Abstract
Objective
To evaluate the clinical presentation, diagnostic procedures, and surgical management of hepatic abscesses in patients with chronic granulomatous disease (CGD).
Summary Background Data
Chronic granulomatous disease is a rare inherited primary immunodeficiency in which phagocytes cannot destroy catalase-positive bacteria and fungi. Defects in the phagocytic cells’ respiratory burst lead to life-threatening infections, including hepatic abscess. These abscesses are recurrent and often multiple and are treated differently from bacterial abscesses in patients without CGD.
Methods
Between 1980 and 2000, 61 cases of hepatic abscess in 22 patients with CGD were treated at the National Institutes of Health. Clinicopathologic features were investigated by retrospective review of the medical records, radiographs, and histopathology.
Results
Twelve of the 61 cases were primary hepatic abscesses. Twenty-nine of the cases were recurrent hepatic abscesses, and 20 cases were persistent hepatic abscesses. The median age at the time of initial hepatic abscess presentation was 14 years. Subjective fever was the most frequent presenting symptom, and the erythrocyte sedimentation rate was elevated in 98% of cases. Fifty-two cases were managed surgically and eight cases were managed with percutaneous drainage. One patient refused surgery. The surgical complication rate was 56%; however, there were no deaths directly related to the hepatic abscesses. Staphylococcus aureus was the most frequent organism identified in culture (88% of positive cultures). Aggressive surgery and antibiotics ultimately resulted in successful treatment of all patients.
Conclusions
Hepatic abscesses occurring in patients with CGD represent a difficult diagnostic and treatment challenge. Early excision and treatment with antibiotics directed against S. aureus is necessary. General surgeons should be aware of this rare immunodeficiency and should aggressively manage hepatic abscesses in these patients.
Chronic granulomatous disease (CGD) is a rare inherited disease of childhood. Mutations in any of the peptide subunits of the NADPH oxidase complex lead to a defective respiratory burst in phagocytes. Four separate genetic defects, one X-linked and three autosomal, may lead to mutations in either cytosolic or membrane-bound components of the NADPH oxidase. 1,2 CGD is characterized by recurrent, life-threatening infections by catalase-positive bacteria and fungi. 1 Patients frequently have recurrent infections involving the lymph nodes, lungs, soft tissue, bones, and liver. Excessive granuloma formation is also typical in this patient population. Most patients die of infectious complications within the first three decades of life. 3
Although hepatic abscess is a common manifestation of this disease, the management of this entity has not been clearly defined in the literature. The characteristics of hepatic abscess in patients with CGD are unique, and principles of management for other forms of hepatic abscess do not necessarily apply. Recommendations for treatment have ranged from antibiotic treatment to percutaneous drainage to open surgical debridement or resection. 1,4,5 We present the largest single-institution experience to date of hepatic abscess in patients with CGD. From our 20-year experience in the diagnosis and management of hepatic abscess in patients with CGD, we discuss the clinical aspects, radiologic evaluation, pathologic and microbiologic characteristics, and our preferred treatment approach for this entity. In addition, we attempt to define factors that can predict the persistence of hepatic abscesses in patients with CGD.
PATIENTS AND METHODS
Patient Population
Between 1980 and 2000, 156 patients with CGD were evaluated at the National Institutes of Health (NIH) on various clinical protocols for the diagnosis and management of CGD within the National Institute of Allergy and Infectious Diseases. Twenty-two of these patients were seen by the surgery service of the National Cancer Institute for management of hepatic abscesses. All of these patients had the diagnosis of CGD confirmed by either a nitroblue tetrazolium reduction or dihydrorhodamine oxidation test. The molecular defect was determined by immunoblotting or molecular typing. A retrospective chart review of these 22 patients was performed, along with a review of the pathology slides and radiologic studies. Patient characteristics from the entire CGD patient population were obtained from a clinical database.
Primary hepatic abscess was defined as an abscess occurring in a patient for the first time. A recurrent abscess (vs. persistent disease) was arbitrarily defined as any abscess presenting at least 3 months after completion of treatment for a previous hepatic abscess (including the period of antibiotic administration). Persistent disease (a failure of treatment) was defined as a persistent abscess or new abscess requiring management in the postoperative period during antibiotic therapy or within 3 months of completing operative antibiotic therapy. Twenty-two patients accounted for 61 separate cases of hepatic abscess. Twelve of the 61 cases were primary abscesses and 29 were recurrent abscesses. Nine patients failed to respond to treatment one or more times, accounting for 20 cases of persistent abscess (Fig. 1).
