Abstract
Objective
To investigate a possible association with plasma platelet activating factor acetylhydrolase (PAF-AH) gene mutation with the risk of abdominal aortic aneurysm (AAA).
Summary Background Data
Plasma platelet activating factor acetylhydrolase is known to catalyze platelet activating factor (PAF), thereby inactivating its inflammatory function. Deficiency of this enzyme is caused by a missense mutation (G994 →T) in exon 9 of the plasma PAF-AH gene.
Methods
We did a case-control study including 131 patients (median age 73.4 [range 50–84] years) and 106 controls matched for age and sex. Genomic DNA was analyzed for the mutant allele by a specific polymerase-chain reaction. Plasma PAF-AH activity was measured in both groups.
Results
The frequency of the mutant allele (T allele) in the plasma PAF-AH gene in AAA patients was significantly higher than in control subjects. The association of the missense mutation with AAA was statistically significant and independent of other risk factors. Among AAA patients with normal genomic type, plasma PAF-AH activity was strongly correlated to the plasma concentration of low density lipoprotein cholesterol (LDL-C), while the correlation was not observed among AAA patients with heterozygotes genotype. Patients having AAA with both T allele and hyperlipidemia were more likely to have other atherosclerotic diseases such as ischemic heart disease, stroke and peripheral arterial occlusive diseases than patients with the normal genomic type and normal lipid level.
Conclusions
The genetic mutation of plasma PAF-AH gene appear to be an independent risk factor for AAA. Our findings need to be confirmed in a larger, prospective study including patients from different populations.
The etiology of abdominal aortic aneurysm (AAA) involves both environmental and genetic factors. Male gender, tobacco use, hypertension, and hyperlipidemia have been considered to be risk factors for AAA. 1–3 Although patients with AAA and atherosclerotic diseases often share these risk factors and manifest both diseases at the same time, the role of atherosclerosis in AAA is still controversial. Even if atherosclerosis does play an important role in AAA, it is likely that additional etiopathologic processes are involved. Much attention has been given to the hypothesis that inflammatory response in the vessel wall causes enzymatic degradation of the connective tissue matrix. The best-studied group of such enzymes involved is the matrix metalloproteinases (MMPs).
Recent studies have identified that mRNA of MMP-1, 2, and 9 are induced by platelet-activating factor (PAF) in epithelial cells and fibroblasts. 4,5 Moreover, PAF receptor antagonist has abolished PAF-induced collagenolysis in the human uterine cervix. 5 Platelet-activating factor is a biologically potent ether phospholipid generated by many types of cells including neutrophils, macrophages, platelets, and endothelial cells. Platelet-activating factor is inactivated by the enzyme PAF acetylhydrolase (PAF-AH), which hydrolyzes the sn-2-acetyl moiety of PAF to lyso-PAF. Platelet-activating factor acetylhydrolase protects low density lipoprotein (LDL) against oxidative modification, 6 which is thought to be important in preventing atherosclerosis. Since an inherited form of PAF-AH deficiency was reported by Miwa et al, 7 this autosomal recessive trait has only been observed in Japanese populations. Recently, the molecular structure of the human plasma PAF-AH gene was determined, 8 in which deficiency of this enzyme is also identified to be a result of a single point mutation (G994→T) in exon 9. In patients who are homozygous for the mutation, enzymatic activity is completely abolished, while heterozygotes have reduced activity. Previous studies have identified that the mutation of the plasma PAF acetylhydrolase gene is associated with severity of asthma attack, 7 nephrotic syndrome in children, 9 and atherosclerotic diseases such as coronary artery disease, 10 stroke 11 and peripheral artery occlusive disease. 12
Based on these findings, we performed a case–control study to see whether there is an association between plasma PAF acetylhydrolase gene mutation and AAA.
METHODS
Plasma PAF-AH genotype analysis was performed on blood samples from 131 patients with AAA treated at Second Department of Surgery of Hamamatsu University Hospital. A diagnosis of AAA was established when an abdominal aortic diameter of 30 mm or more was measured by either computed tomography or abdominal ultrasound. Patients with Marphan’s syndrome were not involved in this study. The control group was chosen from patients who visited the hospital for gastrointestinal screening tests such as gastroscopy and colonoscopy that had no history of stroke, ischemic heart disease, or peripheral arterial occlusive diseases. The control group consisted of individuals living in the same area and matched with patients that had AAA for sex and age. All subjects enrolled in this study were Japanese, and all patients gave informed consent. The Ethics Committee of Hamamatsu University School of Medicine approved this study.
