Abstract
Objective
To define the significance of positive microscopic resection margins in a large cohort treated for soft tissue sarcoma.
Methods
The authors analyzed 2,084 patients with localized primary soft tissue sarcoma (all anatomic sites) treated from 1982 to 2000. Clinicopathologic variables studied included tumor site, size, depth, histologic type, grade, and resection margin status. Treatment other than resection was not analyzed. Study endpoints included local and distant recurrence-free and disease-specific survival rates, estimated by the Kaplan-Meier method. Univariate and multivariate analyses were performed using the log-rank test and the Cox proportional hazards model.
Results
Median follow-up was 50 months. After primary resection, 1,624 (78%) patients had negative and 460 (22%) had positive resection margins. Having positive margins nearly doubled the risk of local recurrence and increased the risk of distant recurrence and disease-related death. Seventy-two percent of patients with positive margins had no recurrence. Resection margin did not predict local control for retroperitoneal sarcomas or fibrosarcomas. Resection margin remained significantly associated with distant recurrence-free survival and disease-specific survival across all subsets after adjusting for other prognostic variables. The overall 5-year disease-specific survival rates for negative and positive margins were 83% and 75%.
Conclusions
Positive microscopic resection margins significantly decrease the local recurrence-free survival rate for other-than-primary fibrosarcoma and retroperitoneal sarcomas, and independently predict distant recurrence-free survival rates and disease-specific survival rates for all patient subsets. Adjuvant therapy should be considered in the management of soft tissue sarcoma to increase local control. Because 72% of positive margins did not equate with inevitable local recurrence, considerable clinical judgment is required in considering additional treatment. Microscopic resection margins should be considered for inclusion in staging systems and treatment algorithms that address local recurrence.
Several relevant prognostic factors have been defined for soft tissue sarcoma (STS). Three of these factors—tumor size, depth relative to the superficial investing muscular fascia, and histologic grade—have been incorporated into the recent stage classification system of the American Joint Committee on Cancer (AJCC). 1 The current staging system does not incorporate other factors that have proven prognostic significance for survival, including anatomic site, positive microscopic surgical margins, and presentation with locally recurrent disease. 2
A previous report defined clinical and pathologic prognostic factors based on a large population of patients with primary and locally recurrent extremity sarcoma. 2 Factors predictive of disease-specific death in that study included tumor size 10 cm or more, high histologic grade, deep location, presentation with local recurrence, and positive microscopic surgical margin. Positive microscopic margins were associated with increased risk for local recurrence in addition to an increased tumor-related death rate.
The prognostic significance of positive microscopic margins may not be generalizable to anatomic primary tumor sites other than extremity. In the current study we focus on the issue of histologic margins and extend the analysis to all anatomic sites initially treated for localized primary STS. The aim of the study was to define the clinical significance of a positive microscopic margin after surgical treatment on a site-specific basis, as well as its relative influence over relapse-free and disease-specific survival rates. This analysis was conducted on a large, well-characterized patient cohort consecutively treated and followed up at a single institution specializing in the treatment of sarcoma.
METHODS
General
In 1982 the Memorial Sloan-Kettering Cancer Center (MSKCC) Sarcoma Database was initiated to prospectively record patient, tumor, treatment, recurrence, and death data on all patients admitted for treatment of STS. This database is maintained by the Department of Surgery and manages demographic, staging, treatment, and outcome data. From this database we analyzed 2,084 consecutive patients (older than 16 years of age) with localized primary STS (all anatomic sites) admitted, treated, and followed up at our institution from July 1982 to January 2000. Patient follow-up was complete and was obtained from clinical chart review, tumor registry information, physician records, patient correspondence, and telephone interviews.
Histopathology
Pathologic review on a weekly basis was recorded prospectively by dedicated sarcoma pathologists and entered primarily into the database. Members of the Department of Pathology confirmed the histologic diagnosis. The histopathology slides were not re-reviewed for the purpose of this study such that redefinition of some cases (for example, malignant fibrous histiocytoma as myxofibrosarcoma) was not made. Molecular diagnosis by genetic characterization was performed only in recent years. Primary histopathologic categories included liposarcoma, malignant fibrous histiocytoma, leiomyosarcoma, fibrosarcoma, synovial sarcoma, malignant peripheral nerve sheath tumor, hemangiopericytoma, gastrointestinal stromal tumor, extraskeletal chondrosarcoma, angiosarcoma, and “other” STS. Information concerning pathologic findings was obtained from the database and confirmed by review of all pathology reports, with particular emphasis on microscopic margin status. Tumor size was measured as the maximum diameter of the fresh resected tumor specimen. Tumors were classified into three size categories: 5 cm or less, more than 5 cm to 10 cm, and more than 10 cm. Tumor depth was characterized as superficial or deep relative to the superficial investing muscular fascia. Tumor histologic grade was classified as low or high based on the degree of cellularity, differentiation, and vascularity, nuclear pleomorphism, number of mitosis per high-power field, and amount of stromal necrosis. 3 Macroscopic margins were assessed at the time of surgery and microscopic margins were assessed histopathologically. Complete resection was defined as the absence of gross residual disease after surgical excision of the tumor. A microscopically negative margin was defined as no tumor at the inked margin. A microscopically positive margin was defined as tumor present at the inked margin. The decision to regard microscopic margins as either negative or positive was influenced by the retrospective nature of this analysis. Margin status was reviewed and margin data were recorded prospectively at weekly pathologic review.