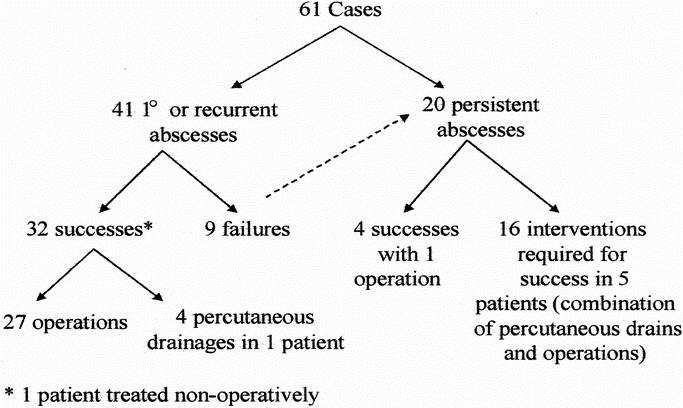
Figure 1. Outline of 61 cases. There were 12 cases of primary hepatic abscesses and 29 cases of recurrent abscesses. Failures in nine patients led to 20 cases of persistent abscesses.
Statistical Analysis
Statistical analysis for prognostic factors was performed using a univariate chi-square analysis or Wilcoxon signed-rank test.
RESULTS
Patient Characteristics
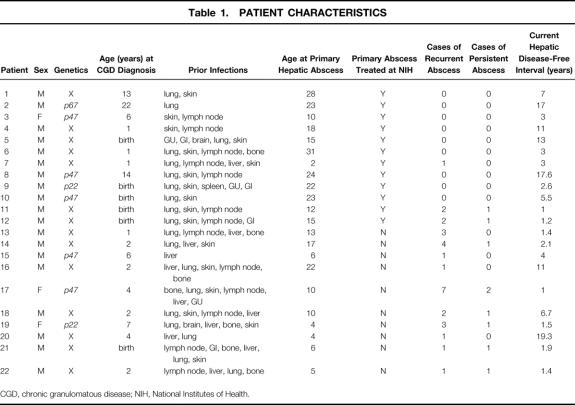
Nineteen of the 22 patients treated were male (86%) and 17 of the 22 patients were white (77%), numbers expected from previous published reports. Fourteen patients had X-linked CGD; two, five, and one patients inherited CGD in an autosomal recessive pattern from defects in the p22phox, p47phox phox, or p67phox components of NADPH oxidase, respectively. No genotype was overrepresented; the distribution of genotypes was similar to that seen in the general population of patients with CGD. The median patient age at the time of the initial CGD diagnosis was 2 years (range 1–22). The median age at the time of the initial hepatic abscess presentation was 14 years (range 2–28). All of the 22 patients had had other infections related to their CGD, including lung (86%), skin (77%), lymph nodes (64%), bone (32%), urinary tract (14%), gastrointestinal tract (14%), and brain (5%) (Table 1). In virtually all patients, infection at some other site preceded development of hepatic abscess. Only 4 of the 156 patients (3%) followed up at the NIH had a hepatic abscess as their initial manifestation of CGD.
Table 1. PATIENT CHARACTERISTICS
CGD, chronic granulomatous disease; NIH, National Institutes of Health.
Symptoms and Signs
For primary and recurrent abscesses, patients reported fever in 78% of cases. The most common other symptoms were abdominal pain (39%), fatigue (22%), weight loss (15%), and night sweats (12%). Uncommon symptoms were shoulder pain, headache, nausea, vomiting, and back pain. Abdominal tenderness and hepatomegaly were observed in 31% and 20% of cases, respectively. Therefore, most cases did not include localizing signs or symptoms to the abdomen.
Laboratory Evaluation
On admission, the white blood cell count was above normal (>9.6 × 109/L) in 46% of cases (median 9 × 109/L, range 4.1–24), and the sedimentation rate (ESR) was elevated (>42 mm in female patients and >25 mm in male patients) in 98% of cases. Alkaline phosphatase was greater than the age-appropriate level in 50% of cases. Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were above normal (ALT > 45 U/L, AST > 36 U/L) in only six and four cases, respectively.
Radiologic Evaluation
A total of 47 computed tomography (CT) scans, 37 ultrasounds, and 28 magnetic resonance (MR) imaging scans were used to evaluate the 61 cases of hepatic abscesses. No patient had a diagnosis of hepatic abscess made incidentally by radiologic imaging obtained for other purposes. Abscesses ranged from 1 to 6 cm. Since intraoperative ultrasound became available in 1987, it has been used in 30 instances.
On precontrast CT images, abscesses were lower in attenuation than the surrounding liver. On postcontrast images, the abscesses typically showed a rim of enhancement with central hypovascularity (Fig. 2). In some instances, intense enhancement of liver parenchyma adjacent to the abscess was seen and may have been caused by venous compression and poor venous drainage.
