Abstract
Objective
To evaluate the pathogenesis of metaplastic processes within the esophagus using a human model in which the exact duration of reflux was known.
Summary Background Data
The pathogenesis of Barrett’s esophagus (BE) is incompletely understood. Patients undergoing esophagectomy and gastric tube reconstruction represent a good model for studying the pathophysiology of columnar cell metaplasia of the human esophagus because the cervical esophagus is rarely or never exposed to gastric contents before the surgical procedure.
Methods
Thirty-two patients underwent manometry, simultaneous 24-hour pH and bilirubin monitoring, and endoscopy with biopsy 3 to 10.4 years after esophagectomy. The presence of columnar mucosa in the cervical esophagus was confirmed on histologic examination. The findings on endoscopy and histology were related to clinical data and the results of pH and bilirubin monitoring 1 cm proximal to the esophagogastrostomy.
Results
Fifteen (46.9%) of the 32 patients had metaplastic columnar mucosa within their cervical esophagus. Metaplasia was significantly more common in patients with a preoperative diagnosis of BE. The length of metaplastic mucosa correlated significantly with the degree of esophageal acid exposure, but the presence of abnormal bilirubin exposure was unrelated to the presence of metaplasia. The prevalence of metaplasia did not change with increasing time. Intestinal metaplasia was found within the columnar-lined segment in three patients 8.5, 9.5, and 10.4 years after esophagectomy. All patients with intestinal metaplasia had abnormal exposure of both acid and bilirubin, but the presence of combined reflux was not significantly higher in these patients compared with patients with nonintestinalized segments of columnar mucosa.
Conclusions
Esophageal columnar metaplasia is a common complication after gastric pull-up esophagectomy. Metaplasia is more likely to develop in patients with previous BE than other patients. Metaplasia develops in response to squamous epithelial injury in predisposed individuals.
The clinical significance of Barrett’s esophagus (BE) lies in its association with esophageal adenocarcinoma. The risk for this lethal malignancy has been reported to be 40 to 125 times greater in patients with BE compared with the normal population. 1–3 The pathogenesis of BE is incompletely understood, but the condition is commonly believed to be the result of damaged squamous epithelium being replaced by cardiac- or junctional-type mucosa within which, over time and with persistent inflammation, intestinal metaplasia develops. 4–9
Patients who have undergone esophagectomy and gastric tube reconstruction have an anastomosis between the cervical esophagus and the stomach in which the normal squamous epithelium of the esophagus is interposed to the acid-secreting mucosa of the gastric body. The reconstruction allows acid and duodenal juice to reflux from the gastric conduit to the remaining proximal esophagus, acting as model of gastroesophageal reflux. Both experimental and clinical studies suggest that reflux of gastric and duodenal juice and the duration of reflux are important factors in the pathogenesis of Barrett’s metaplasia and esophageal adenocarcinoma. 8,10,11 Thus, the gastric pull-up esophagectomy can be used to study the development of columnar metaplasia in vivo in humans. Previous histologic examination of the removed esophagus and stomach guarantees tumor- and metaplasia-free anastomotic edges. The exact time for the occurrence of reflux is known because the proximal esophagus to which the gastric tube is anastomosed has rarely or never been exposed to reflux before the surgical procedure. By evaluating patients operated on at different times, correlations between time and the presence and type of metaplasia may be found.
The aim of this study was to evaluate the pathogenesis of metaplastic processes within the esophagus using a human model in which the exact duration of reflux was known. This was done by characterizing and comparing physiologic, demographic, and clinical data in patients with and without metaplastic columnar mucosa in the cervical esophagus after esophagectomy and gastric tube reconstruction.
PATIENTS AND METHODS
Patients
Between December 1989 and October 1997, 142 consecutive patients underwent esophagectomy and gastric tube reconstruction at the Department of Surgery, Lund University Hospital. By March 2000, 82 patients had died and 60 were still alive. All surviving patients were invited to take part in the study. Twenty-eight patients declined to participate because of other coexisting medical illness, old age, or unwillingness to undergo the tests. The remaining 32 patients (18 men and 14 women, median age 64 years [range 46–84]) were evaluated by manometry, simultaneous pH and bilirubin monitoring, and endoscopy with biopsy. Symptoms of heartburn and regurgitation were graded from 0 to 3 (absent to severe). Cervical heartburn was defined as a burning sensation in the chest or throat.