Treatment
Surgical treatment decisions were based on review of pathologic material obtained from biopsy or prior resections, and the size and anatomic location of the primary tumor. The goal of surgical treatment was excision of the tumor with negative gross and microscopic margins of resection. All patients were treated with appropriate structure- and function-sparing surgical resection. The majority of patients (92%) with primary extremity sarcomas underwent limb-preserving resection; only 8% (91/1,156) required primary amputation. Some received adjuvant radiation and/or chemotherapy (generally doxorubicin and ifosfamide) based on prognostic factors predicting an increased risk of local or distant disease failure at the discretion of the Multidisciplinary Soft Tissue Sarcoma Group or as part of clinical trials. Adjuvant therapy was not uniformly prospectively randomized but included both patients treated as part of clinical trials and those given standard of care based on prognosis. Inclusion of these treatment-related covariates would confound the impact of other factors studied. Thus, although these treatment-related factors are reported, they were excluded from the statistical analyses because they were not uniformly applied. Patients were treated according to the standard of care at MSKCC.
Data
Primary endpoints included time to first local recurrence, distant metastasis, and disease-specific death. Clinicopathologic factors were correlated with study endpoints. Patient variables analyzed included age at diagnosis (younger than 50, or 50 or older), gender, presentation status (biopsy or no treatment, or incomplete or complete prior excision), and anatomic site. Anatomic sites were classified as head and neck, upper and lower extremity, superficial trunk, retroperitoneum, visceral gastrointestinal, visceral gynecologic, visceral genitourinary, and thoracic. Tumor variables included size (≤5 cm, >5–10 cm, or >10 cm), and depth (superficial or deep). Pathologic factors analyzed were histologic type, histopathologic grade (low or high), and status of microscopic surgical margins (negative or positive).
Definitions
A primary tumor was defined as a localized lesion previously untreated or biopsied (incisional or inadequate excisional biopsy) before definitive surgical therapy. Primary tumor was defined according to the AJCC. 1
Sixty-six (3.2%) patients who could not undergo complete resection of all grossly evident disease because tumors were in proximity to major neurovascular anatomy were resected with margins limited by the paucity of nonvital surrounding soft tissues. In our effort to define the clinical significance of a positive microscopic margin after surgical treatment and its relative influence on disease-free and disease-specific survival, we conducted the outcome analysis including and excluding those in whom complete resection of gross disease at the time of primary surgery could not be achieved.
Local recurrence was defined as the first clinically, radiologically, or pathologically evident tumor of the same histologic type, within or contiguous to the previously treated tumor bed, 3 or more months after primary therapy. Nodal recurrence was defined by pathologically confirmed metastases to regional draining lymph nodes. Distant metastasis was defined by clinical or radiologic evidence of systemic disease spread outside the primary tumor basin. Regional nodal recurrences were included as part of distant metastasis for the purpose of analysis. Follow-up was calculated from the time of first surgery for the primary sarcoma to the date of last follow-up.
Statistical Analysis
Summary statistics were obtained using established methods. When analyzing a factor, only categories that represented at least 5% of the cases were considered. Categories with less than 5% of the cases were either combined or excluded from the analysis. Associations between categorical factors were studied with the Fisher exact test or the chi-square test as appropriate.
Outcome was classified according to sites of first disease recurrence as defined above: local and distant recurrence (including nodal metastases). The clinical outcomes studied were local recurrence, distant recurrence, and disease-specific survival. Time to recurrence and disease-specific survival were calculated from the date of primary surgery. Median time to recurrence or tumor-related death was the time when 50% of patients had had recurrence or died of disease. For this study, any patient with a clinical event within 3 months after definitive treatment at MSKCC was excluded. For local recurrence, only the first local recurrence that was not preceded by any other recurrences was considered; all other local recurrences were censored. In defining distant recurrence, any regional nodal or distant recurrence after definitive surgery at MSKCC was considered. Synchronous local and distant first recurrences were considered separately as an event for both local recurrence and distant metastasis. Because desmoid tumors have low to negligible potential for developing distant recurrence, all patients with this histologic type were excluded from analyses involving distance recurrence and disease-related death. Patients whose deaths resulted from sarcoma were considered to have died of disease and were considered in disease-related death calculations. Those who died of other causes in the absence of sarcoma were considered to have been alive without evidence of disease and were censored at the time of death.
The rate of recurrence or death was estimated using the Kaplan-Meier product limit method. The univariate influence of prognostic factors on study endpoints was analyzed using the log-rank test. Multivariate analysis based on the Cox proportional hazards regression model was used to associate covariates to time-dependent endpoints. Only factors identified to be potentially significant in the univariate analysis were evaluated in the multivariate analysis to assess the independent prognostic effect of these variables. Statistical analysis was performed using JMP and SAS software (JMP and SAS, Cary, NC). P < .05 was considered significant.
To assess the independent predictive effect of a covariate (anatomic site or histologic grade, for example) for a nominal response (in this case microscopic margin), a logistic regression model was constructed and parameters were estimated using maximum likelihood. The Wald test statistic was computed for each effect in the model. Confidence limits and odds ratios were calculated for the maximum likelihood parameter estimates.
The following factors were studied: patient age, gender, tumor location, size, depth, histologic type, grade, and status of the microscopic and gross margins. A two-step approach was used to examine the independent prognostic value of margins of resection on clinical outcome. In the first step, a Cox model, excluding margin status, was developed for each clinical endpoint. The Cox model for each clinical endpoint was chosen using a best subset selection procedure. In this procedure, Cox models are fitted using all possible subsets of combinations of one factor, two factors, up to all the factors under consideration. The best model for each subset represents the best-fitting model to the data using the same number of factors. For reason of conservatism, using as few factors as possible in this study, we used the best-fitting Cox model based on five factors. In the second step, we took the best-fitting five-factor model in the first step and determined whether margin status added prognostic value to the model. In this second step, the possible interactions between margin status and all factors in the five-factor model were studied. An interaction of margins with a factor exists when the influence of margins of resection changes with the level of the factor.
