Abstract
Objective
To evaluate experience with isolated orthotopic liver transplantation in children with liver failure associated with short bowel syndrome (SBS).
Summary Background Data
Infants who have liver failure as a result of SBS are frequently referred for consideration for combined liver and small bowel transplantation. In a few patients the liver disease develops despite a seemingly adequate bowel, which if given time and appropriate management has the potential for full enteral adaptation. There is a limited literature suggesting the utility of OLT without replacement of the native bowel. The advantages over combined liver and small bowel transplantation are clear: organ availability is greater, liver-reduction techniques are well established, lower immunosuppression is required, and there is greater experience in the care of children after orthotopic liver transplantation.
Methods
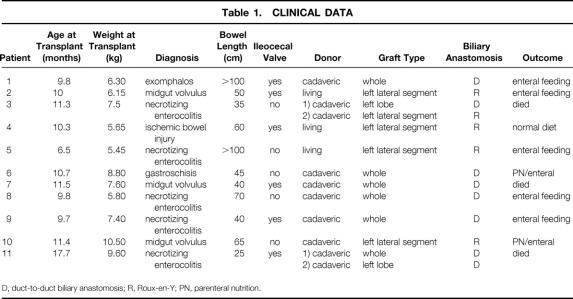
Eleven infants, considered to have a good prospect of eventual gut adaptation to full enteral nutrition if it were not for their advanced liver disease, underwent isolated orthotopic liver transplantation. Age range was 6.5 to 17.7 months. All patients had been dependent on parenteral feeding but had also shown significant enteral tolerance at some time before listing for transplantation. Advanced liver disease was apparent both clinically and on histologic examination. All were jaundiced and had low albumin levels, and most had coagulopathy. As a group the infants had growth retardation. Estimated remaining length of small bowel beyond the ligament of Treitz was in the range of 25 to more than 100 cm. Six infants retained their ileocecal valve.
Results
Thirteen liver transplants were performed in the 11 patients. A combination of whole livers (n = 6) and reduced-size grafts, of which three were from living-related donors, were used. Biliary anastomosis was duct-to-duct in eight instances and involved a short Roux limb in the others. Eight patients are alive with follow-up of 15 to 66 months. Three deaths have occurred after transplantation as a result of sepsis. Of eight surviving patients, only two continue to receive intravenous support and in both there is increasing enteral tolerance. Since transplantation, all surviving children have shown adequate growth with maintenance of pretransplant centiles.
Conclusions
In selected infants with liver failure secondary to short bowel syndrome in whom complete enteral autonomy is anticipated, isolated liver transplantation can offer long-term survival.
The ability of patients with intestinal failure resulting from short bowel syndrome (SBS) to achieve nutritional autonomy depends on the capacity of the gut remnant to compensate or adapt. Intestinal adaptation requires increasing the functional absorptive surface by growth in length and diameter as well as in villous height and crypt depth. 1 If fibrotic liver disease develops before enteral autonomy is achieved, a downward spiral occurs, with decreased enteral tolerance, nutritional impairment, and progression to liver failure. The deterioration of bowel function is related to several factors, including anorexia, intestinal malabsorption secondary to luminal bile acid deficiency, ascites, bowel wall edema and portal hypertensive exudative enteropathy, and gastrointestinal bleeding. 2 Typically patients are then considered for intestinal or combined liver and small bowel transplantation. In selected patients, if it were not for the appearance of severe liver disease, there might still be considerable optimism that adaptation of the bowel to full enteral tolerance could be achieved in time. In these circumstances, is there a role for isolated liver transplantation? Anecdotal accounts have circulated that liver transplantation alone should not be attempted because in these patients cholestasis will inevitably develop in the allograft after transplantation and that the prospect of intestinal adaptation is negligible. Despite these dire warnings, several groups have successfully carried out isolated liver transplantation in children with end-stage liver disease related to SBS, although reports in the literature are few. 2–4 We now report the largest experience with isolated liver transplantation in children with liver failure associated with SBS.