Surgical Procedure
The abdomen was explored through an upper midline incision. After standard dissection, which included removal of fat and regional lymph nodes, the gastric tube was created using a linear cutting stapler (TLC 55, Ethicon, Stockholm, Sweden, or GIA, Autosuture, Stockholm, Sweden). The stapler was fired multiple times from the lesser curvature approximately 7 cm cranial to the pylorus along the axis of the greater curvature, creating a 5-cm-wide tube. A pyloroplasty was performed when deemed necessary (n = 7). A right posterolateral thoracotomy provided access for dissection of the thoracic esophagus and the mediastinal lymph nodes. The esophageal substitutes were placed in the posterior mediastinum. Anastomoses in the apex of the right chest (n = 25) were stapled using a circular stapling device (Premium CEEA, Autosuture). A 90-mm linear stapling device (RL 90, Ethicon) was used to resect the remaining stomach, including the gastrostomy previously used for the introduction of the circular stapler. Seven patients had hand-sutured end-to-end anastomoses in the neck using a single continuous all-layer monofilament 4-0 suture (PDS II, Ethicon). The cervical end of the resected esophagus was inspected to make sure that the squamous epithelium was normal and that no Barrett’s mucosa was present above the esophagogastrostomy. The absence of metaplastic columnar mucosa in the upper resection line was confirmed by histopathologic examination.
Stationary Manometry
Standard stationary manometry was performed after an overnight fast to evaluate the function of the esophageal remnant and to determine the position of the esophagogastrostomy. A manometry catheter consisting of three channels placed at 5-cm intervals was passed transnasally and was positioned in the gastric tube. The catheter was withdrawn from the stomach and through the anastomosis to the cervical esophagus in 1-cm increments. The patients were given wet swallows of 5 mL water at each level to detect esophageal contractions as the sensors entered the cervical esophagus. The position of the esophagogastrostomy and the contraction amplitudes of the cervical esophagus were calculated from the mean of three recordings.
Combined Ambulatory Esophageal pH and Spectrometric Bilirubin Monitoring
Esophageal bilirubin monitoring was performed simultaneously with pH monitoring. Medications known to influence gastric acid secretion were not discontinued in an attempt to record the degree of esophageal acid and bilirubin exposure representative of the time after esophagectomy. An antimony catheter (Medtronic Synectics, Shoreview, MN) and a fiberoptic probe designed to detect bilirubin (Bilitec 2000, Medtronic Synectics) were passed transnasally and positioned 1 cm above the manometrically defined esophagogastrostomy. Esophageal pH was recorded on a portable digital data recorder and was analyzed as previously described. 12 The subjects were instructed to carry out their normal daily activities but to avoid strenuous exertion. A diary was kept of food and fluid intake, symptoms, and time spent in supine and upright positions. Based on reports of esophageal acid exposure in normal volunteers, abnormal acid exposure was diagnosed when more than 4.4% of the monitored time was spent below pH 4.0. 12
Esophageal bilirubin exposure was measured by spectrophotometry based on the specific light absorption of bilirubin at a wavelength of 453 nm and recorded on a portable opticoelectric data logger. 13–15 The patients were instructed to follow a special diet, which involved restriction to three meals a day and no food with an absorbance similar to that of bilirubin. 10 The fiberoptic probe was calibrated in water before and after monitoring. An absorbance threshold of 0.2 was selected and bilirubin exposure was quantified as the percentage of time above this threshold. 16 Records with a bilirubin absorbance drift equal to or greater than 0.15 were discarded. Twenty-four-hour bilirubin absorbance data were analyzed with a software program (Gastrosoft, Irving, TX) to calculate the total percentage of time the bilirubin absorbance exceeded 0.2 during the total monitored period. Based on reports of esophageal bilirubin exposure in normal volunteers, abnormal bilirubin exposure was diagnosed when more than 1.7% of the monitored time was spent above the absorbance threshold of 0.2. 17
Endoscopy
Upper gastrointestinal endoscopy was performed with the pH and Bilitec catheters still in the esophageal remnant. The position of the catheters and their relationship to the position of the esophagogastrostomy were noted. The presence of metaplastic columnar mucosa in the esophagus was suspected when circumferential areas or tongues of pink-appearing glandular mucosa extended from the esophagogastrostomy into the white-appearing squamous epithelium of the cervical esophagus. The extent of esophageal columnar mucosa was defined as the distance from the esophagogastrostomy to the highest point of the squamocolumnar junction. Multiple biopsy samples were obtained from areas of metaplastic columnar mucosa. The presence of a columnar-lined esophagus was confirmed on histologic evaluation of the biopsy specimens. The presence of erosive esophagitis was noted and biopsy samples were also obtained from these areas to rule out the presence of metaplastic columnar mucosa. The gastric tube was inspected and multiple biopsy samples were obtained from the gastric antrum for the analysis of gastric intestinal metaplasia and Helicobacter pylori infection.
Histologic Assessment
All biopsy specimens underwent routine fixation and staining with hematoxylin and eosin. Cardiac-type mucosa was characterized by a mucosa lined by gastric-type surface and foveolar cells with glands composed entirely of mucous cells without any parietal or chief cells. Oxyntocardiac mucosa was defined by a mucosa lined by gastric-type surface and foveolar cells with glands containing a mixture of mucous, oxyntic, and chief cells. 18 Specialized intestinal metaplasia was identified by the presence of well-defined goblet cells within columnar epithelium. The biopsy specimens were evaluated for the presence of H. pylori infection using a Giemsa stain.
