volume in portal veins after transplantation among those without preoperative PVT, but no difference was observed in hepatic artery flows. A significant difference was found in recipient age and hospital stay. No significant difference was found with respect to anesthesia time or length of intensive care unit stay. We also compared (data not shown) packed red blood cell, platelet, fresh-frozen plasma, and cryoprecipitate transfusions among both groups. Although volumes transfused were lower for the PVT group in all categories, the differences were not significant. Hematocrit and prothrombin time values (data not shown) at postoperative days 0, 1, 3, and 7 showed no significant differences among the groups. There was, however, a difference (P < .001) in platelet counts in the immediate postoperative period among the PVT group and the control group. Corresponding values (103/mm3) at days 0, 1, 3, and 7, respectively, were 76 versus 88, 65 versus 94, 43 versus 73, and 83 versus 110.
Table 1. ETIOLOGY OF LIVER FAILURE
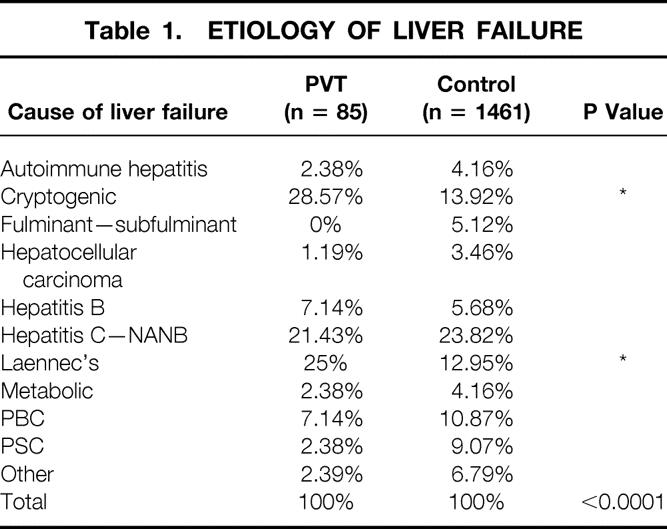
Table 2. CHARACTERISTICS FOR BOTH GROUPS
Of the 85 patients who underwent portal vein thrombendvenectomy at transplantation, 10 (12%) had a history of surgical portosystemic shunts. Two of them had patent shunts at the time of surgery; these are the same two in whom rethrombosis of the portal vein developed. Of the remaining eight, seven had their shunts occluded or defunctionalized by the time of transplantation and one had no record of the shunt status at the time of surgery. Of the control group of patients (n = 1,461) without PVT at transplantation, 62 (4%) had portosystemic shunts.
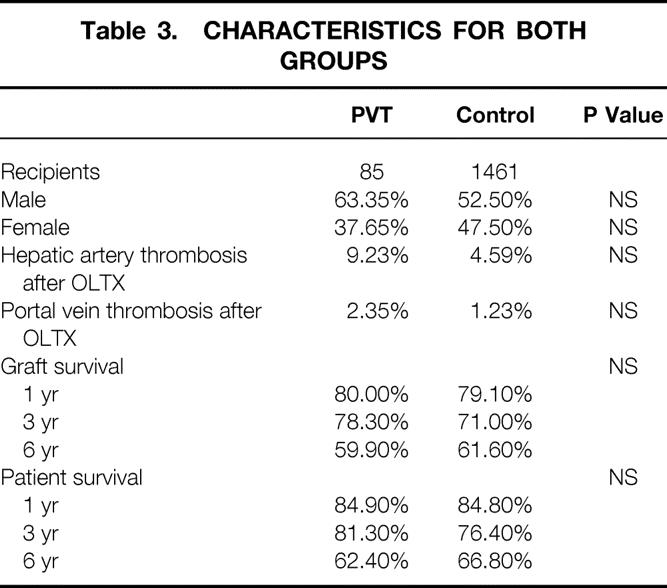
Table 3 lists differences in gender, incidence of postoperative hepatic artery and portal vein thrombosis, and graft and patient survival rates. No significant difference among the two groups was reached by any of these parameters.