PATIENTS AND METHODS
Subjects
Eleven patients who were referred for evaluation for combined small bowel and liver transplantation were selected to undergo isolated liver transplantation over a 6-year period. Patient characteristics are listed in Table 1. Liver transplantation was carried out at a median age of 10.3 months (range 6.5–17.7). All had SBS and end-stage liver disease. All 11 infants had jaundice with hepatosplenomegaly on physical examination, and all had cirrhosis shown on biopsy either before transplantation or at explantation. Median prothrombin time, after vitamin K administration, was 17.1 seconds (range 13–32.9), median plasma albumin was 2.3 g/dL (range 1.9–3.2), median bilirubin was 19.8 mg/dL (range 13.9–35.6), and median platelet count was 39 × 109/L (range 13–86). As a group the infants had significant growth retardation, with a median z-score for weight of −2.33 (range −3.19–0.68) and a median z-score for length of −2.92 (range −3.67 to −0.31). Radiologic evaluation showed a residual small bowel length of median 50 cm (range 25 to more than 100 cm). Six patients retained their ileocecal valve, and all subjects retained most or all of their colon. Each infant had a history of considerable enteral tolerance either at the time of evaluation or before the progression of their liver disease. Median maximal enteral tolerance, at or before evaluation, was 70% of nutritional requirements (range 40–80%). Assessment of intestinal motility was based on clinical assessment of enteral tolerance, stool output, and radiologic transit time of oral contrast. Formal small bowel manometry was not carried out. No subject showed significant dilatation of any segment of small bowel, although one patient had a short jejunal stricture.
Table 1. CLINICAL DATA
D, duct-to-duct biliary anastomosis; R, Roux-en-Y; PN, parenteral nutrition.
Statistical Analysis
The group was small, so normal distribution of parameters within the group was not assumed. Standard deviation scores (z-scores) were used to compare growth parameters from before and after transplantation. Z-scores were calculated using National Center for Health Statistics growth data. 5 Data are expressed as median and range, and paired growth data on surviving infants were compared using the Wilcoxon signed ranks test. Nominal data were analyzed using the Fisher exact test.
RESULTS
Eleven patients underwent 13 liver transplants with six whole organs and seven reduced-size liver grafts (see Table 1). In three instances a relative donated the left lateral segment allograft. Biliary reconstruction was duct-to-duct in eight transplants and choledochojejunostomy with a short Roux-en-Y in five transplants. The duct-to-duct biliary reconstruction was created using standard techniques. Interrupted monofilament absorbable sutures were used. The anastomosis was fashioned over a biliary stent. The (biliary) stent was a 3F or 5F feeding tube, depending on the diameter of the duct. No T tubes were used. The stent was either removed before completion of the anastomosis or passed into the duodenum through the anastomosis. The Roux-en-Y limbs were created using the shortest possible segment of small bowel that could be brought up without tension. This was typically 10 to 15 cm long. The method of the biliary construction was dictated by the patient’s underlying anatomy.
Immunosuppression for the first patient consisted of cyclosporin and steroids. All other patients received tacrolimus and steroids for primary immunosuppression according to our current protocol for orthotopic liver transplantation in children. Tacrolimus is administered enterally within 24 hours of transplantation, and initially we aim for blood levels of 12 to 15 ng/mL for the first month. Ease of administration and ease of maintaining target plasma levels were not noticeably different from infants receiving liver transplantation for other indications.
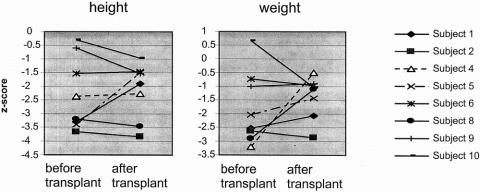
Eight patients are alive with a median duration of survival of 27 months (range 15–66). Survival was not influenced by the presence of an ileocecal valve (P = 1.0) or type of biliary anastomosis (P = .24). Six subjects have been weaned fully from parenteral nutrition at a median of 7 months after liver transplantation (range 1.5–18). Only patient 4 eats an entirely normal diet; the remaining five patients continue to require supplemental tube feeding. Linear growth for survivors has continued along their pretransplant centile rather than showing catch-up in the majority of subjects. Median change in z-score for height is −0.01 (range −0.95–1.56) and median change in z-score for weight is 0.36 (range −1.69–2.95) (Fig. 1).