RESULTS
Patient, Tumor, and Treatment Characteristics
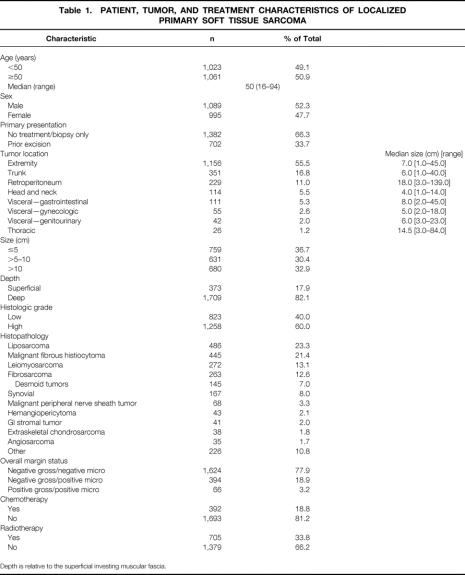
Patient, tumor, and treatment characteristics are shown in Table 1. The majority of the 2,084 patients presented with previously biopsied or untreated (66%) primary tumors. Nearly three fourths of sarcomas (73%) originated in the extremity or superficial trunk. Approximately 78% of patients had margin-negative primary resections. Primary amputation was rarely indicated in the 1,156 patients with extremity sarcoma (n = 91 [8%]).
Table 1. PATIENT, TUMOR, AND TREATMENT CHARACTERISTICS OF LOCALIZED PRIMARY SOFT TISSUE SARCOMA
Depth is relative to the superficial investing muscular fascia.
Overall Recurrence Rates, Follow-Up, and Survival
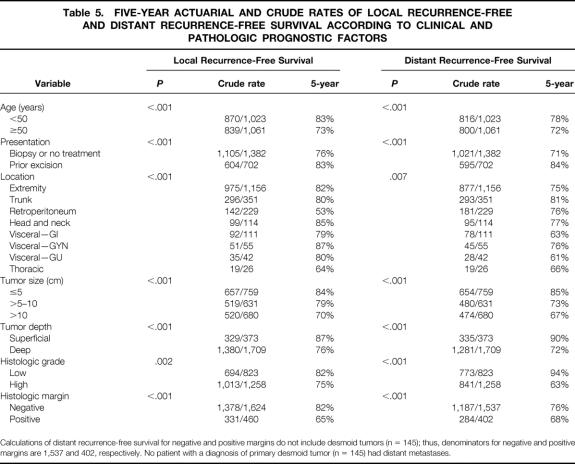
Median follow-up for patients alive at the time of this study was 50 months (range 3–266). Local recurrences were diagnosed in 375 (18%) patients (Table 2). Of the 375 patients with local recurrence, 246 (66%) had negative and 129 (34%) had positive microscopic margin resections of the primary tumors (Table 3). The 5-year local recurrence-free survival rate for the entire cohort was 79%.
Table 2. LOCALIZED PRIMARY SOFT TISSUE SARCOMA, RECURRENCE, FOLLOW-UP STATUS, AND SURVIVAL
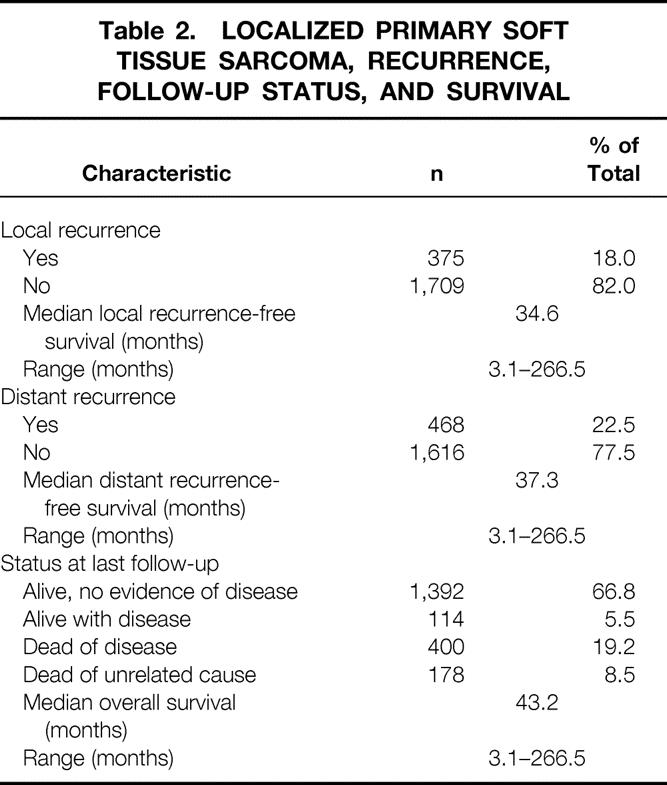
Table 3. DISTRIBUTION OF DISEASE STATUS ACCORDING TO MICROSCOPIC MARGIN STATUS
Calculations for distant recurrence and sarcoma related death exclude desmoid tumors (n = 145); thus, denominators for negative and positive margins are 1,537 and 402, respectively. No patient with desmoid tumor had distant metastases or died of disease.
Distant recurrences were diagnosed in 468 (23%) of patients. Of the 468 patients with distant recurrence, 350 (75%) had negative and 118 (25%) had positive microscopic margin resections of primary sarcomas (see Table 3). The 5-year distant recurrence-free survival for the entire study group was 75%.
Median overall survival was 43 months (range 3–267); median disease-specific survival had not been reached. Overall and disease-specific 5-year survival rates for the study cohort were 73% and 79%, respectively. At last follow-up, 1,392 (67%) patients were alive without evidence of disease and 400 (19%) had died of disease (see Table 3).
Impact of Clinical and Pathologic Variables on Microscopic Margins
Tumors arising in the head and neck and retroperitoneum were at significantly increased likelihood of having involved margins after primary resection. Positive microscopic margins were identified in 19% of extremity/trunk, 30% of head and neck, and 45% of retroperitoneal resected primary tumor specimens (P = .005). The rates of positive microscopic margins increased progressively with increasing primary tumor size. Rates of positive microscopic margins according to primary tumor size were as follows: 5 cm or less, 13%; more than 5 to 10 cm, 19%; more than 10 to 15 cm, 26%; more than 15 to 20 cm, 37%; and more than 20 cm, 43% (P < .001).