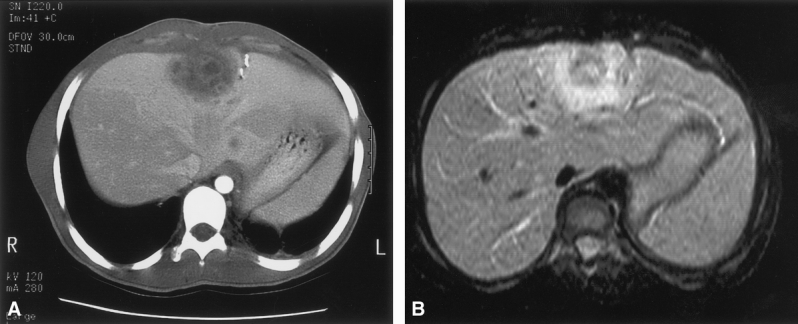
Figure 2. (A) An abdominal computed tomography scan with contrast showing a loculated, heterogenous, centrally located hepatic abscess in a patient with chronic granulomatous disease. (B) T1-weighted magnetic resonance image of the same abscess showing a uniform ring of intense enhancement surrounding central heterogeneous enhancement.
On T1-weighted MR images, abscesses showed low signal intensity relative to the surrounding liver. On fat-suppressed or short τ inversion recovery (STIR) T2-weighted images, the abscesses showed moderately high signal intensity. Intense enhancement of abscesses appeared as a uniform ring of high signal intensity with central heterogeneous enhancement on all postgadolinium T1-weighted MRIs (see Fig. 2). Smaller abscesses showed homogeneous enhancement without central hypovascularity. Extensive enhancement was observed in adjacent liver segments in some patients, likely reflecting inflammation or edema around the abscess. In contrast to active abscesses, chronic scarring or previous sites of abscess resection showed low signal intensity on T2-weighted images and did not enhance after gadolinium administration.
On sonography, abscesses appeared solid and hypoechoic. There was little flow identified by Doppler within the abscess, and the abscess was usually heterogeneous in echotexture. Scars and previous sites of surgery could not be differentiated from abscess on sonography. Intraoperative ultrasound was sensitive for lesions smaller than 5 mm in diameter and could detect small abscesses not visible with preoperative imaging (Fig. 3).
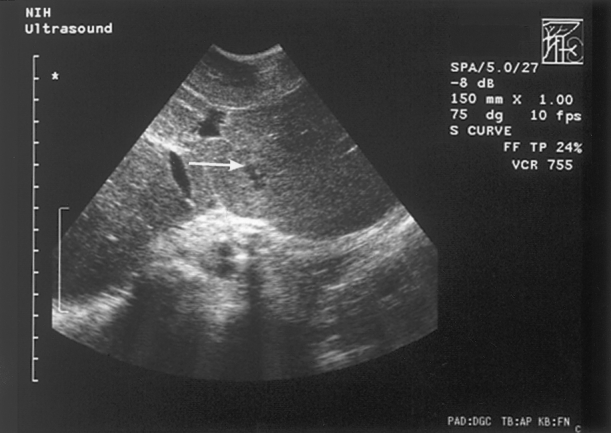
Figure 3. Intraoperative ultrasound showing a hepatic abscess smaller than 5 mm (white arrow).
When comparing preoperative imaging with surgical findings and intraoperative ultrasound for detection of lesions, the sensitivity of preoperative CT, MRI, and ultrasound was 60%, 62%, and 66%, respectively. The difference among these modalities was not significant.
Treatment
The 22 patients had 61 cases of hepatic abscesses, resulting in 52 separate surgical procedures and 8 percutaneous drainages. Sixty-two percent of cases consisted of more than one abscess; 10% consisted of more than four abscesses. The median number of abscesses as determined during surgery was 2 (range 1–14).
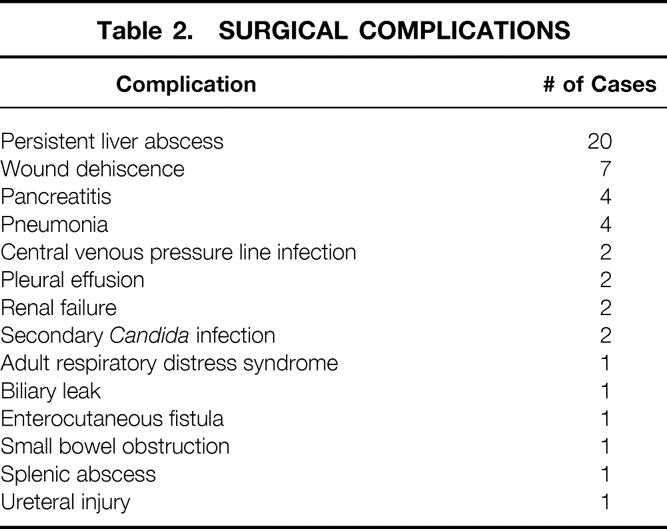
Surgical exploration was performed on 135 abscesses in the 52 surgical cases. Of the 135 abscesses, 85 were treated by debridement and 50 were treated by complete excision. Thirty of these 52 procedures were performed on patients with prior liver operations. The median number of prior liver operations in this series was 1 (range 1–6). Intraoperative ultrasound, in use since 1987, was used in the most recent 30 cases. The median surgical time was 6.5 hours (range 1–12.1); median blood loss for the procedure was 1,000 mL (range 100–8,000). Drains were placed in all but one patient. The mean duration of the drains was 24 days (range 3–90). The median hospital stay was 60 days (range 18–334). No patient died as a complication of the operation. The overall surgical complication rate was 56%, including seven cases of wound dehiscence and four cases each of pancreatitis and pneumonia (Table 2).