Figure 1. Height and weight standard deviation scores before and after liver transplantation in surviving patients with short bowel syndrome.
Three patients died after liver transplantation. Patient 3 had hepatic artery thrombosis necessitating retransplantation, after which generalized sepsis with splenic gangrene developed. Patient 7 underwent stricturoplasty at the time of transplantation. Fulminant gram-negative sepsis developed on day 4 after transplantation and progressed to multiple organ failure. In the final patient, centrilobular necrosis and acute liver failure developed on postoperative day 7, unrelated to hepatic artery thrombosis or rejection. She was retransplanted 9 days after the first transplant operation and made an excellent recovery. One month later she was tolerating more than 50% of requirements enterally, but 35 days after retransplantation she deteriorated acutely. Blood cultures revealed Candida, and respiratory syncytial virus was cultured from a bronchoalveolar lavage specimen. She died 8 days later.
Several complications have been encountered in the survivors. Posttransplant lymphoproliferative disorder in patient 1 was diagnosed 2 months after transplantation and responded completely to adenotonsillectomy, low-dose cyclophosphamide therapy, and temporary withdrawal of immunosuppression. Patient 2 had cytomegaloviral hepatitis with full recovery on intravenous ganciclovir. In patient 5 an intraabdominal abscess developed almost a year after transplantation, related to a stricture in her colonic remnant, which required surgical drainage. In patient 10 a biliary stricture developed that responded fully to percutaneous dilatation.
Two patients still receive intravenous fluids. At the time patient 6 presented to us, he was severely jaundiced and coagulopathic with bleeding and showed signs of developmental regression, probably associated with encephalopathy. His listing for isolated liver transplantation was based on a history of 50% enteral tolerance before liver decompensation and a nonstrictured 45-cm-long small bowel. He is now 24 months after liver transplantation with normal liver function. Parenteral nutrition supplies 60% of calories and fluid requirements. Patient 10 continues to receive intravenous fluids; he is now making steps toward nutritional autonomy 15 months after liver transplantation.
DISCUSSION
The survival rate for patients with SBS requiring long-term parenteral nutrition is approximately 60% at 5 years. 6 About 15% of these deaths are directly related to parenteral nutrition as a consequence of sepsis or liver disease, and possibly half of all deaths in children receiving parenteral nutrition are due to liver failure. 7 The etiology of parenteral nutrition-induced liver disease is multifactorial. 8–14 The conventional hypothesis of direct hepatotoxicity by parenteral nutrition or deficiency of individual nutrient components is now considered incomplete. Extensive intestinal resection may set the stage for hepatobiliary sepsis and secondary liver disease through the combination of an altered luminal microbial environment and loss of gut-associated lymphoid mass. 15 The emphasis now is on the susceptibility of the liver, especially in infants, to sepsis-induced dysfunction. 16–18 Whatever the process, it eventually leads to cholestasis and cirrhosis. Once end-stage liver disease develops, survival is about 1 year without transplantation.
The selection of patients suitable for isolated liver transplantation with SBS requires a careful functional assessment of past and present bowel function. Maximal enteral tolerance is used as an indicator of best absorptive ability of the bowel. Formal tests of intestinal absorption or motility are not useful because the advanced liver disease gives a false impression of excessively poor bowel function. Bowel length estimates are imprecise using only measurement of bowel shown on contrast radiology. However, consistency of approach enables us to compare relative bowel lengths between subjects, and no other noninvasive method has been shown to be superior. 19 We use these two pieces of information in addition to routine clinical assessment to estimate the likelihood of eventual full enteral adaptation after successful restoration of normal hepatic function. 20 Further, because adaptation of the small intestine after neonatal resection tends to be complete by 3 or 4 years of age, we would suggest, as a general rule, that this technique is applicable only to infants and very young children.