Patients were considered smokers if they had a smoking index (years × amount of smoking [number of cigarettes] per day) > 100 and current smoking status. They were considered to have hyperlipidemia if their fasting total plasma cholesterol level was ≥ 220 mg/dL or they had already been treated with cholesterol-lowering drugs. They were considered to have hypertension if they met the criteria of the World Health Organization or were already being treated with antihypertensive agents. Diabetes mellitus was defined if they met the diagnostic criteria of the World Health Organization or were already currently using insulin or oral hypoglycemic agents. Medical history concerning stroke, ischemic heart disease, and peripheral arterial occlusive disease were obtained. The presence of ischemic heart disease was determined by history of myocardiac infarction or angina, and by abnormal results on electrocardiogram. Peripheral arterial occlusion was also checked by measuring the ankle brachial pressure index (ABI) and confirmed by angiography. In patients with AAA, total cholesterol, triglyceride, LDL-cholesterol (LDL-C), and HDL-cholesterol (HDL-C) were measured.
Genotype Determination
Genomic DNA was extracted from 2 mL of whole blood with a GenTLE kit (Takara Biomedicals, Ohtsu, Japan) according to the manufacturer’s instructions. The genotype of plasma PAF-AH was determined with an allele-specific PCR method, as described previously. 8 The sequences of the sense primer (Sense primer A) and three antisense primers (Antisense primers B,C,D) were as follows:
Sense primer A: 5'-CTATAAATTTATATCATGCTT-3'
Antisense primer B: 5'-TTTACTATTCTCTTGCTTTAC-3'
Antisense primer C: 5'-TCACTAAGAGTCTGAATAAC-3'
Antisense primer D: 5'-TCACTAAGAGTCTGAATAAA-3'
Genotypes were designated as GG (normal), GT (heterozygous), and TT (homozygous deficient).
Assay for Plasma PAF Acetylhydrolase Activity
Venous blood was collected into a tube containing EDTA and centrifuged at 2,000 x g for 15 minutes at 4°C. Plasma samples were stored at −70°C until the time of assay. The activity of plasma PAF-AH was determined using the method described by Miwa et al. 7
Statistical Analysis
Data on age are presented as mean ±SD. The difference between the groups was analyzed using the unpaired Student’s t test. The differences in frequencies of smoking, hypertension, hyperlipidemia, diabetes mellitus, and frequencies of the mutant allele were analyzed by Fisher exact test. χ2 analysis was used to test deviations of genotype distribution from Hardy-Weinberg equilibrium and to determine allele or genotype frequencies between patients and control groups. P values were two-sided. Statistical analysis was performed with Statview (version 4.5, Abacus Concepts, Inc., Berkely, CA). Univariate analysis of odds ratios were calculated to analyze risk factors for AAA. Furthermore, logistic regression methods were used to calculate each odds ratio and 95% CI estimating the relative risk for AAA associated with T allele, which was adjusted for sex, age, smoking, diabetes, hyperlipidemia, and hypertension (JMP, version 3.1, SAS Institute Inc, Cary, NC). In addition we calculated odds ratios separately among patients having AAA with/without hyperlipidemia and/or T allele to estimate relative risks for other atherosclerotic diseases. Plasma PAF-AH activity was compared by one-way ANOVA with the Scheffe test. A value of P < .05 was taken to be statistically significant. Simple correlations between plasma PAF-AH activity, and total cholesterol, triglyceride, HDL-C, and LDL-C were determined.
RESULTS
The Clinical characteristics of patients with AAA and control subjects are shown in Table 1. There was no significant difference in age and sex between the groups. Among the atherosclerotic risk factors examined, the patients with AAA were more likely than controls to have hyperlipidemia and hypertension.
Table 1. CHARACTERISTICS OF AAA AND CONTROL SUBJECT

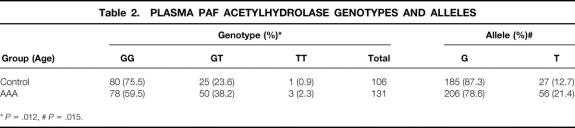
The genotypic and allelic frequencies of the G/T mutation of PAF-AH gene are summarized in Table 2. These data are consistent with the distribution predicted by the Hardy-Weinberg equilibrium. Among controls, the frequencies for the G and the T allele were 0.87 and 0.13, respectively, and are similar to the frequency previously reported for Japanese individuals. 10,11 The prevalence of the GT+TT genotype was significantly more frequent in patients with AAA than in control subjects. There was also a significant difference in allele frequencies between patients with AAA and control subjects (Table 2). The odds ratio of the GT+TT versus the GG genotype of PAF-AH gene between patients with AAA and control subjects was 2.48 (95% CI, 1.36–4.65). The association of this mutation with AAA patients was statistically significant and independent of other atherosclerotic risk factors when subjected to logistic regression analysis (Table 3).