Table 2. SURGICAL COMPLICATIONS
Concurrent Therapy
All patients received postoperative antibiotics and 23% of cases received antifungal therapy. For persistent fever or clinical evidence of infection, white blood cell transfusions (56% of cases) and/or interferon-γ (17% of cases) was used. One patient was treated successfully by intralesional instillation of granulocytes and interferon-γ to control multiple hepatic abscesses after surgical debridement. 6 Patients were treated with corticosteroids (27% of cases) to minimize granuloma formation at the surgical incision and facilitate wound healing.
Microbiology
Blood cultures were obtained at presentation before intravenous antibiotics in all 41 cases of either primary or recurrent abscess. Blood cultures were positive in only two cases (5%); both grew Staphylococcus aureus. Sixteen of the 41 cases had preoperative CT-guided percutaneous aspiration of the abscesses for organism identification. All patients had hepatic abscess material sent for microbiology at the time of surgery.
Results of all cultures were as follows: 23 cases (88% of all positive cultures) were S. aureus, of which 2 were resistant to methicillin; 1 case each of Nocardia and Lactobacillus; and 1 case consisting of multiple organisms (Candida glabrata, Streptococcus mitis, and S. aureus). No organism was cultured in 15 (37%) of the cases.
Histologic and Pathologic Results
Excised liver abscess specimens from patients with CGD showed a variety of pathologic changes that reflected both the acute infectious problem and the underlying disorder. Sectioning through the abscess cavity usually revealed multiple loculated foci, varying in size from a few millimeters to several centimeters. The cavities were filled with thick, purulent material and lined by pale fibrous tissue. The fibrous cuff around the abscess was often a centimeter or more thick and gradually merged into the liver parenchyma (Fig. 4).
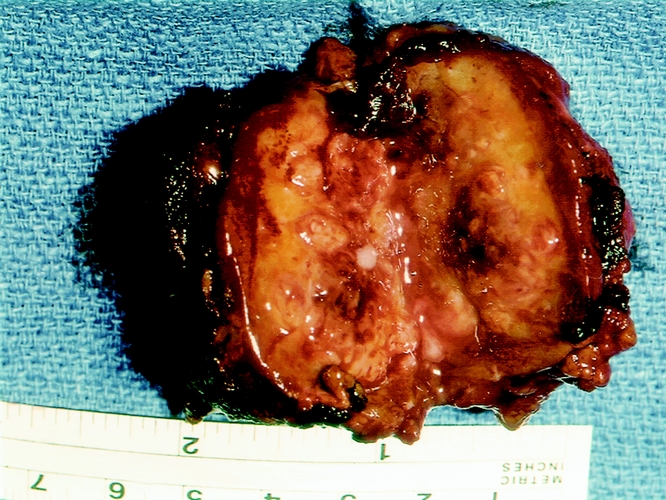
Figure 4. Typical appearance of a hepatic abscess in a patient with chronic granulomatous disease. Note the fibrous cuff surrounding the abscess and the thick, purulent center.
Microscopic sections of the abscesses showed necrotic, fibrinopurulent debris. The edges of the cavities were lined by a chronic inflammatory infiltrate consisting of epithelioid macrophages, lymphocytes, eosinophils, and neutrophils (Fig. 5). The fibrous tissue around the abscess cavity contained a sparser infiltrate as well as small necrotizing and nonnecrotizing granulomas. Residual portal areas were sometimes trapped in the fibrous tissue. The grossly normal liver showed typical changes of chronic granulomatous disease: mild chronic inflammation without significant interface hepatitis in the portal areas and numerous pigmented macrophages characteristic of CGD, both singly and in small clusters, within the portal areas and hepatic sinuses. The pigmented material took up the periodic acid-Schiff stain and may reflect inadequate intracellular digestion of cell debris by the macrophages. Poorly formed, nonnecrotizing granulomas were sometimes found within portal areas and in the parenchyma. Portal and central veins in the vicinity of the abscess sometimes showed intraluminal thrombi, but ischemic necrosis of the liver was not often seen. Rarely was there bile duct inflammation in the nonabscessed liver, but in at least two cases this led to chronic cholestatic changes.