Statistical Analysis
Data are reported as median and interquartile ranges unless otherwise stated. The Fisher exact test was used to compare proportions and the Mann-Whitney test was used to compare continuous data between two groups. The ethical research committee of Lund University approved the study.
RESULTS
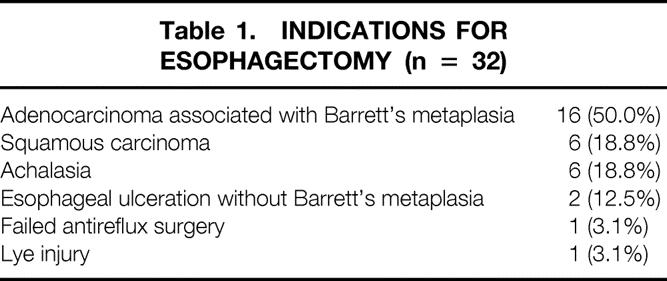
Table 1 shows the indications for esophagectomy in the study population. Sixteen (50.0%) patients had a diagnosis of BE before surgery. The median time after esophagectomy was 4.9 years (range 3.0–10.4). At the time of the study, 10 of the 32 patients (31.2%) were receiving no acid-suppression therapy; the remaining 22 (68.8%) were being treated with chronic acid-suppression medication for symptoms of reflux. Eight of these patients were being treated with a single daily dose of proton pump inhibitors and 12 were receiving proton pump inhibitors twice daily. Two patients were being treated with H2 receptor antagonists.
Table 1. INDICATIONS FOR ESOPHAGECTOMY (n = 32)
Fifteen (46.9%) of the 32 patients had segments of metaplastic columnar mucosa in the cervical esophagus on endoscopy, which was confirmed on histologic evaluation of the biopsy specimens. The median length of the columnar-lined segment was 0.5 cm (range 0.5–4).
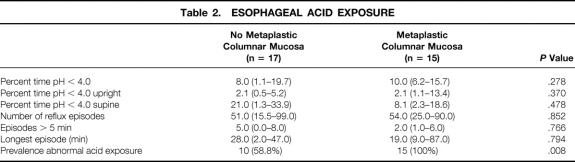
Table 2 shows the degree and pattern of esophageal acid exposure in patients with and without metaplastic columnar mucosa within the cervical esophagus. Despite the use of potent acid-suppression medication, the median percentage of time spent below pH 4.0 was high in both groups. There was no difference in the median percentage of time below pH 4.0, but the prevalence of abnormal acid exposure was significantly higher in patients with columnar metaplasia. Acid exposure occurred mainly in the supine position. The majority of patients had no or mild symptoms of heartburn and regurgitation after esophagectomy. The relationship between the severity of these symptoms and the degree of acid exposure in the cervical esophagus is shown in Figures 1 and 2. The median percentage of time below pH 4.0 was high also in the absence of heartburn and regurgitation, indicating that severe reflux is common also in asymptomatic patients after esophagectomy and gastric tube reconstruction.
Table 2. ESOPHAGEAL ACID EXPOSURE
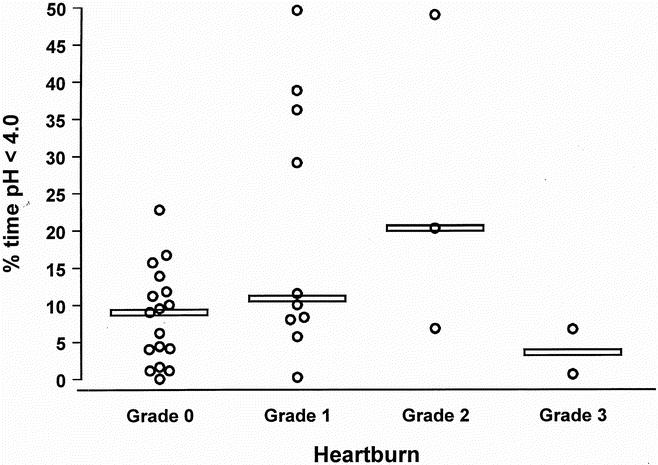
Figure 1. Relationship between the severity of cervical heartburn and the degree of acid exposure in the cervical esophagus. Values for each subject are plotted and the horizontal line denotes the median of each group.