Table 2. PLASMA PAF ACETYLHYDROLASE GENOTYPES AND ALLELES
*P = .012,
#P = .015.
Table 3. RISK FACTORS FOR AAA

* Calculated by multiple logistic regression analyses.
The average value of plasma PAF-AH activity in each group is shown in Figure 1. Plasma PAF-AH activity in GT heterozygotes was almost half of that in subjects with the GG genotype. No PAF-AH activity was detected in TT homozygotes. Plasma PAF-AH activity was significantly higher in patients with AAA than in control subjects in GG genotype subgroups.
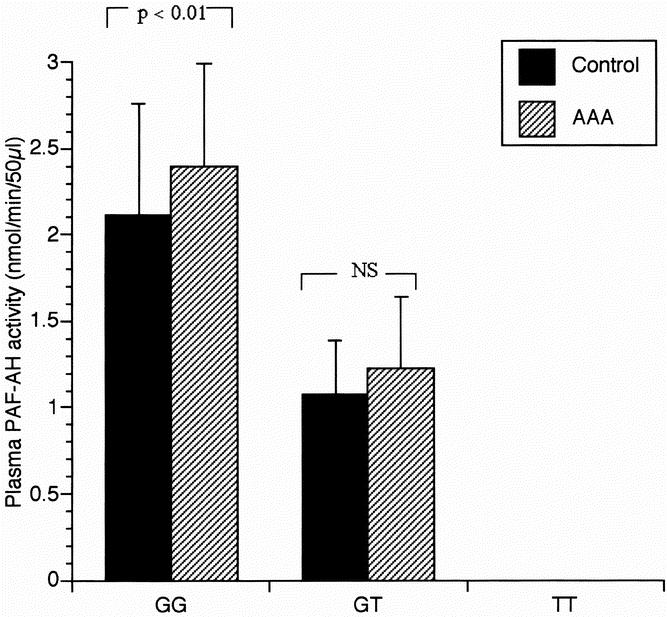
Fig. 1. Plasma PAF acetylhydrolase activity and Genotype. Error bars correspond to ± standard deviation of mean.
Maximal abdominal aortic diameter was measured by CT in patients with AAA. The diameters in GG and GT+TT patients were 49.9 ± 11.2 mm and 50.3 ± 11.8 mm, respectively. There was no significant difference between the groups.
Table 4 shows correlations between plasma PAF-AH activity and total cholesterol, triglyceride, HDL-C, and LDL-C in AAA patients with GG or GT genotype. In patients with GG genotype, plasma PAF-AH activity is related to total cholesterol and LDL-C by simple regression analysis (r = 0.53 P < .001, 0.59 P < .001. respectively). However, after adjusting the PAF-AH activity for LDL-C by linear regression, the correlation between PAF-AH activity and total cholesterol in GG genotype patients was almost completely abolished. Figure 2 shows the simple correlation of LDL-C with PAF-AH activity in AAA patients with GG genotype or GT genotype. Unlike patients with GG genotype, there was no significant coefficient between LDL-C and PAF-AH activity in patients with GT heterozygotes. The levels of LDL-C in GG genotype patients were significantly higher than those in patients with GT or TT genotypes (126.2 ± 37.6, 111.3 ± 33.0 mg/dL, respectively;P < .05).