Considering both primary size and depth in terms of AJCC T stage, the rate of positive margins was similar for T1a (≤5 cm, superficial; 13%), T1b (≤5 cm, deep; 14%), and T2a (>5 cm, superficial; 10%) tumors. Overall margin-positive rates for stage T1a, T1b, and T2a tumors were significantly lower than those for T2b (>5 cm, deep) lesions (12% vs. 27%, P < .001). Patients who previously underwent resection and were then referred for subsequent re-resection (two “primary” rather than one definitive operations) had a significantly lower rate of microscopic margin involvement by tumor than patients who had undergone only biopsy or no treatment before presentation to MSKCC (10% vs. 29%, P < .001). This may be attributable to the finding that patients who presented after biopsy only or with no prior treatment had a greater proportion of larger (>5 cm, 73% vs. 23%, P < .001) and deep (91% vs. 65%, P < .001) primary tumors than those who had prior excisions.
Margin-positive rates according to histologic type were as follows: fibrosarcoma, 32%; liposarcoma, 29%; leiomyosarcoma, 18%; malignant fibrous histiocytoma, 18%; synovial, 12%; other, 12% (P < .001, fibro- or liposarcoma vs. others). This may be attributable to the desmoid group (40% margin positive) in the case of fibrosarcoma, and liposarcoma’s predilection for the retroperitoneum as an anatomic site of origin, because 43% of margin-positive liposarcomas were retroperitoneal.
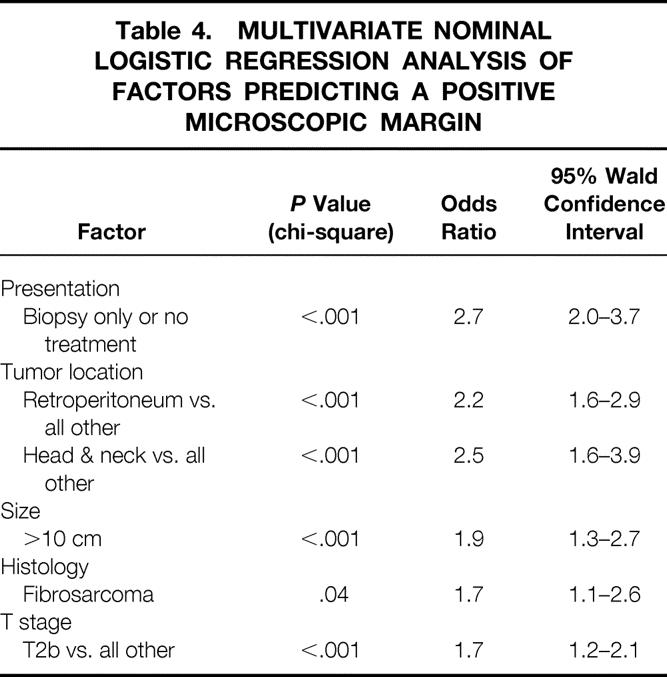
On multivariate analysis, independent predictors of a positive microscopic resection margin were presentation with no prior treatment or biopsy alone, retroperitoneal or head and neck primary tumor site, tumor size more than 10 cm, T2b (>5 cm, deep) stage, and fibrosarcoma (including desmoid) histopathology (Table 4).
Table 4. MULTIVARIATE NOMINAL LOGISTIC REGRESSION ANALYSIS OF FACTORS PREDICTING A POSITIVE MICROSCOPIC MARGIN
Rate of Developing Local Recurrence
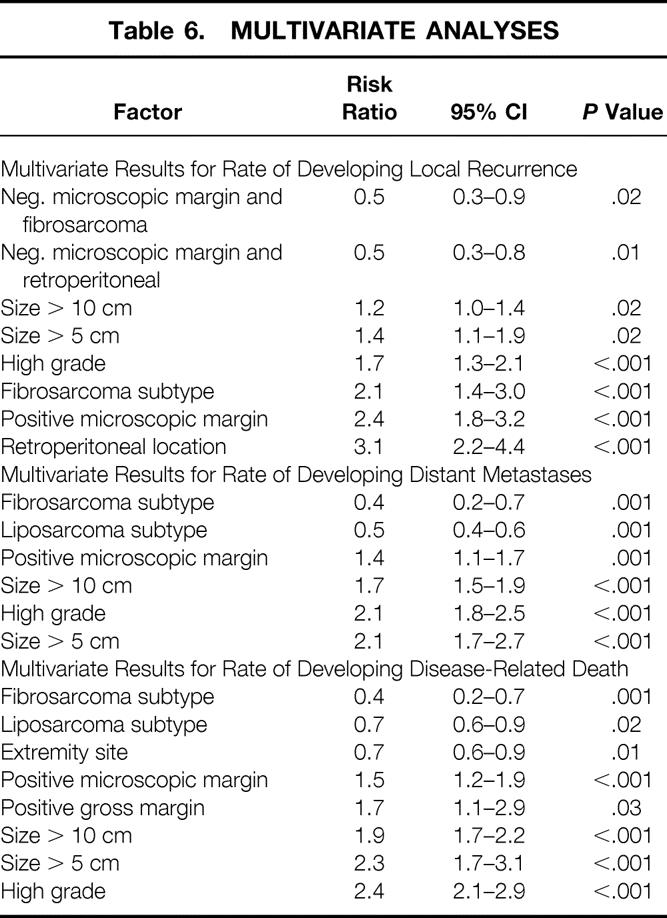
Of the 2,084 patients, 1,624 (78%) had negative and 460 (22%) had positive microscopic margins after primary resection (see Table 3). The risk of local recurrence with a negative margin was 15%; the risk with a positive margin was 28% (P < .001). Even with a positive histologic margin, however, 72% of patients did not have local recurrence. By univariate analysis, a positive microscopic margin was associated with a higher rate of local recurrence (P < .001). The 5-year local recurrence-free survival rates for negative and positive microscopic margins were 82% and 65%, respectively (Table 5, Fig. 1). The best five-factor Cox model predicting local recurrence consisted of the following factors: histologic grade, size more than 5 cm, size more than 10 cm, retroperitoneal location, and fibrosarcoma (including desmoid) histologic subtype. Microscopic margin status remained significantly associated with local recurrence by multivariate analysis (P < .001) after adjusting for these five factors (Table 6).