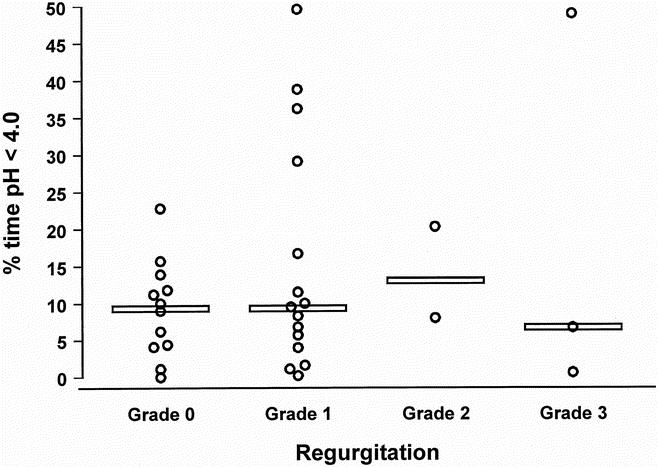
Figure 2. Relationship between the severity of regurgitation and the degree of acid exposure in the cervical esophagus. Values for each subject are plotted and the horizontal line denotes the median of each group.
Figure 3 relates the degree of esophageal acid exposure to the extent of metaplastic mucosa within the esophagus. There was a direct correlation between the length of the metaplastic segment and the percentage of time the cervical esophagus was exposed to pH less than 4.0.
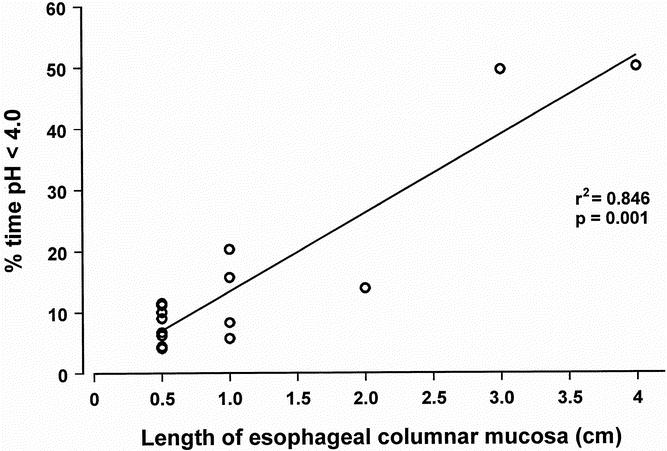
Figure 3. Relationship between extent of metaplasia in the cervical esophagus (cm) and the percentage of time spent below pH 4.0 on 24-hour esophageal pH monitoring (n = 15).
The degree of esophageal acid exposure was significantly higher in patients with esophageal mucosal injury (erosive esophagitis or metaplastic columnar mucosa) compared with those without (11.2 vs. 4.0, P = .030). However, the contraction amplitude of the cervical esophagus did not appear to be an important factor for clearing refluxed gastric juice. This was suggested by similar median contraction amplitudes in patients with and without mucosal injury (62.2 vs. 40.0 mm Hg, P = .253).
Esophageal exposure to duodenal juice in patients with and without esophageal columnar metaplasia is shown in Table 3. The median percentage of time spent above bilirubin absorbance of 0.2 was similar in the two groups. In contrast to acid reflux, reflux of duodenal juice occurred more frequently in the upright position. The prevalence of abnormal bilirubin exposure was similar in patients with and without pyloroplasty (71.4% vs. 64.0%, P = 1.00). Further, patients with a pyloroplasty had a similar prevalence of esophageal columnar metaplasia compared with those without a pyloroplasty (42.9% vs. 48.0%, P = 1.00).
Table 3. ESOPHAGEAL BILIRUBIN EXPOSURE
The prevalence of esophageal columnar metaplasia was significantly higher in patients with a preoperative diagnosis of BE compared with those without. Metaplasia was found in 11 of the 16 patients (68.8%) with BE at the time for esophagectomy and in 4 of the 16 patients (25.0%) with no previous BE (P = .032). There was no difference in the median time spent below pH 4.0 between patients with and without BE before the esophagectomy (8.7% vs. 9.8%, P = 1.00). The susceptibility of the squamous epithelium to erosive esophagitis in response to increased acid exposure in the cervical esophagus was similar in the two groups (43.8% vs. 50.0%, P = 1.00). The relationships between the prevalence of erosive esophagitis, esophageal columnar metaplasia, and the degree of esophageal acid exposure in patients with and without preoperative BE are shown in Figures 4 and 5. The prevalence of esophagitis was similar in the two groups, but metaplasia was found significantly more often in patients with BE before surgery. There was no increase in the prevalence of these types of mucosal injury with increasing degree of acid exposure in the cervical esophagus. Figure 6 shows the prevalence of esophageal columnar metaplasia in patients with varying lengths of time since surgery. The prevalence of metaplasia did not increase with time.