Table 4. CORRELATIONS BETWEEN PLASMA PAH-AH ACTIVITY, AND PLASMA LIPID AND LIPOPROTEIN CONCENTRATIONS IN AAA PATIENTS
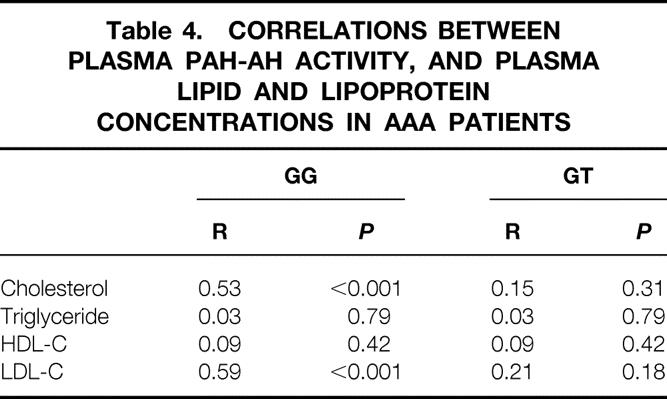
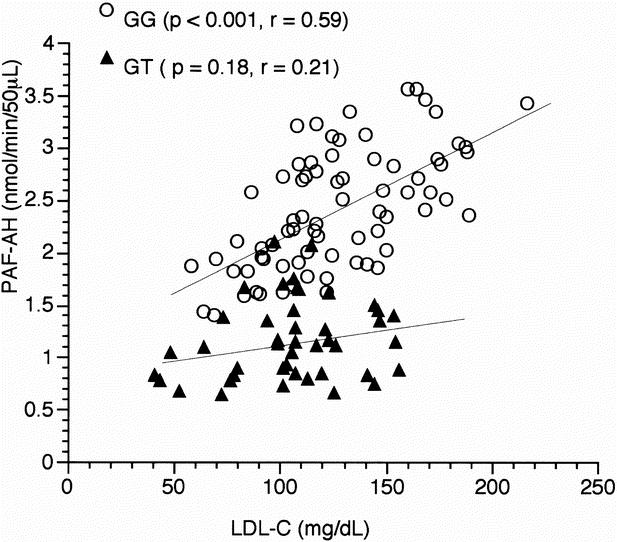
Fig. 2. Scatterplot of plasma PAF-AH activity and LDL-C concentration in AAA patients with normal (GG) or heterozygotes (GT) genotype.
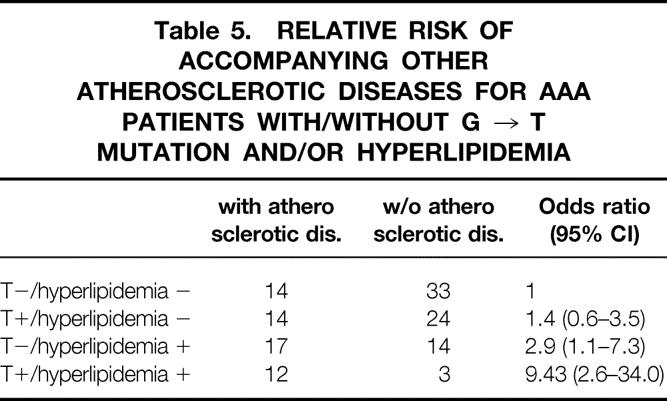
We calculated the relative risk for accompanying either history or concomitance of atherosclerotic diseases such as ischemic heart disease, stroke, or peripheral arterial occlusive diseases for AAA patients with T allele (GT or TT) stratified by the presence or absence of hyperlipidemia. This time, hyperlipidemia was defined as either total cholesterol ≥ 220 or LDL-C ≥ 140. We found that the relative risk for accompanying atherosclerotic disease for AAA patients with both T allele and hyperlipidemia was 9.4 (95% CI 2.6–34.0) compared with patients with normal genotype and normal lipid level (Table 5).
Table 5. RELATIVE RISK OF ACCOMPANYING OTHER ATHEROSCLEROTIC DISEASES FOR AAA PATIENTS WITH/WITHOUT G → T MUTATION AND/OR HYPERLIPIDEMIA
DISCUSSION
Our results suggest that the G994 →T mutation in the plasma PAF-AH gene is associated with AAA. We found a significantly higher prevalence of individuals who have the mutation among patients with AAA than among a control group with matched age and sex. These findings are further supported by the results of our multivariate analysis, which adjusts for the presence of tabulated atherosclerotic risk factors, and which shows an independent association between T allele and AAA. As the plasma activity of the enzyme is determined by the genotype, low or no activity of the enzyme may be an important factor in genetic susceptibility to AAA. In addition, hyperlipidemia in combination with the presence of T allele led to a higher risk of other atherosclerotic diseases among patients with AAA, suggesting that an interaction between PAF-AH and lipoprotein may play a crucial role in the pathogenesis of atherosclerotic diseases in Japanese people.