The number of patients presented in this series is insufficient for us to define strict criteria for the application of isolated liver transplantation in infants with end-stage liver disease secondary to SBS. It is generally acknowledged that a small bowel length of less than 25 cm (beyond the ligament of Treitz) is unlikely to result in full adaptation. Therefore, this is probably the lower limit at which an isolated liver transplant can be contemplated in this situation. Similarly, we cannot define a precise lower limit for previous enteral tolerance below which there would be no hope of intestinal adaptation after successful orthotopic liver transplantation. We have used 50% enteral tolerance as our benchmark. If a patient has tolerated an enteral intake at any time before end-stage liver failure that approaches or exceeds 50%, then the opportunity to consider isolated liver transplantation exists. Most patients referred to our transplant program for consideration for combined liver and intestinal transplantation (and all the patients in this series were referred for such combined transplantation) have considerably less than 20% enteral tolerance and small bowel lengths that range from 0 cm beyond the ligament of Treitz to full lengths of dysfunctional small bowel.
This report describes the largest experience using liver replacement alone for the treatment of parenteral nutrition-induced liver failure with associated SBS. Eight of 11 patients with SBS and end-stage liver disease survived isolated liver transplantation. This is similar to results obtained in other young children receiving liver transplants for other causes of liver disease, such as biliary atresia. 21 The decision to treat patients with SBS and associated liver failure with isolated liver transplantation has been based on the expectation of full weaning from parenteral nutrition. All of the patients had shown 40% to 80% enteral nutrition at some point. We did not perform liver transplants to act as a “bridge” to later combined liver and small bowel transplantation, as has been recently proposed by others. 4 Despite the successful liver transplants described in this series, the majority of children with SBS accompanied by end-stage liver disease are not suitable for this form of treatment and will require combined liver and small bowel transplantation.
Liver transplantation allowed the progression of small bowel adaption to occur, permitting either freedom from parenteral nutrition or a reduction in its use. All survivors have made progress on enteral feedings, although two remain on intravenous support. Growth has been maintained, although it is not possible in this group to compare growth at defined time points after transplantation. Among surviving subjects with significantly low pretransplant weights, four of five patients have improved their z-score for weight since the transplant. Those already within the normal range have generally maintained their centile. One patient has had a significant deterioration in z-score for weight since transplantation, but this patient had marked fluid retention with ascites before transplantation. After transplantation his weight decreased, but he is now gaining weight along the 15th centile. Linear growth has tended to follow the pretransplant centiles rather than displaying catch-up. The lack of catch-up growth is disappointing but in no way implies that the patients in this series would have been better off with intestinal transplantation. In our intestinal transplant patients, we also see no catch-up in linear growth if the child is stunted going into transplantation. 22 There are also published data indicating that infants and children who are stunted before liver transplantation for other indications may also not show adequate catch-up growth. 23 One patient eats a normal diet, but the dependence on supplemental enteral tube feeding in five of the six patients off parenteral nutrition is not unexpected. Many children with SBS who have been weaned from parenteral nutrition before progressive liver disease develops continue to require the same kind of supplemental feedings to maintain their growth and nutritional status. 1
Sepsis remains a serious concern in this group of patients. All three deaths were the result of infection, and several of the surviving patients have had systemic bacterial sepsis while still requiring parenteral nutrition. However, there have been no significant bacterial infections in any of the patients once the central venous catheter has been removed. Apart from bacterial sepsis, other posttransplant complications have been similar to those seen in patients with other indications for liver transplantation. In particular, acute rejection rates appeared to be comparable in frequency and severity with those seen in other liver transplant populations.