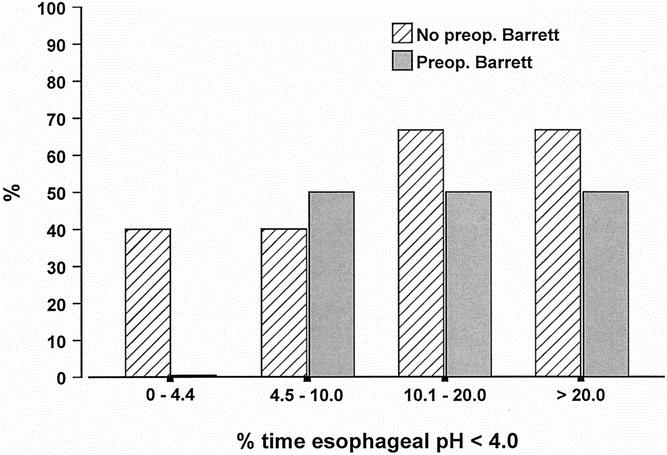
Figure 4. Relationship between the prevalence of erosive esophagitis and the degree of esophageal acid exposure in patients with and without Barrett’s esophagus before esophagectomy and gastric tube reconstruction.
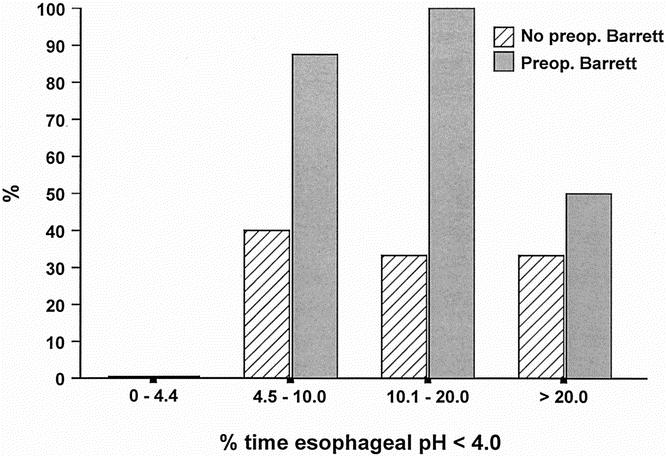
Figure 5. Relationship between the prevalence of columnar metaplasia within the cervical esophagus and the degree of esophageal acid exposure in patients with and without Barrett’s esophagus before esophagectomy and gastric tube reconstruction.
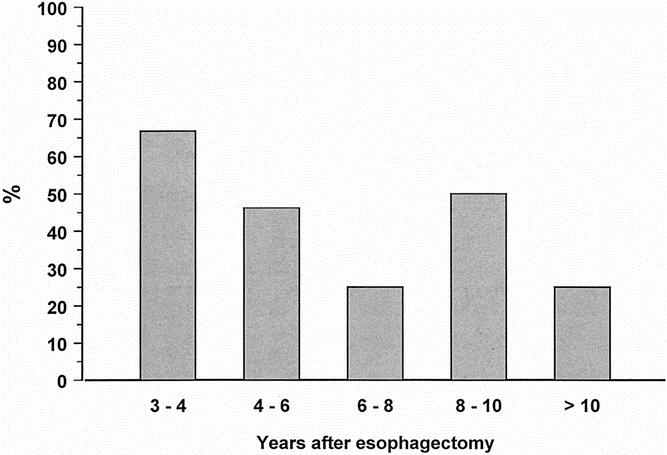
Figure 6. Prevalence of columnar metaplasia within the cervical esophagus in patients with varying lengths of postoperative period after esophagectomy and gastric tube reconstruction.
Histopathologic evaluation of the metaplastic columnar mucosa showed pure cardiac mucosa in nine patients (60.0%) and oxyntocardiac mucosa in three patients (20.0%). Goblet cells, the hallmark of intestinal metaplasia, were found in three patients 8.5, 9.5, and 10.4 years after esophagectomy. No patient with dysplastic columnar mucosa was found. The median postoperative period was significantly longer in patients with intestinal metaplasia compared with those without (9.5 vs. 4.2 years, P = .004). All patients with intestinal metaplasia had abnormal esophageal exposure of both acid and bilirubin, but the presence of combined reflux was not significantly higher in these patients compared with patients with nonintestinalized segments of columnar mucosa (3/3 [100%] vs. 4/12 [33.3%], P = .077).
H. pylori was found in only one patient on the histologic evaluation of the biopsy samples from the gastric antrum and was hence unassociated with the presence of metaplastic columnar mucosa in the cervical esophagus (P = .452). One patient without esophageal columnar metaplasia had intestinal metaplasia in the gastric antrum but no evidence of H. pylori infection.
DISCUSSION
Metaplastic columnar mucosa is a well-known phenomenon after esophagectomy and gastric tube reconstruction. 19–21 In this study patients who underwent this surgical procedure were studied to evaluate the pathophysiology of esophageal columnar metaplasia, thus acting as a human model of gastroesophageal reflux where the exact time for the induction of reflux to squamous epithelium was known.