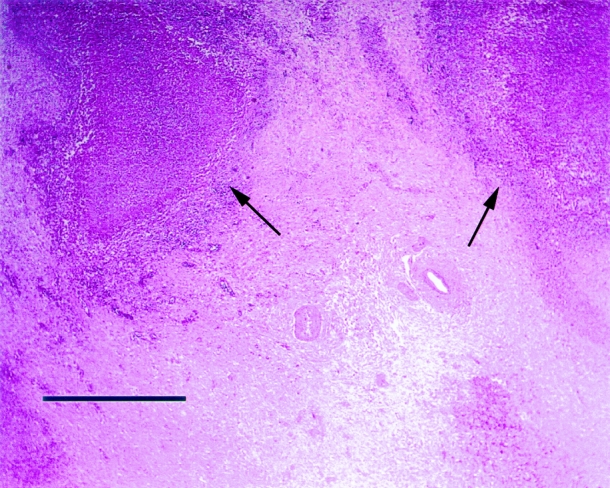
Figure 5. The photomicrograph shows a portion of a typical hepatic abscess in a patient with chronic granulomatous disease. The center of the abscess contains amorphous cell debris. The edge of the abscess is defined by a thin rim of epithelioid histiocytes and other inflammatory cells further surrounded by a thick zone of dense fibrous scar tissue (black arrows). When organisms are seen on special stains, they are always found within the cavity rather than in the surrounding scar or liver tissue. Because of the thickness of the scarred area, no normal liver parenchyma is shown. (Bar = 1 mm)
Treatment Results
Of the 41 cases with primary or recurrent abscesses, 27 were managed successfully by surgery and 4 by percutaneous drainage. One patient was successfully managed without surgery with antibiotics alone. Nine patients failed to respond to initial surgical management and required a total of 20 subsequent procedures for abscess management, including 16 operations and 4 percutaneous drainages. Four of these nine patients required only one further operation to control their disease. The remaining five patients required multiple procedures to manage abscesses (see Fig. 1). Two representative histories are summarized below.
A 23-year-old man had an initial surgical debridement of seven abscesses in both right and left lobes. Two abscesses persisted in the right lobe, requiring two additional operations and a final percutaneous drainage to eradicate disease. The patient remained free of disease in the liver for 5 years and then died of an unrelated complication of his CGD.
An 18-year-old boy was managed initially by wedge resections of three left lobe abscesses and debridement of a right lobe abscess. Five subsequent operations were performed over 1.5 years for persistent and new disease occurring in both lobes. After a complicated postoperative course, he has remained free of hepatic abscesses for 3 years.
Percutaneous drainage was performed eight times on four patients with hepatic abscesses. One p47phox-deficient patient underwent successful percutaneous drainage on four separate occasions for management of recurrent abscesses. On three of these occasions, one percutaneous drain was placed; the fourth time, five drains were placed. All abscesses eventually resolved, and the patient has not had a recurrence of liver abscesses in 7 years. Cultures obtained from all four drainage procedures were positive for S. aureus. Three other patients underwent percutaneous drainage procedures for persistent disease after failed surgical management. In two patients, percutaneous drainage was a successful adjunct. The last patient deteriorated clinically after a failed percutaneous attempt and required an emergent surgical intervention.
A patient with CGD coinfected with HIV refused surgical treatment for a Nocardia hepatic abscess. He received 3 months of intravenous antibiotics and the Nocardia resolved without complication. The patient is now 7 years since treatment without a hepatic abscess recurrence.
Follow-Up
The average potential follow-up was 8.9 years after treatment of a patient’s first abscess at the NIH. None of the 22 patients in this series died as a result of their hepatic abscesses or complications resulting from their treatment. However, four of these patients have subsequently died as a result of other complications of CGD.
Prognostic Factors for Treatment Success
Table 3 shows potential prognostic factors associated with treatment success. Previous hepatic abscess predicted failure of treatment (P = .017). No other prognostic factor was statistically significant for predicting failure of treatment.
Table 3. PROGNOSTIC VARIABLES FOR PREDICTING PERSISTENT HEPATIC ABSCESS
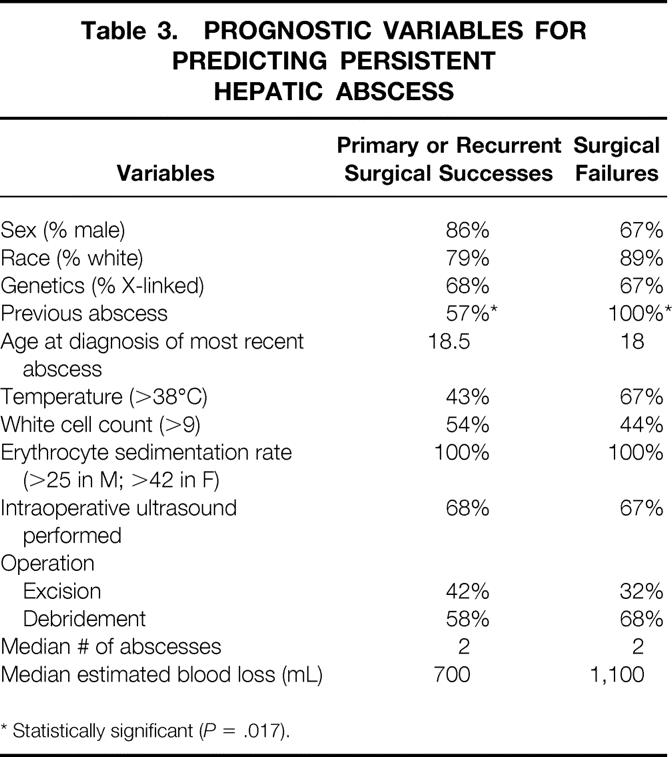
* Statistically significant (P = .017).