The mechanism by which the point mutation led to an increase in genetic susceptibility to AAA is not known. Recent studies have highlighted the important role of enzymes that degrade collagen and elastin in formation of AAA. Among the enzymes, the matrix metaloproteinases (MMP) have been identified in AAA tissue, and are known to degrade both elastin and collagen in the aortic wall. 13,14 Platelet activating factor was demonstrated to increase the activity of MMPs, in which the reaction was blocked by PAF antagonist. On the other hand, histologic examination of AAA tissue shows a prominent inflammatory response. Leukocytes, primarily macrophages and lymphocytes, can be seen invading the intimal plaque and advantitia. 15 Platelet activating factor is also a potent inflammatory mediator for migration of these cells. These inflammatory cells, especially macrophages, are believed to be a major source of MMP-1 and MMP-9 in AAA. 16 Taken together, the lack or low level of PAF-AH caused by genetic mutation may fail to inactivate PAF, accelerating inflammation and MMP production, which may contribute to formation of AAA. This idea is further supported by the observation that recombinant plasma PAF-AH markedly inhibits PAF-induced inflammation in rats. 17
In addition, PAF-AH may also play a pivotal role in preventing atherosclerosis. Although the pathogenesis of atherosclerosis is complex, oxidative modification of LDL is involved in the development of atherosclerosis. Oxidized LDL injures the endothelium and helps monocytes infiltrating the endothelium to differentiate into macrophages. Oxidized LDL generates large amounts of antigens during the progression of atherosclerotic diseases, which stimulate not only T-cell migration but also induce antibody production. Therefore, atherosclerosis may involve an autoimmune response to oxidized LDL. 18 Platelet activating factor acetylhydrolase protects LDL from oxidation and prevents internalization by macrophages. 6 This action may inhibit the initial development of atherosclerosis. 6,19 Therefore, the deficiency of plasma PAF-AH may advance LDL-associated atherosclerosis. In this study, plasma PAF-AH activity was strongly correlated with the plasma concentration of LDL-C among AAA patients with normal genotype. This result was quite similar to data reported in a previous study performed in the United States. 20 These findings suggest that the correlating elevation of PAF-AH with LDL-C level may be one of the defense mechanisms against atherosclerosis. Conversely, plasma PAF-AH was not related to LDL among patients with T allele. Indeed, the results of a subgroup analysis indicate an interaction of the genetic mutation and hyperlipidemia to increase risks for having history or other concurrent atherosclerotic diseases for patients with AAA. These findings suggest that patients with the genetic mutation may lack the PAF-AH-related defensive mechanism against atherosclerosis. Therefore, the genetic mutation and associated deficiency of PAF-AH activity may increase the risk of atherosclerotic diseases in AAA patients. However, whether the combination of T-allele and hyperlipidemia increase the risk of atherosclerosis for patients without AAA is unknown, and similar studies for patients without AAA (such as in ischemic heart disease) or stroke patients, as well as healthy controls, are needed.
The role of oxidized LDL in the pathogenesis of AAA has yet to be elucidated. In this study, the levels of LDL-cholesterol in many patients with AAA were not markedly high, and the levels of LDL-cholesterol in patients with the normal (GG) gene were significantly higher than those in patients with the mutation (GT or TT). Therefore, the cholesterol pathway might be more involved in increasing the risk of atherosclerotic diseases in patients with AAA rather than in the mechanisms of AAA formation. Because the causes of both AAA and atherosclerosis are multifactorial, we speculate that plasma PAF-AH deficiency is not a definitive but a relative risk factor for susceptibility to these diseases. Further studies are needed to define the role of PAF-AH interacting with other risk factors in a complex multifactorial disorder. Larger populations of the patients, including more diverse groups and people from ethnic origins, need to be studied prospectively to investigate the role of PAF-AH. If confirmed, our findings will have important implications for identifying patients at higher risk and will provide a rationale for systemically assessing atherosclerotic diseases.
Footnotes
This work was supported in part by a Public Trust Surgical Research grant (2001), Japan.
Correspondence: Naoki Unno, MD, Second Department of Surgery, Hamamatsu University School of Medicine, 1-20-1 Handayama, Hamamatsu, 431-3192, Japan.
E-mail: unno@hama-med.ac.jp
Accepted for publication May 22, 2001.