We have shown that after esophagectomy and gastric tube reconstruction, the cervical esophagus is exposed to high amounts of acid despite the use of potent acid-suppression therapy and the absence of severe symptoms. Further, metaplastic esophageal columnar mucosa is a common complication secondary to reflux-induced injury to the squamous epithelium after gastric tube reconstruction. The short lengths of columnar metaplasia found in many of the patients in this study raise the question of whether these changes represent true metaplasia or not. However, we believe that the finding of pure cardiac or oxyntocardiac mucosa within the cervical esophagus is clear evidence of esophageal columnar metaplasia because the region of the gastroesophageal junction, where these mucosal types may be found, 22–24 is resected during esophagectomy.
Although the study population represents only a significant subset of the overall group having esophageal resections (32/60), we do not believe there is a significant risk of bias in our results. The majority of the 28 patients who declined to participate did so because of old age or other coexisting medical illness. It is possible that the patients included in the study were more symptomatic compared with the patients who declined to participate. However, metaplastic changes in the cervical esophagus were unrelated to the presence as well as the severity of symptoms, suggesting that the same prevalence of metaplastic changes may be expected in a less symptomatic group.
Metaplastic esophageal columnar mucosa is believed to develop as a result of a peculiar type of healing from reflux-induced injury to squamous epithelium. In this study the degree of esophageal acid exposure and the prevalence of erosive esophagitis were similar in patients with and without preoperative BE. This indicates that the susceptibility of the squamous epithelium to erosive damage in response to refluxed gastric juice is similar in the two groups. In contrast to the erosive manifestations, there was a sharp distinction in the propensity to develop metaplastic mucosa within the esophagus in response to mucosal injury. This was manifested by the prevalence of columnar metaplasia, which was significantly higher in patients with a diagnosis of BE before esophagectomy. These results suggest that some individuals are less prone to metaplastic changes within the esophagus despite the presence of severe reflux and erosive esophagitis, whereas others, in whom metaplasia develops, have the predisposition required for metaplasia development. It is unlikely that the difference in metaplasia development is explained by differences in the composition of the refluxate. Bilirubin is just a marker for duodenoesophageal reflux, and potentially injurious compounds of duodenal juice, such as the amount and the type of the individual bile acids, pancreatic enzymes, and lysolecithin are not measured by this technique. Although it is possible, it is unlikely that these other factors are essential for the development of columnar metaplasia because esophageal exposure of duodenal juice was similar in patients with and without metaplasia. Most likely metaplasia develops in response to erosive squamous epithelial injury in patients with the required underlying genetic traits, regardless of the composition of the refluxate. Epidemiologic support for the importance of genetic factors in the development of metaplastic changes is provided by reports of strong familial expression of BE. 25–28 The observation that metaplasia primarily develops in patients with a certain predisposition has important implications for understanding the pathogenesis of BE and managing patients with reflux disease.
The prevalence of metaplasia was similar at different times after esophagectomy. This observation suggests that the prevalence of metaplasia does not increase with time but that metaplasia develops early in response to mucosal injury in patients with the required underlying predisposition. The observation that metaplasia develops early is emphasized by one of the patients in this study who had a 1-cm-long segment of metaplastic columnar mucosa on a routine endoscopic control 13 months after surgery. At the time of this study, 39 months later, the metaplastic segment was 2 cm long. The biopsy samples confirmed the presence of cardiac-type mucosa, but no intestinal metaplasia was found. In this study there was a correlation between the length of the columnar-lined segment and the degree of esophageal acid exposure. This confirms the results of previous reports 29 and suggests that the length of metaplasia is determined by the degree of esophageal acid exposure. It is possible that the length of the metaplastic columnar mucosa increases if the severity of reflux disease worsens, but longitudinal studies would be needed to test this hypothesis.
A recent study by Öberg et al 8 reported that patients with intestinal metaplasia in short segments of columnar lining, in addition to longer duration of reflux symptoms, had a significantly higher prevalence of abnormal duodenoesophageal reflux than patients with no intestinal metaplasia. It was suggested that the presence of duodenoesophageal reflux and the duration of reflux might be important factors in the pathogenesis of intestinal metaplasia. The importance of duodenoesophageal reflux for the development of intestinal metaplasia was not confirmed in this study, even though there was a tendency toward a higher prevalence of abnormal bilirubin exposure in the patients with intestinalized metaplastic mucosa. Abnormal bilirubin exposure was seen in all three patients with intestinal metaplasia. Further studies are needed to clarify the role of duodenal reflux in the pathogenesis of intestinal metaplasia. Intestinal metaplasia was detected 8.5, 9.5, and 10.4 years after esophagectomy, and the median length of the postoperative period was significantly longer in these patients compared with patients without intestinal metaplasia. These results confirm the observations by Öberg et al 8 and indicate that intestinal metaplasia develops over time and under the proper luminal conditions in already established segments of columnar metaplasia as a second step in the pathogenesis of BE. The hypothesis that there is a sequential development from squamous epithelium to cardiac-type mucosa to intestinal metaplasia in response to acid-induced injury is also supported by the observations of Hamilton and Yardley. 19 They reported on the development of Barrett’s mucosa in the esophagus above the anastomosis in three patients after esophagogastrectomy. In two, they documented progression from squamous epithelium to cardiac mucosa and subsequently intestinal metaplasia over 6.3 and 10 years.