Table 5. FIVE-YEAR ACTUARIAL AND CRUDE RATES OF LOCAL RECURRENCE-FREE AND DISTANT RECURRENCE-FREE SURVIVAL ACCORDING TO CLINICAL AND PATHOLOGIC PROGNOSTIC FACTORS
Calculations of distant recurrence-free survival for negative and positive margins do not include desmoid tumors (n = 145); thus, denominators for negative and positive margins are 1,537 and 402, respectively. No patient with a diagnosis of primary desmoid tumor (n = 145) had distant metastases.
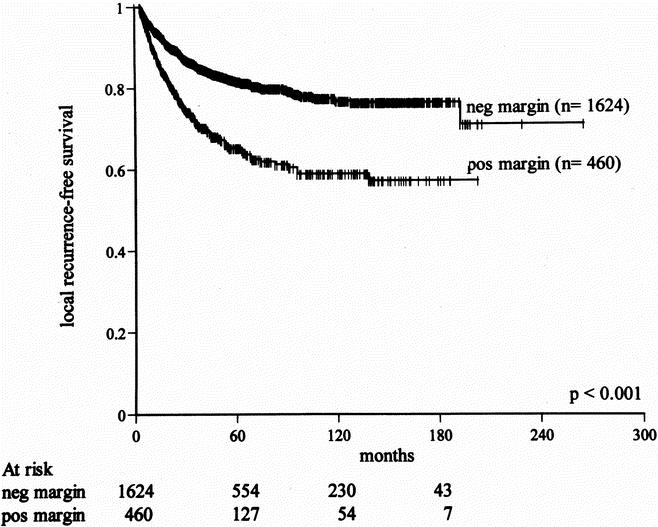
Figure 1. Effect of microscopic resection margin on local recurrence-free survival for primary soft tissue sarcoma (all anatomic sites).
Table 6. MULTIVARIATE ANALYSES
Regarding the significance of tumor size, both tumors more than 5 cm and more than 10 cm were significant prognostic factors, suggesting that any tumor more than 5 cm has an unfavorable impact compared with those less than 5 cm, and those larger than 10 cm carry an additional risk. The risk ratio of local recurrence for tumors larger than 5 cm was 1.4; for those larger than 10 cm it was 1.2. The model illustrates that if a patient has a tumor larger than 5 cm, then there is 1.4 times the risk of developing a local recurrence compared with one smaller than 5 cm. If the tumor is actually larger than 10 cm, then the risk of local recurrence is 1.2 times in addition to 1.4. Therefore, the risk for a tumor larger than 10 cm is 1.2 times the risk compared with a tumor between 5 and 10 cm and 1.4 × 1.2 = 1.7 compared with a tumor smaller than 5 cm.
We found an interaction between microscopic margin status and tumor location (P = .01) and histologic subtype (P = .02). This indicates that the influence of microscopic margin was not uniform across different levels of these factors. In particular, the relative risk for developing local recurrence between positive and negative margins was 2.4 (95% confidence interval 1.8–3.2) for patients with sarcomas other than fibrosarcoma in a location other than the retroperitoneum. For patients with fibrosarcoma, this relative risk was reduced to approximately 2.4 × 0.5 = 1.2. This implies that there was no significant influence of margin status for patients with that histology for the endpoint of local recurrence-free survival; however, desmoid tumors were included in the analysis. The lack of influence of histologic margins on local disease control was evident when patients with desmoid tumors (P = .65) and those with nondesmoid fibrosarcomas (P = .23) were analyzed separately. Similarly, the impact of microscopic margin was minimal for patients with retroperitoneal tumors in terms of local control of disease (relative risk = 2.4 × 0.5 = 1.2).
Rate of Developing Distant Recurrence
By univariate analysis, positive microscopic margin was associated with a higher rate of distant recurrence (27% vs. 23%, P < .001, Fig. 2). This influence, although significant, was small and was due to differences in late development (beyond 2 years) of distant metastases. The 5-year distant recurrence-free survival rates for primary tumors resected with negative and positive microscopic margins were 76% and 68%, respectively (see Table 5). By multivariate analysis, microscopic margin remained significantly associated with the rate of distant recurrence (P = .001) after adjusting for grade, size more than 5 cm, size more than 10 cm, and liposarcoma and fibrosarcoma histology (see Table 6). Gross margin was not significant by multivariate analysis (P = .8), likely related to the rarity (3.2%) of grossly positive resection margins in this patient cohort. A positive microscopic margin conferred a similar unfavorable risk on development of distant metastases across all subsets of patients (relative risk = 1.4, 95% confidence interval 1.1–1.7).
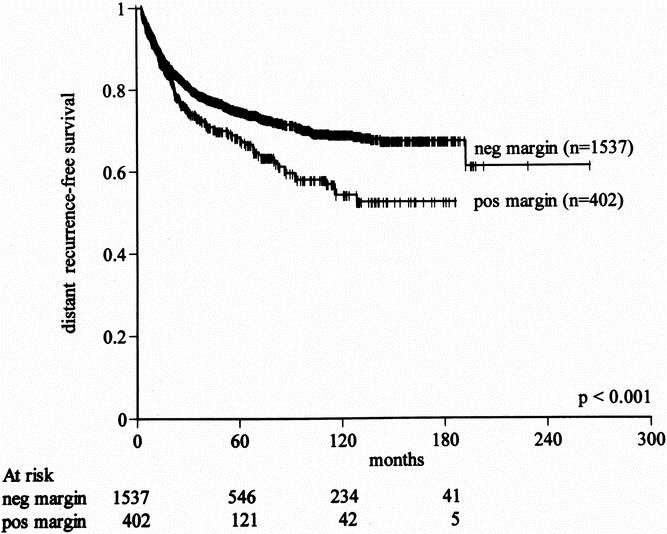
Figure 2. Effect of microscopic resection margin on distant recurrence-free survival for primary soft tissue sarcoma (all anatomic sites).