Where possible, biliary anastomosis was duct-to-duct so as not to further reduce the functional length of the small bowel with the fashioning of a Roux-en-Y loop. When duct-to-duct anastomosis was not technically feasible (for example, in living-donor liver transplantation), a short Roux loop was used and did not appear to affect the patient outcome or predispose the patient to ascending cholangitis. Novel methods for biliary drainage have been discussed such as hepaticoduodenostomy or hepaticogastrostomy, but we did not feel the need to consider such options. 4
The alternative to isolated liver transplantation in these patients would be combined liver and small bowel transplantation. However, death rates for children on the waiting list for combined liver small bowel transplantation approach 50%. 24 The number of liver and small bowel grafts suitable for infants such as those described in this series, weighing less than 10 kg, is pitifully small, and the waiting list death rate for this group of patients is greater still. 25 Death rates after combined liver and small bowel transplantation also exceed those of isolated liver transplantation. 1,26–28 Therefore, if long-term survival with a liver graft alone can be reasonably expected, then the opportunity should be seized.
In conclusion, isolated liver transplant can offer a long-term solution for patients with end-stage liver disease requiring total parenteral nutrition in carefully selected patients who have shown historical evidence of enteral feeding tolerance and who have sufficient small bowel that complete enteral adaptation can be reasonably expected. Experience with both transplantation and nutritional support of children with SBS is probably essential for a successful outcome in such situations.
Footnotes
Correspondence: Simon P. Horslen, MB, ChB, FRCPCH, 983285 Nebraska Medical Center, Omaha, NE 68198-3285.
E-mail: shorslen@surgery.unmc.edu
Accepted for publication August 16, 2001.
References
- 1.Vanderhoof JA, Langnas AN. Short-bowel syndrome in children and adults. Gastroenterology 1997; 113: 1767–1778. [DOI] [PubMed] [Google Scholar]
- 2.Gottrand F, Michaud L, Bonnevalle M, et al. Favorable nutritional outcome after isolated liver transplantation for liver failure in a child with short bowel syndrome. Transplantation 1999; 67: 632–634. [DOI] [PubMed] [Google Scholar]
- 3.Lawrence JP, Dunn SP, Billmire DF, et al. Isolated liver transplantation for liver failure in patients with short bowel syndrome. J Pediatr Surg 1994; 29: 751–753. [DOI] [PubMed] [Google Scholar]
- 4.Muiesan P, Dhawan A, Novelli M, et al. Isolated liver transplant and sequential small bowel transplantation for intestinal failure and related liver disease in children. Transplantation 2000; 69: 2323–2326. [DOI] [PubMed] [Google Scholar]
- 5.Hamill PV, Drizd TA, Johnson CL, et al. Physical growth: National Center for Health Statistics percentiles. Am J Clin Nutr 1979; 32: 607–629. [DOI] [PubMed] [Google Scholar]
- 6.Messing B, Lemann M, Landais P, et al. Prognosis of patients with nonmalignant chronic intestinal failure receiving long-term home parenteral nutrition. Gastroenterology 1995; 108: 1005–1010. [DOI] [PubMed] [Google Scholar]
- 7.Kelly DA. Liver complications of pediatric parenteral nutrition–epidemiology. Nutrition 1998; 14: 153–157. [DOI] [PubMed] [Google Scholar]
- 8.Hofmann AF. Defective biliary secretion during total parenteral nutrition: probable mechanisms and possible solutions. J Pediatr Gastroenterol Nutr 1995; 20: 376–390. [DOI] [PubMed] [Google Scholar]
- 9.Drongowski RA, Coran AG. An analysis of factors contributing to the development of total parenteral nutrition-induced cholestasis. J Parenter Enteral Nutr 1989; 13: 586–589. [DOI] [PubMed] [Google Scholar]