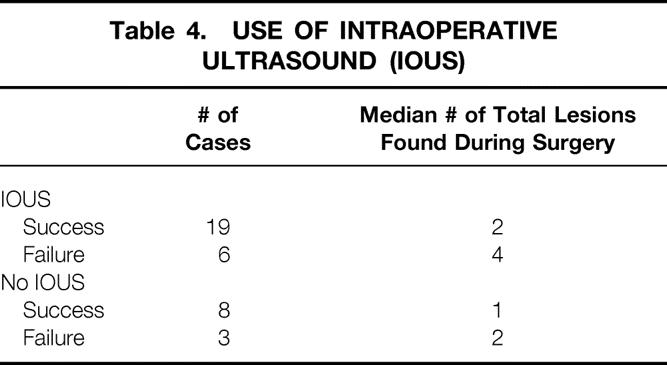
Intraoperative ultrasound was performed for the management of primary and recurrent abscess in 25 cases. In 19 of these 25 cases (76%), a successful surgical outcome (defined as nonrecurrence of an abscess within 3 months of completion of treatment) was achieved. Eleven operations were performed without the use of intraoperative ultrasound for primary and recurrent abscesses. Eight of these 11 cases (73%) were successfully treated without intraoperative ultrasound. Table 4 shows an increased detection of hepatic lesions during surgery when intraoperative ultrasound was performed. The increase in the median number of hepatic lesions detected with intraoperative ultrasound did not lead to an increased operative success rate.
Table 4. USE OF INTRAOPERATIVE ULTRASOUND (IOUS)
DISCUSSION
Chronic granulomatous disease is the clinical manifestation of genetic defects that lead to dysfunction of the NADPH oxidase, the enzyme responsible for the phagocyte production of superoxide and its metabolites: hydrogen peroxide, hydroxyl radical, and bleach. Four separate genetic mutations (one X-linked and three autosomal recessive) result in defects in distinct peptide subunits of the NADPH oxidase. All four mutations render the phagocyte unable to kill certain microorganisms. 1–3 The organisms involved are almost always catalase-positive, which enables them to destroy both cellularly and bacterially produced hydrogen peroxide. In contrast, most catalase-negative pathogens produce hydrogen peroxide and, thereby, supply metabolites to the CGD leukocytes, leading to the local formation of bleach. 7
The diagnosis of CGD is made by demonstrating an absent or exceedingly low respiratory burst in leukocytes. This is best determined by measuring superoxide (02−) or its metabolites produced by phagocytes in response to stimulation, such as phorbol-12-myristate acetate or opsonized zymosan. The most reliable tests for CGD diagnosis are superoxide production or dihydrorhodamine oxidation. 8 The clinical course of patients with CGD is characterized by recurrent, severe bacterial and fungal infections. The death rate in CGD is 2% to 5% per year, depending on the genotype (X-linked has a worse prognosis). 3
Few reports describe the management of hepatic abscesses in CGD. 4,5,9–15 Roback et al in 1971 outlined treatment options gathered from their management of 6 hepatic abscesses in 25 patients with CGD. They recommended partial hepatic lobectomy for peripheral abscesses and full and extensive debridement for central abscesses. They also recommended that drains be left for months and withdrawn slowly. 5 Mouy et al managed 14 hepatic abscesses in 48 patients with CGD over a 16-year period. Nine cases required operation; two were managed successfully with percutaneous drainage. 12 Eckert et al reported two hepatic abscesses occurring in 10 patients with CGD, but outcome was not discussed. 13 Chusid described one case of hepatic abscess and reviewed 26 others in patients with CGD, and emphasized the need for surgery as the only acceptable treatment modality. 14 Mulholland et al followed up 20 patients with CGD over 19 years; in 9 patients, 16 separate abscesses developed. Fourteen of the 16 abscesses were managed surgically by debridement in addition to antibiotics. They recommended hepatotomy, evacuation of the exudate, and external drainage. They did not perform hepatic resections. Only one abscess in their series, occurring immediately after surgery, was managed by percutaneous drainage. 5 In the largest multiinstitution review to date, Johnston et al reported 69 hepatic abscesses in 168 patients with CGD. They recommended surgical drainage and long-term continuous antibiotic therapy. 15
In our series of 156 patients with CGD followed up at a single center, the 14% incidence of hepatic abscesses was quite low compared with other series. 4,5,12 Signs and symptoms were highly variable. Most patients presented with fevers and chills. Abdominal tenderness was present in 31% of patients. Laboratory values suggested infection: most patients had a slight leukocytosis and an increased erythrocyte sedimentation rate.