The vast majority of the patients in this study were satisfied with the results of surgery, despite the need for chronic acid-suppression therapy. However, the high degree of acid exposure in the cervical esophagus and the high prevalence of metaplastic columnar mucosa after esophagectomy and gastric tube reconstruction raise the question of whether the stomach should be a preferred organ for reconstruction after esophagectomy. Importantly, the clinical significance of the metaplastic changes found in this study is unclear. Although other substitutes offer theoretic advantages, these remain to be proven. Possibly, young individuals, especially those with known BE and those with a good probability of permanent cure from malignant disease, should be offered reconstruction using a colon interposition.
It is unclear whether a pyloroplasty results in higher degrees of duodenoesophageal reflux and whether it should be performed routinely during esophagectomy and gastric tube reconstruction. In this study there was no significant difference in the prevalence of abnormal bilirubin exposure in patients with and without pyloroplasty. However, because the study population was small and only seven patients had a pyloroplasty, the impact of a pyloroplasty on the reflux of duodenal juice is still unclear. Future studies are needed.
We conclude that after esophagectomy and gastric tube reconstruction, the cervical esophagus is exposed to high amounts of acid despite the use of potent acid-suppression therapy and the absence of severe symptoms. Esophageal columnar metaplasia is a common complication after gastric pull-up esophagectomy. The presence of abnormal bilirubin exposure was unrelated to the presence of esophageal columnar metaplasia but may be an important factor in the development of intestinal metaplasia. Patients with previous BE esophagus are more prone to metaplasia than other patients. These findings suggest that metaplasia develops in response to erosive squamous epithelial injury in predisposed individuals, regardless of the composition of the refluxate. It is possible that in addition to reflux and esophageal mucosal injury, certain genetic traits may be necessary for the development of metaplastic changes, but genetic studies are needed to test this hypothesis. The observation that an underlying predisposition may be important for the development of metaplastic changes within the esophagus introduces a new concept to the pathogenesis of esophageal columnar metaplasia that may greatly increase our understanding of this controversial condition.
Footnotes
Correspondence: Stefan Öberg, MD, Department of Surgery, Lund University Hospital, 221 85 Lund, Sweden.
E-mail: stefan.oberg@skane.se
Accepted for Publication August 7, 2001.
References
- 1.Hameeteman W, Tytgat GN, Houthoff HJ, et al. Barrett’s esophagus: development of dysplasia and adenocarcinoma. Gastroenterology 1989; 96: 1249–1256. [DOI] [PubMed] [Google Scholar]
- 2.Cameron AJ, Ott BJ, Payne WS. The incidence of adenocarcinoma in columnar-lined (Barrett’s) esophagus. N Engl J Med 1985; 313: 857–859. [DOI] [PubMed] [Google Scholar]
- 3.Van der Veen AH, Dees J, Blankensteijn JD, et al. Adenocarcinoma in Barrett’s oesophagus: an overrated risk. Gut 1989; 30: 14–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldman MC, Beckman RC. Barrett syndrome: case report with discussion about concepts of pathogenesis. Gastroenterology 1960; 39: 104–110. [PubMed] [Google Scholar]
- 5.Bremner CG, Lynch VP, Ellis FHJ. Barrett’s esophagus: congenital or acquired? An experimental study of esophageal mucosal regeneration in the dog. Surgery 1970; 68: 209–216. [PubMed] [Google Scholar]
- 6.Hage E, Pederson SA. Morphological characteristics of the columnar epithelium lining the lower esophagus in patients with Barrett’s syndrome. Virchows Arch 1972; 357: 219–229. [DOI] [PubMed] [Google Scholar]
- 7.Weinstein WM, Ippoliti AF. The diagnosis of Barrett’s esophagus: goblets, goblets, goblets. Gastrointest Endosc 1996; 44: 91–95. [DOI] [PubMed] [Google Scholar]