Rate of Disease-Specific Death
The presence of a positive margin was associated with a 1.6-fold increase in sarcoma-related death (18% to 29%, P < .001). By univariate analysis, positive microscopic margin was associated with a worse disease-specific survival rate (P < .001). This influence was more significant for late death, presumably because margin status has only a late influence on the development of distant recurrence, which is a surrogate for disease-related death in most patients (Fig. 3). The 5year disease-specific survival rates for primary tumors resected with negative and positive microscopic margins were 80% and 70%, respectively (Table 7). By multivariate analysis, microscopic margin remained significantly associated with disease-specific survival (P < .001, relative risk = 1.5, 95% confidence interval 1.2–1.9) after adjusting for grade, size more than 5 cm, size more than 10 cm, histology, and extremity site (see Table 6). Gross margin status was also marginally significant (P = .03, relative risk = 1.7, 95% confidence interval 1.1–2.9). The increased risk of disease-related death for positive margins was uniform across all subsets of patients.
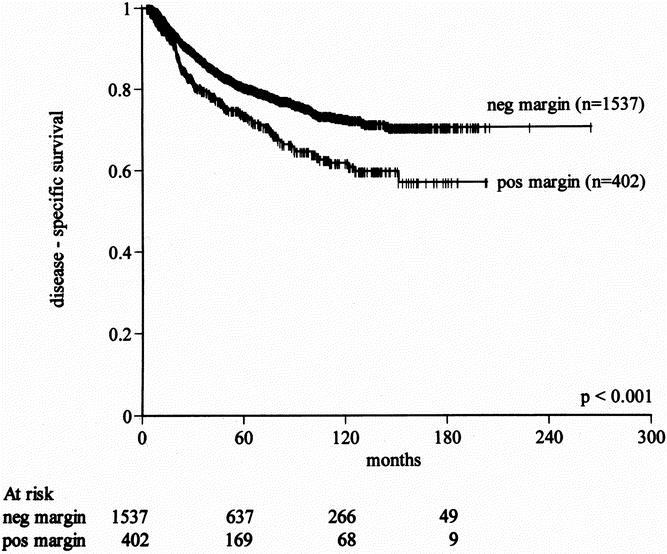
Figure 3. Effect of microscopic resection margin on disease-specific survival for primary soft tissue sarcoma (all anatomic sites).
Table 7. FIVE-YEAR ACTUARIAL AND CRUDE RATES OF DISEASE-SPECIFIC SURVIVAL ACCORDING TO CLINICAL AND PATHOLOGIC PROGNOSTIC FACTORS
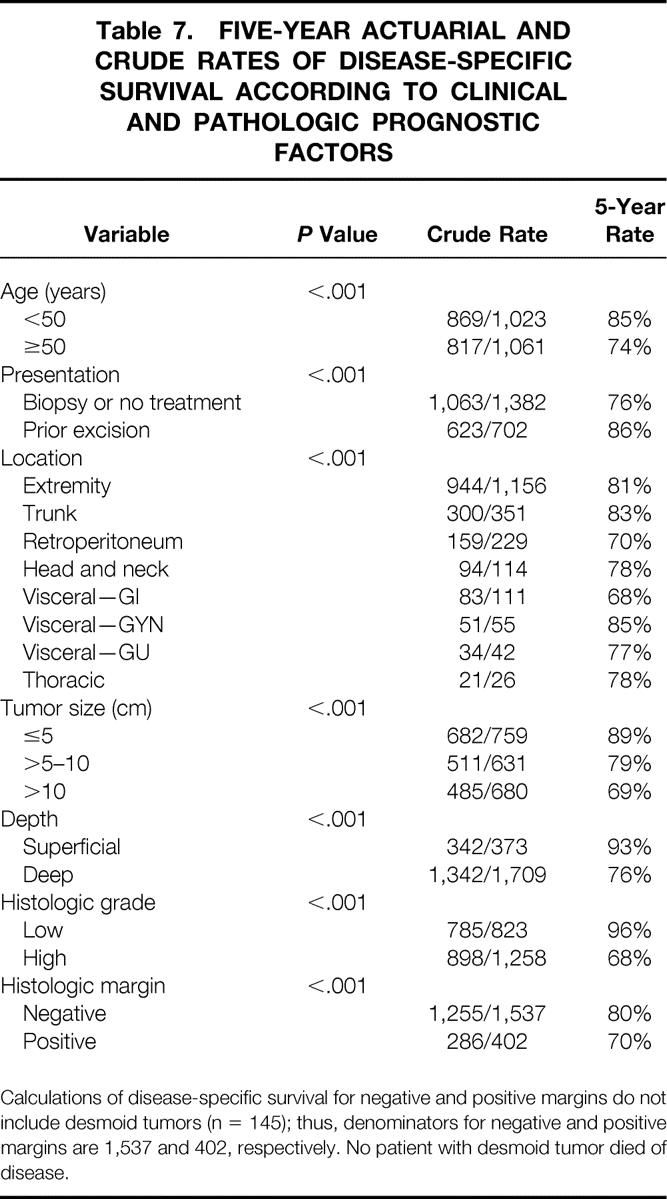
Calculations of disease-specific survival for negative and positive margins do not include desmoid tumors (n = 145); thus, denominators for negative and positive margins are 1,537 and 402, respectively. No patient with desmoid tumor died of disease.