A high index of suspicion for hepatic abscess as well as a low threshold for radiologic imaging is needed in patients with CGD. We have not identified a clear advantage to any specific radiologic imaging modality. Our sensitivity for CT, MRI, and ultrasound was 60%, 62%, and 66%, respectively, when compared with intraoperative findings and intraoperative ultrasound. These modalities underestimated the number of abscesses in 30%, 19%, and 10% of cases, respectively. The performance of intraoperative ultrasound did not lead to an improved outcome, despite the detection of more hepatic abscesses when intraoperative ultrasound was performed (see Table 4). Twice as many hepatic abscesses were discovered during surgery with the use of intraoperative ultrasound. However, similar percentages of patients (76% vs. 73%) had successful surgical outcomes with or without intraoperative ultrasound. Although intraoperative ultrasound detected many abscesses missed by preoperative imaging, these abscesses may not have needed to be treated surgically. Abscesses detected by intraoperative ultrasound and not by preoperative imaging are generally less than 5 mm, and antibiotics may be adequate for these small abscesses.
Studies of the management of pyogenic hepatic abscesses in patients without CGD suggest that intravenous antibiotics and percutaneous drainage are appropriate first-line treatments. 16,17 Pyogenic abscesses from sources other than CGD are generally filled with homogenous purulent material. Hepatic abscesses in patients with CGD are unique, however, and must be managed differently. CGD abscesses are dense, septated masses with a fibrous pseudocapsule and thick, inspissated fluid. Therefore, they usually cannot be treated with percutaneous drainage. In our series, percutaneous drainage was attempted under extenuating circumstances in four cases only. It was successful in three of these instances. Similarly, antibiotics alone were infrequently tried, and less often successful. It is our concern that inadequately treated abscesses may seed new areas within the liver, eventually leading to unresectable, untreatable disease. Therefore, we practice aggressive surgical management of hepatic abscesses as soon as possible after diagnosis.
Recommended surgical procedures for hepatic abscess in CGD vary widely in the literature, as discussed above. We believe the best surgical management is enucleation of the abscesses using a Kelley clamp tissue crushing technique on the outside of the pseudocapsule while maintaining inflow occlusion (Pringle maneuver) and a low central venous pressure (to minimize venous back-bleeding). 18 Dissecting along the pseudocapsule plane minimizes injury to major vessels and allows complete excision of the abscess without spillage of its purulent content. We believe this minimizes the risk for recurrence and may eliminate the need for long-term external drainage. For deep abscesses, the liver can be split along intersegmental planes to expose the pseudocapsule for enucleation. This minimizes loss of hepatic parenchyma and leaves the vascular anatomy intact for future excisions. Preservation of hepatic parenchyma is important because at least 58% of our patients with hepatic abscess required more than one hepatic surgery. In difficult situations where major portal triads or hepatic venous branches are involved, debridement of the abscess with external drainage may still be required.
Drains were placed in all but one of the operations. However, it is unlikely that patients derive any benefit from drains in the setting of complete abscess excision. Our management of drains has changed recently. Currently, drains are used only after debridement but not after excision. Further, we have recently seen two cases of secondary hepatic infection with C. glabrata, probably resulting from drains.
Many patients had percutaneous aspiration of a hepatic abscess in an effort to isolate an organism before antibiotics and surgical management, especially if surgery was likely to be delayed more than 24 hours from presentation. After preoperative aspiration, we typically administer ceftriaxone and vancomycin with or without rifampin pending cultures. S. aureus was the most common isolate from hepatic abscesses. This result is consistent with other series. 3,4 Blood cultures were rarely positive. After surgery, intravenous antibiotic therapy in our center is typically of 4 to 8 weeks’ duration, with frequent imaging to verify resolution of all surgical sites and the absence of new lesions.
In our series, the surgical complication rate was 56%, but there were no deaths. Mulholland reported a similar complication rate of 50%. 5 The complication rate is higher than in standard hepatic surgery because all patients are immunodeficient and many have had prior hepatic operations, resulting in possibly intense perihepatic scar tissue. In addition, the underlying disease may lead to increased complications: wound healing is impaired in CGD and is paradoxically steroid-responsive. When patients exhibited slow wound healing as a result of granuloma formation, steroids were administered. In our series, steroids were used in 27% of cases for poor wound healing and were deemed beneficial in most cases. Other techniques in the management of hepatic abscess include white cell transfusions, systemic interferon-γ administration, and instillation of leukocytes directly into the abscess cavity. 6
In summary, the management of patients with CGD is difficult, burdened with complications, and characterized by repeated hospital admissions. Recurrent infections lead to multiple operations. Intraoperative ultrasound is technically helpful, but small abscesses (<5 cm) may be treated adequately with antibiotics alone. Aggressive surgical management of hepatic abscesses in patients with CGD is crucial to patient survival. Steroids may be administered for postoperative wound healing problems. Although CGD is relatively rare, management of its hepatic abscesses is distinct from pyogenic hepatic abscesses occurring in other diseases. Thus, general surgeons should be aware of the unique characteristics of liver abscesses occurring in CGD.