References
- 1.Wilmink TBM, Quick CRG, Day NE. The association between cigarette smoking and abdominal aortic aneurysm. J Vasc Surg 1999; 30: 1099–1105. [DOI] [PubMed] [Google Scholar]
- 2.Reed D, Reed C, Stemmermann G, et al. Are aortic aneurysms caused by atherosclerosis? Circulation 1992; 85: 205–211. [DOI] [PubMed] [Google Scholar]
- 3.Watt HC, Law MR, Wald NJ, et al. Serum triglyceride: a possible risk factor for ruptured abdominal aortic aneurysm. Int J Epidemiol 1998; 27: 949–952. [DOI] [PubMed] [Google Scholar]
- 4.Shan L, Nishimura Y, Kotani Y, et al. Platelet-activating factor increases the expression of metalloproteinase-9 in human bronchial epithelial cells. Eur J Pharmacol 1999; 374: 147–156. [DOI] [PubMed] [Google Scholar]
- 5.Sugano T, Nasu K, Narahara H, et al. Plarelet-activating factor induces an imbalance between matrix metalloproteinase-1 and tissue inhibitor of metalloproteinase-1 expression in human uterine cervical fibroblasts. Biol Reprod 2000; 62: 540–546. [DOI] [PubMed] [Google Scholar]
- 6.Watson AD, Navab M, Hama SY. Effect of platelet activating factor-acetylhydrolase on the formation and action of minimally oxidized low density lipoprotein. J Clin Invest 1995; 95: 774–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miwa M, Miyake T, Yamanaka T, et al. Characterization of serum platelet-activating factor (PAF) acetylhydrolase: Correlation between deficiency of serum PAF acetylhydrolase and respiratory symptoms in asthmatic children. J Clin Invest 1988; 82: 1983–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stafforini DM, Satoh K, Atkinson DL, et al. Platelet-activating factor acetylhydrolase deficiency: A missense mutation near the active site of an anti-inflammatory phospholipase. J Clin Invest 1996; 97: 2784–2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu H, Iijima K, Shiozawa S, et al. Platelet-activating factor acetylhydrolase gene mutation in Japanese nephrotic children. Kidney Int 1998; 54: 1867–1871. [DOI] [PubMed] [Google Scholar]
- 10.Yamada Y, Ichihara S, Fujimura T, et al. Identification of the G994 - T missence mutation in exon 9 of the plasma platelet-activating factor acetylhydrolase gene as an independent risk factor for coronary artery disease in Japanese men. Metabolism 1998; 47: 177–181. [DOI] [PubMed] [Google Scholar]
- 11.Hiramoto M, Yoshida H, Imaizumi T, et al. A mutation in plasma platelet-activating factor acetylhydrolase (Val279 - Phe) is a genetic risk factor for stroke. Stroke 1997; 28: 2417–2420. [DOI] [PubMed] [Google Scholar]
- 12.Unno N, Nakamura, T, Kaneko H, et al. Plasma platelet-activating factor acetylhydrolase deficiency is a genetic risk factor for atherosclerotic occlusive disease in Japan. J Vasc Surg 2000; 32: 263–267 [DOI] [PubMed] [Google Scholar]
- 13.Newman KM, Malon AM, Shin RD. Matrix metalloproteinases in abdominal aortic aneurysm: Characterization, purification, and their possible sources. Connect Tissue Res 1994; 30: 265–276. [DOI] [PubMed] [Google Scholar]
- 14.Freestone T, Turner RJ, Coady A. Inflammation and matrix metalloproteinases in the enlarging abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol 1995; 15: 1145–1151. [DOI] [PubMed] [Google Scholar]
- 15.Koch AE, Haines GK, Rizzo RJ. Human abdominal aortic aneurysms: Immunophenotypic analysis suggesting an immune-mediated response. Am J Pathol 1990; 137: 1199–1213. [PMC free article] [PubMed] [Google Scholar]
- 16.Thompson RW, Holmes DR, Mertens RA. Production and localization of 92-kilodalton gelatinase in abdominal aortic aneurysms: An elastolytic metalloproteinase expressed by aneurysm-infiltrating macrophages. J Clin Invest 1995; 96: 318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tjoelker LW, Wilder C, Eberhardt C, et al. Anti-inflammatory properties of a platelet-activating factor acetylhydrolase. Nature 1995; 374: 549–553. [DOI] [PubMed] [Google Scholar]
- 18.Stemme S, Faber B, Holm J, et al. T lymphocytes from human atherosclerotic plaques recognize oxidized low density lipoprotein. Proc Natl Acad Sci USA 1995; 92: 3893–3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stafforini DM, Zimmerman GA, McIntyre TM. The platelet-activating factor acetylhydrolase from human plasma prevents oxidative modification of low-density lipoprotein. Trans Assoc Am Physicians. 1992; 105: 44–63. [PubMed] [Google Scholar]
- 20.Guerra R, Zhao B, Mooser V, et al. Determinants of plasma platelet-activating factor acetylhydrolase: heritability and relationship to plasma lipoproteins. J Lipid Res 1997; 38: 2281–2288. [PubMed] [Google Scholar]