Table 3. CHARACTERISTICS FOR BOTH GROUPS
DISCUSSION
Despite early criteria that excluded candidates with PVT from liver transplantation, OLTx is possible provided that hepatopedal portal flow can be reestablished. Portal vein inflow has been previously accomplished in patients with PVT using a variety of techniques. Vein grafting to the superior mesenteric vein, to the splenomesenteric confluence, or to other mesenteric veins and implantation of the donor portal vein at the splenomesenteric confluence or onto a varix are probably the most frequent ones. Previous studies had found a high incidence of PVT in recipients with autoimmune chronic active hepatitis, chronic active hepatitis, trauma, previous dissection of the porta hepatis, tumors, cryptogenic cirrhosis, hypercoagulable states, and previous splenectomy. 6,9 Based on our data, there seems to be a significant association only with cryptogenic cirrhosis and with Laennec’s cirrhosis. Although some authors observed a higher rate of postoperative complications 9 and diminished graft survival 1 when compared with those recipients without PVT, we found no significant difference in patient or graft survival rates. Other series have found that patients with PVT had greater intraoperative blood loss and coagulopathy. 1,9 Blood requirements varied from 22 to 36 units of packed red blood cells per patient in some series 3,10 and from 35 to 15 units in others. 8 Mean operative time reported was 9.7 ± 4.8 hours. 3 Our mean packed red blood cell requirement was 6.23 units, which is not significantly different from that seen in the control group. No difference was observed in our study with respect to anesthesia time (mean <7 hours in all patients), length of intensive care unit stay, postoperative transfusions, prothrombin time, and hematocrit values. The lowered platelet values in the PVT group during the postoperative period are probably associated with the administration of dextran. Published results vary as to deaths and complications associated with the presence of preoperative PVT. 5 In our series, although patients with PVT were older than those in the control group, their length of hospital stay was significantly shorter. There was a three times higher incidence of surgical portosystemic shunts in patients with PVT at transplantation than in our control population (12% vs. 4%). Although not significant, we observed that the incidence of hepatic artery thrombosis after transplantation was higher in patients with preoperative PVT. We believe this could be explained by the fact that the number of patients in the PVT group was much smaller than that in the control group. Portal vein rethrombosis rates range from 6% to 40%, with death rates ranging from 35% to 100%. 2,6,9,12 One large series 13 found that PVT patients have a higher incidence of postoperative complications, higher in-hospital death rates, and a diminished 5-year survival rate. We found, however, that patient and graft survival rates for recipients with preoperative PVT was no different than that for the general transplant population when a meticulous thrombendvenectomy was done, carefully removing the injured intima. Our incidence of portal vein rethrombosis was not significantly different from that of the general transplant population. We have had no deaths among those few who rethrombosed. Postoperative anticoagulation and Doppler ultrasound evaluations have been recommended for patients with preoperative PVT. 8,12 We prefer to use dextran after surgery for 48 hours and to subsequently continue with a daily aspirin for 3 months because of their effect on platelets.
We have successfully transplanted patients with varying degrees of PVT by performing thrombendvenectomy rather than complex venous reconstructions at the time of OLTx. Today, the need for such reconstruction is highly unusual. We believe that thrombendvenectomy permits a prompt and rapid resolution of an otherwise complex problem, and it is our procedure of choice in patients with preoperative PVT. We have not encountered any intra- or postoperative complications with this technique.
Footnotes
Correspondence: Goran B. Klintmalm, MD, PhD, Baylor University Medical Center, Transplantation Services, 3500 Gaston Avenue, Dallas, TX 75246.
E-mail: gb.klintmalm@baylordallas.edu
Accepted for publication August 10, 2001.
References
- 1.Gayowski TJ, Marino IR, Doyle HR, et al. A high incidence of native portal vein thrombosis in veterans undergoing liver transplantation. J Surg Res 1996; 60: 333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moreno Gonzalez E, Garcia Garcia I, Gomez Sanz R, et al. Liver transplantation and portal thrombosis. Minerva Chir 1990; 45: 1415–1419. [PubMed] [Google Scholar]
- 3.Langnas AN, Marujo WC, Stratta RJ, et al. A selective approach to preexisting portal vein thrombosis in patients undergoing liver transplantation. Am J Surg 1992; 163: 132–136. [DOI] [PubMed] [Google Scholar]
- 4.Nonami T, Yokoyama I, Iwatsuki S, et al. The incidence of portal vein thrombosis at liver transplantation. Hepatology 1992; 16: 1195–1198. [PMC free article] [PubMed] [Google Scholar]
- 5.Cherqui D, Duvoux C, Rahmouni A, et al. Orthotopic liver transplantation in the presence of partial or total portal vein thrombosis: problems in diagnosis and management. World J Surg 1993; 17: 669–674. [DOI] [PubMed] [Google Scholar]
- 6.Davidson BR, Gibson M, Dick R, et al. Incidence, risk factors, management, and outcome of portal vein abnormalities at orthotopic liver transplantation. Transplantation 1994; 57: 1174–1177. [DOI] [PubMed] [Google Scholar]
- 7.Lerut JP, Mazza D, van Leeuw V, et al. Adult liver transplantation and abnormalities of splanchnic veins: experience in 53 patients. Transplant Int 1997; 10: 125–132. [DOI] [PubMed] [Google Scholar]
- 8.Seu P, Shackleton CR, Shaked A, et al. Improved results of liver transplantation in patients with portal vein thrombosis. Arch Surg 1996; 131: 840–845. [DOI] [PubMed] [Google Scholar]
- 9.Shaked A, Busuttil RW. Liver transplantation in patients with portal vein thrombosis and central portocaval shunts. Ann Surg 1991; 214: 696–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stieber AC, Zetti G, Todo S, et al. The spectrum of portal vein thrombosis in liver transplantation. Ann Surg 1991; 213: 199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirsch JP, Howard TK, Klintmalm GB, et al. Problematic vascular reconstruction in liver transplantation. Part II. Portovenous conduits. Surgery 1990; 107: 544–548. [PubMed] [Google Scholar]
- 12.Moreno Gonzalez E, Garcia Garcia I, Gomez Sanz R, et al. Liver transplantation in patients with thrombosis of the portal, splenic or superior mesenteric vein. Br J Surg 1993; 80: 81–85. [DOI] [PubMed] [Google Scholar]
- 13.Yerdel MA, Gunson B, Mirza D, et al. Portal vein thrombosis in adults undergoing liver transplantation: risk factors, screening, management, and outcome. Transplantation 2000; 69: 1873–1881. [DOI] [PubMed] [Google Scholar]