Footnotes
Correspondence: David Bartlett, MD, Surgery Branch, National Cancer Institute/NIH, Building 10, Rm. 2B38, Bethesda, MD 20892.
E-mail: david_bartlett@nih.gov
Accepted for publication August 15, 2001.
References
- 1.Segal BH, Leto TL, Gallin JI, et al. Genetic, biochemical, and clinical features of chronic granulomatous disease. Medicine 2000; 79 (3): 170–200. [DOI] [PubMed] [Google Scholar]
- 2.Lekstrom-Himes JA, Gallin JI. Immunodeficiency diseases caused by defects in phagocytes. N Engl J Med 2000; 343 (23): 1703–1714. [DOI] [PubMed] [Google Scholar]
- 3.Winkelstein JA, Marino MC, Johnston RB, et al. Chronic granulomatous disease. Report on a national registry of 368 patients. Medicine 2000; 79 (3): 155–169. [DOI] [PubMed] [Google Scholar]
- 4.Roback SA, Weintraub WH, Good RA, et al. Chronic granulomatous disease of childhood: surgical considerations. J Pediatr Surg 1971; 6: 601–611. [DOI] [PubMed] [Google Scholar]
- 5.Mulholland MW, DeLaney JP, Simmons RL. Gastrointestinal complications of chronic granulomatous disease: surgical implications. Surgery 1983; 94: 569–575. [PubMed] [Google Scholar]
- 6.Lekstrom-Himes JA, Holland SM, DeCarlo ES, et al. Treatment with intralesional granulocyte instillations and interferon-gamma for a patient with chronic granulomatous disease and multiple hepatic abscesses. Clin Infect Dis 1994; 19: 770–773. [DOI] [PubMed] [Google Scholar]
- 7.Forrest CB, Forehand JR, Axtell RA, et al. Clinical features and current management of chronic granulomatous disease. Hematol Oncol Clin North Am 1988; 2: 253–266. [PubMed] [Google Scholar]
- 8.Vowells SJ, Sekhsaria S, Malech HL, et al. Flow cytometric analysis of the granulocyte respiratory burst: a comparison study of fluorescent probes. J Immunol Methods 1995; 178: 89–97. [DOI] [PubMed] [Google Scholar]
- 9.Barton LL, Moussa SL, Villar RG, et al. Gastrointestinal complications of chronic granulomatous disease: case report and literature review. Clin Pediatr 1998; 37: 231–236. [DOI] [PubMed] [Google Scholar]
- 10.Johnston RB, Jr., McMurray JS. Chronic familial granulomatosis. Report of five cases and review of the literature. Am J Dis Child 1967; 114: 370–378. [DOI] [PubMed] [Google Scholar]
- 11.Perry HB, Boulanger M, Pennoyer D. Chronic granulomatous disease in an adult with recurrent abscesses. Arch Surg 1980; 115: 200–202. [DOI] [PubMed] [Google Scholar]
- 12.Mouy R, Fischer A, Vilmer E, et al. Incidence, severity, and prevention of infections in chronic granulomatous disease. J Pediatr 1989; 114: 555–560. [DOI] [PubMed] [Google Scholar]
- 13.Eckert JW, Abramson SL, Starke J, et al. The surgical implications of chronic granulomatous disease. Am J Surg 1995; 169: 320–323. [DOI] [PubMed] [Google Scholar]
- 14.Chusid MJ. Pyogenic hepatic abscess in infancy and childhood. Pediatrics 1978; 62: 554–559. [PubMed] [Google Scholar]
- 15.Johnston RB, Newman SL. Chronic granulomatous disease. Pediatr Clin North Am 1977; 24: 365–376. [DOI] [PubMed] [Google Scholar]
- 16.Branum GD, Tyson GS, Branum MA, et al. Hepatic abscess. Changes in etiology, diagnosis, and management. Ann Surg 1990; 212: 655–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang CJ, Pitt HA, Lipsett PA, et al. Pyogenic hepatic abscess. Changing trends over 42 years. Ann Surg 1996; 223: 600–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baer HU, Dennison AR, Mouton W, et al. Enucleation of giant hemangiomas of the liver. Technical and pathologic aspects of a neglected procedure. Ann Surg 1992; 216: 673–676. [DOI] [PMC free article] [PubMed] [Google Scholar]