- 10.Bell RL, Ferry GD, Smith EO, et al. Total parenteral nutrition-related cholestasis in infants. J Parenter Enteral Nutr 1986; 10: 356–359. [DOI] [PubMed] [Google Scholar]
- 11.Merritt RJ. Cholestasis associated with total parenteral nutrition. J Pediatr Gastroenterol Nutr 1986; 5: 9–22. [DOI] [PubMed] [Google Scholar]
- 12.Teitelbaum DH, Han-Markey T, Drongowski RA, et al. Use of cholecystokinin to prevent the development of parenteral nutrition-associated cholestasis. J Parenter Enteral Nutr 1997; 21: 100–103. [DOI] [PubMed] [Google Scholar]
- 13.Yip YY, Lim AK, Tan KL. A multivariate analysis of factors predictive of parenteral nutrition- related cholestasis (TPN cholestasis) in VLBW infants. J Singapore Paediatr Soc 1990; 32: 144–148. [PubMed] [Google Scholar]
- 14.Iyer KR, Spitz L, Clayton P. BAPS prize lecture: New insight into mechanisms of parenteral nutrition-associated cholestasis: role of plant sterols. British Association of Paediatric Surgeons. J Pediatr Surg 1998; 33: 1–6. [DOI] [PubMed] [Google Scholar]
- 15.Bianchi A. Longitudinal intestinal lengthening and tailoring: results in 20 children. J R Soc Med 1997; 90: 429–432.9306995 [Google Scholar]
- 16.Sondheimer JM, Asturias E, Cadnapaphornchai M. Infection and cholestasis in neonates with intestinal resection and long-term parenteral nutrition. J Pediatr Gastroenterol Nutr 1998; 27: 131–137. [DOI] [PubMed] [Google Scholar]
- 17.Wolf A, Pohlandt F. Bacterial infection: the main cause of acute cholestasis in newborn infants receiving short-term parenteral nutrition. J Pediatr Gastroenterol Nutr 1989; 8: 297–303. [PubMed] [Google Scholar]
- 18.Braxton C, Lowry SF. Parenteral nutrition and liver dysfunction–new insight? J Parenter Enteral Nutr 1995; 19: 3–4. [DOI] [PubMed] [Google Scholar]
- 19.Nightingale JM, Bartram CI, Lennard-Jones JE. Length of residual small bowel after partial resection: correlation between radiographic and surgical measurements. Gastrointest Radiol 1991; 16: 305–306. [DOI] [PubMed] [Google Scholar]
- 20.Sondheimer JM, Cadnapaphornchai M, Sontag M, Zerbe GO. Predicting the duration of dependence on parenteral nutrition after neonatal intestinal resection. J Pediatr 1998; 132: 80–84. [DOI] [PubMed] [Google Scholar]
- 21.Chardot C, Carton M, Spire-Bendelac N, et al. Prognosis of biliary atresia in the era of liver transplantation: French national study from 1986 to 1996. Hepatology 1999; 30: 606–611. [DOI] [PubMed] [Google Scholar]
- 22.Iyer K, Horslen S, Iverson A, et al. Nutritional outcome and growth following intestinal transplantation in children. J Pediatr Surg 2001 (in press). [DOI] [PubMed]
- 23.McDiarmid SV, Gornbein JA, DeSilva PJ, et al. Factors affecting growth after pediatric liver transplantation. Transplantation 1999; 67: 404–411. [DOI] [PubMed] [Google Scholar]
- 24.Transplant Patient DataSource. (2000, February 16). Richmond, VA. United Network for Organ Sharing. Retrieved [December 6, 2000] http://207.239.150.13/tpd/
- 25.Beath SV, Needham SJ, Kelly DA, et al. Clinical features and prognosis of children assessed for isolated small bowel or combined small bowel and liver transplantation. J Pediatr Surg 1997; 32: 459–461. [DOI] [PubMed] [Google Scholar]
- 26.Grant D. Intestinal transplantation: 1997 report of the international registry. Intestinal Transplant Registry. Transplantation 1999; 67: 1061–1064. [DOI] [PubMed] [Google Scholar]
- 27.Kumar N, Grant D. Gastrointestinal transplantation: An update. Liver Transplant 2000; 6: 515–519. [DOI] [PubMed] [Google Scholar]
- 28.Pinna AD, Weppler D, Nery J, et al. Intestinal transplantation at the University of Miami: five years of experience. Transplant Proc 2000; 32: 1226–1227. [DOI] [PubMed] [Google Scholar]