DISCUSSION
Soft tissue sarcomas are markedly heterogeneous tumors in terms of anatomic primary tumor site, histopathology, and biologic behavior. Local control of extremity and superficial trunk sarcomas can be achieved in excess of 90% of patients with limb-sparing multimodality treatment. 4 Sarcomas arising in other anatomic sites are often more difficult to control because of anatomic constraints, delayed disease presentation, proximity to critical neurovascular and osseous structures, and difficulty with the administration of radiation. In light of these considerations, one would predict that the clinical significance of positive histologic margins after surgical treatment would differ among various anatomic sites. Most studies thus far have focused on extremity sarcomas to define clinicopathologic predictors of outcome. Although histologic residual disease has been shown to have prognostic significance for extremity sarcomas, the relationship between pathologic margin status and the natural history of sarcoma has not been well defined for tumors arising in other anatomic sites. 2
In this study of 2,084 patients with primary STS, the presence of a positive margin nearly doubled the risk of subsequent local failure at the primary tumor site (28% vs. 15%). The impact of margin status on the development of distant metastases was less pronounced. The presence of a positive margin was associated with a 1.6-fold increased risk of sarcoma-related death (18% vs. 29%). The difference in disease-specific survival was far less in magnitude than that observed with, say, small (≤5 cm) and large (>10 cm) high-grade STS (5-year disease-specific survival, 82% vs. 52%).
The presence of viable residual disease in the postresection primary tumor bed cannot be defined with certainty. Although imperfect, the benchmark for determining microscopic local residual disease after surgical treatment is pathologic assessment of resection margins. Local control is compromised in the presence of residual viable disease after resection. The Massachusetts General Hospital and M. D. Anderson Cancer Center experiences show that patients with extremity sarcomas who have involved microscopic surgical margins are at a significantly increased risk of local disease failure. 5,6 The largest single-institution analysis of prognostic factors for primary and locally recurrent extremity sarcomas extended the understanding of the clinical importance of viable residual disease; in that study, positive microscopic margins were significantly associated with tumor-related death. 2
Microscopic margin status is an independent predictor of local recurrence-free survival; however, our data suggest that the influence of margin on local control of disease is not uniform across all histologic subsets or anatomic locations. There was no observed difference in local recurrence-free survival among patients with fibrosarcoma or those with primary STS originating in the retroperitoneum who had involved or uninvolved microscopic surgical margins. The absence of margin influence on local control is evident for both desmoid tumors and nondesmoid fibrosarcomas.
Local recurrence-free survival was no different among patients with positive or negative microscopic margins after resection of primary desmoid tumors in this study (5-year local recurrence-free survival rate, 76% vs. 73%, P = .65). No patient with desmoid tumor died of disease. The association of positive microscopic margins of desmoid tumors with local recurrence remains controversial. These data suggest that a positive microscopic margin after complete resection of desmoid tumor does not uniformly predict local failure: 76% with patients with positive margins did not have local recurrence. However, the influence of selective use of radiotherapy on decreasing local recurrence confounds this analysis, because prospective randomized data indicate that radiation treatment can improve local disease control. The natural history of desmoid tumors, although favorable overall, remains enigmatic; these tumors have been found to remain unchanged and even regress spontaneously when managed by observation alone. 7
Margin status did not predict local control of retroperitoneal STS but was found to have significant prognostic value for distant recurrence-free and disease-specific survival. We found that the rate of histologically positive margins increased significantly with increasing primary tumor size (P < .001). Our highest rates of positive microscopic margin resections were for tumors arising in the retroperitoneum. This can be explained by the relatively large size at primary presentation of tumors originating in this location. The determination of microscopic margin status for retroperitoneal tumors is problematic based on large tumor surface area alone and perhaps the methodology of pathologic sampling. Areas that appear grossly suspicious are typically selected for sampling, along with multiple random sections, in an effort to define histologic margins. Thus, our ability to define microscopic residual disease in the retroperitoneum may be limited. The presence of gross residual disease after resection has been shown to be the most significant predictor of tumor-related death for retroperitoneal sarcoma. 8–11 Gross margin status had a marginally significant influence on survival in this study because only 3.2% of patients treated had gross residual disease after primary treatment.
The relatively high incidence of microscopically positive margins after complete resection of gross disease in the retroperitoneum led us to question what factors could reliably predict histologic residual disease. Retroperitoneal and head and neck sites were independent predictors of a positive histologic margin. In the retroperitoneum, tumor volume, deep location, and proximity to vital organs may explain this phenomenon. Similarly, wide-margin excision of primary head and neck sarcomas often requires resection of a considerable volume of tissue that may have important functional or cosmetic implications. Other factors predictive of positive microscopic resection margins were primary tumor size more than 10 cm, stage T2b (size >5 cm and deep location), fibrosarcoma histology, and preoperative biopsy or no prior surgical treatment (one definitive resection). It is intuitive that small primary tumors (T1a/1b, T2a) are predictive of negative resection margins. We have previously reported that patients with primary extremity sarcomas who underwent re-resection after initial resection had significantly better metastases-free and disease-specific survival rates than those treated with a single primary resection. 12 We found re-resection with two “primary” operations in the current study to be an independent predictor of negative microscopic margins for all subsets of patients. Although not included in our best-fitting five-factor Cox model predicting distant metastases and tumor-related death, analysis of the prognostic effect of re-resection after adjusting for the five independent prognostic variables confirms the previous findings for extremity sarcoma and extends those findings to the other anatomic sites evaluated in this study. We found that re-resection of primary STS (all anatomic sites) had no effect on local recurrence-free survival (P > .05). This phenomenon is difficult to explain, considering that re-resection predicts a negative margin but has no influence on local recurrence-free survival, yet favorably affects distant metastasis-free and disease-specific survival. Re-resection may eradicate histopathologically inapparent local residual disease, resulting in loss of dormancy in subclinical systemic disease. 12 Across all subsets of patients studied, we found single primary resection (no prior treatment or biopsy) to be an independent adverse prognostic variable for distant metastasis and sarcoma-related death. The favorable effect on distant metastases-free and disease-specific survival is presumably due to the selection of a “better-risk” group who can undergo resection.