- 8.Öberg S, Peters JH, DeMeester TR, et al. Determinants of intestinal metaplasia within the columnar-lined esophagus. Arch Surg 2000; 135: 651–655. [DOI] [PubMed] [Google Scholar]
- 9.Weston AP, Krmpotich P, Makdisi WF, et al. Short segment Barrett’s esophagus: clinical and histological features, associated endoscopic findings, and association with gastric intestinal metaplasia. Am J Gastroenterol 1996; 91: 981–986. [PubMed] [Google Scholar]
- 10.Vaezi MF, Richter JE. Role of duodenogastroesophageal reflux in gastroesophageal reflux disease. Gastroenterology 1996; 111: 1192–1199. [DOI] [PubMed] [Google Scholar]
- 11.Ireland AP, Peters JH, Smyrk TC, et al. Gastric juice protects against the development of esophageal adenocarcinoma in the rat. Ann Surg 1996; 224: 358–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jamieson JR, Stein HJ, DeMeester TR, et al. Ambulatory 24-h esophageal pH monitoring: normal values, optimal thresholds, specificity, sensitivity, and reproducibility. Am J Gastroenterol 1992; 87: 1102–1111. [PubMed] [Google Scholar]
- 13.Bechi P, Pucciani F, Baldini F, et al. Long-term ambulatory enterogastric reflux monitoring Validation of a new fiberoptic technique. Dig Dis Sci 1993; 38: 1297–1306. [DOI] [PubMed] [Google Scholar]
- 14.Vaezi MF, Lacamera RG, Richter JE. Validation studies of Bilitec 2000: an ambulatory duodenogastric reflux monitoring system. Am J Physiol 1994; 267: G1050–1057. [DOI] [PubMed] [Google Scholar]
- 15.Kauer WK, Burdiles P, Ireland AP, et al. Does duodenal juice reflux into the esophagus of patients with complicated GERD? Evaluation of a fiberoptic sensor for bilirubin. Am J Surg 1995; 169: 98–103. [DOI] [PubMed] [Google Scholar]
- 16.Fein M, Ritter MP, Peters JH, et al. The optimal absorbance threshold for spectrophotometric bilirubin monitoring (Bilitec) in the esophagus. Gastroenterology 1997; 112: A115. [Google Scholar]
- 17.Fein M, Ireland AP, Ritter MP, et al. Duodenogastric reflux potentiates the injurious effects of gastroesophageal reflux. J Gastrointest Surg 1997; 1: 27–33. [DOI] [PubMed] [Google Scholar]
- 18.Chandrasoma P. Norman Barrett: So close, yet 50 years away from the truth. J Gastrointest Surg 1999; 3: 7–14. [Google Scholar]
- 19.Hamilton SR, Yardley JH. Regenerative of cardiac type mucosa and acquisition of Barrett mucosa after esophagogastrostomy. Gastroenterology 1977; 72: 669–675. [PubMed] [Google Scholar]
- 20.Lindahl H, Rintala R, Sariola H, et al. Cervical Barrett’s esophagus: a common complication of gastric tube reconstruction. J Pediatr Surg 1990; 25: 446–448. [DOI] [PubMed] [Google Scholar]
- 21.Gutschow C, Collard JM, Romagnoli R, et al. Denervated stomach as an esophageal substitute recovers intraluminal acidity with time. Ann Surg 2001; 233: 509–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayward J. The lower end of the oesophagus. Thorax 1961; 16: 36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chandrasoma PT, Der R, Ma Y, et al. Histology of the gastroesophageal junction: an autopsy study. Am J Surg Pathol 2000; 24: 402–409. [DOI] [PubMed] [Google Scholar]
- 24.Kilgore SP, Ormsby AH, Gramlich TL, et al. The gastric cardia: fact or fiction? Am J Gastroenterol 2000; 95: 921–924. [DOI] [PubMed] [Google Scholar]
- 25.Fahmy N, King JF. Barrett’s esophagus: an acquired condition with genetic predisposition. Am J Gastroenterol 1993; 88: 1262–1265. [PubMed] [Google Scholar]
- 26.Eng C, Spechler SJ, Ruben R, et al. Familial Barrett esophagus and adenocarcinoma of the gastroesophageal junction. Cancer Epidemiol Biomarkers Prev 1993; 2: 397–399. [PubMed] [Google Scholar]
- 27.Prior A, Whorwell PJ. Familial Barrett’s oesophagus? Hepatogastroenterology 1986; 33: 86–87. [PubMed] [Google Scholar]
- 28.Crabb DW, Berk MA, Hall TR, et al. Familial gastroesophageal reflux and development of Barrett’s esophagus. Ann Intern Med 1985; 103: 52–54. [DOI] [PubMed] [Google Scholar]
- 29.Öberg S, DeMeester TR, Peters JH, et al. The extent of Barrett’s esophagus depends on the status of the lower esophageal sphincter and the degree of esophageal acid exposure. J Thorac Cardiovasc Surg 1999; 117: 572–580. [DOI] [PubMed] [Google Scholar]