The relative prognostic importance of microscopic margins was most evident after the first 2 years after resection of the primary tumor. A recent study of patients with primary extremity sarcomas surviving more than 5 years after initial resection found histologic margin at initial surgery to be an independent predictor of tumor-related death for those alive at 5 years. 13 Histologic grade had a profound impact on early survival but did not influence late survival. Microscopic margin may have greater prognostic influence later (>2 years after primary treatment) in the natural history of the disease. Thus, long-term follow-up of patients with positive histologic resection margins is important.
A prospective randomized controlled trial performed at MSKCC compared surgery alone with surgery and adjuvant brachytherapy for patients with primary and recurrent extremity and superficial trunk STS. Brachytherapy was associated with a significant improvement in local control (89% vs. 66%, P = .025) for patients with high-grade tumors only. There was no significant difference in 5-year disease-specific survival rates between the two treatment arms (84% vs. 81%). 14 A recent National Cancer Institute randomized prospective trial has shown that adjuvant external-beam radiotherapy improves local control for extremity STS of both low and high histologic grade. 15 Because the presence of a positive microscopic resection margin significantly increases the risk of local recurrence for primary nonretroperitoneal sarcomas, radiotherapy after resection is recommended in an effort to optimize local control of these tumors.
In this study we defined the clinical significance of a positive microscopic margin after surgical treatment of primary STS. Microscopic resection margin appears to be an independent predictor of distant recurrence-free and disease-specific survival for all patient subsets. With the exception of fibrosarcoma subtype and retroperitoneal primary tumor location, margin status appears to have pivotal prognostic value in predicting local disease control. These findings have important clinical implications for staging and treatment. Adjuvant therapy should be considered in the management of STS to decrease local recurrence. Because a positive microscopic resection margin did not equate with inevitable local recurrence in 72% of patients, considerable clinical judgment is required in considering additional treatment. The results of this study underscore the importance of histologic resection margin as a prognostic factor and support the inclusion of margin status as a discriminating variable in future randomized clinical trials that stratify patients according to clinical and pathologic prognostic factors in the analysis of outcome.
Acknowledgment
The authors thank, for their major contributions during the past 20 years, their colleagues in pathology, especially Dr. Steven Hajdu, Dr. James Woodruff, and Dr. Cristina Antonescu. They are further grateful to all of their colleagues who have helped care for these patients and provided essential support in data accrual.
Footnotes
Supported by NIH Grant CA47179 (M.F.B.).
Correspondence: Murray F. Brennan, MD, Chairman, Department of Surgery
Memorial Sloan-Kettering Cancer Center, 1275 York Ave, New York, NY 10021.
E-mail: brennanm@mskcc.org
Reprints will not be available from the author.
Accepted for publication August 27, 2001.
References
- 1.Soft tissue sarcoma, AJCC Cancer Staging Manual, 5th ed. Philadelphia: Lippincott Williams & Wilkins, 1998:140–146.
- 2.Pisters PWT, Leung DHY, Woodruff J, et al. Analysis of prognostic factors in 1041 patients with localized soft tissue sarcoma of the extremity. J Clin Oncol 1996; 14: 1679–1689. [DOI] [PubMed] [Google Scholar]
- 3.Hajdu SI, Shiu MH, Brennan MF. The role of the pathologist in the management of soft tissue sarcomas. World J Surg 1988; 12: 326–331. [DOI] [PubMed] [Google Scholar]
- 4.O’Sullivan B, Wylie J, Catton CN, et al. The local management of soft tissue sarcoma. Semin Radiat Oncol 1999; 9: 328–348. [DOI] [PubMed] [Google Scholar]
- 5.Sadoski C, Suit HD, Rosenberg A, et al. Preoperative radiation, surgical margins, and local control of extremity sarcomas of soft tissue. J Surg Oncol 1993; 52: 223–230. [DOI] [PubMed] [Google Scholar]
- 6.Tanabe KK, Pollock RE, Ellis LM, et al. Influence of surgical margins on outcome in patients with preoperatively irradiated extremity soft tissue sarcomas. Cancer 1994; 73: 1652–1659. [DOI] [PubMed] [Google Scholar]
- 7.Rock MG, Pritchard DJ, Reiman HM, et al. Extra-abdominal desmoid tumors. J Bone Joint Surg 1984; 66: 1369–1374. [PubMed] [Google Scholar]
- 8.Cody HS, Turnbull AD, Fortner JG, et al. The continuing challenge of retroperitoneal sarcomas. Cancer 1981; 47: 2147–2152. [DOI] [PubMed] [Google Scholar]
- 9.Jaques DP, Coit DG, Hajdu S, et al. Management of primary and recurrent soft tissue sarcoma of the retroperitoneum. Ann Surg 1990; 212: 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singer S, Corson JM, Demetri GD, et al. Prognostic factors predictive of survival for truncal and retroperitoneal soft-tissue sarcoma. Ann Surg 1995; 221: 185–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lewis JJ, Leung D, Woodruff JM, et al. Retroperitoneal soft-tissue sarcoma: Analysis of 500 patients treated and followed at a single institution. Ann Surg 1998; 228: 355–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewis JJ, Leung D, Espat J, et al. The effect of re-resection in extremity soft tissue sarcoma. Ann Surg 2000; 231: 655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewis JJ, Leung D, Casper ES, et al. Multifactorial analysis of long term follow-up (more than 5 years) of primary extremity sarcoma. Arch Surg 1999; 134: 190–194. [DOI] [PubMed] [Google Scholar]
- 14.Pisters PWT, Harrison LB, Leung DHY, et al. Long-term results of a prospective randomized trial of adjuvant brachytherapy in soft tissue sarcoma. J Clin Oncol 1996; 14: 859–868. [DOI] [PubMed] [Google Scholar]
- 15.Yang JC, Chang AE, Baker AR, et al. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcoma of the extremity. J Clin Oncol 1998; 16: 197–203. [DOI] [PubMed] [Google Scholar]