Abstract
Objective
To investigate cyclooxygenase-2 (COX-2) mRNA expression in curatively resected non-small cell lung cancer (NSCLC) and to determine its association with prognosis.
Summary Background Data
Lung cancer is one of the most common malignancies in the world. Despite improvements in the diagnosis and treatment of NSCLC, the 5-year survival rate remains less than 15%. Identification of prognostic predictors based on molecular alterations could lead to additional diagnostic tools and eventually to more effective therapeutic options. Overexpression of COX-2 has been reported in several human malignancies, including lung cancer, but the prognostic importance of this overexpression has not been elucidated.
Methods
COX-2 mRNA expression was analyzed using a quantitative real-time polymerase chain reaction (Taqman) method in surgically resected tumor specimens from 89 patients with curatively resected NSCLC.
Results
COX-2 mRNA was detectable in all 89 (100%) tumor tissues. High COX-2 expression in tumors was significantly associated with inferior survival. Multivariate analysis showed that high COX-2 expression is an independent predictor of worse survival in patients with NSCLC.
Conclusions
High COX-2 mRNA expression is an important biomarker for biologically aggressive disease in NSCLC and might be helpful in identifying patients who would benefit from additional therapies for controlling their disease.
Lung cancer is the most common cause of cancer-related death in the United States, accounting for more deaths than from prostate, breast, and colorectal cancer combined. 1 Each year approximately 170,000 new cases of lung cancer are diagnosed in the United States. 1 Radical surgery offers the only chance for cure in patients with non-small cell lung cancer (NSCLC), but despite improvements in the detection and treatment of lung cancer in the past two decades, the 5-year survival rate remains less than 15%. 2 To improve the outcome of patients with NSCLC, the development of a prognostic classification based on molecular alterations will be crucial. Such a classification could provide additional accurate and useful diagnostic tools and, eventually, more effective therapeutic options.
Epidemiologic studies have shown that prolonged use of nonsteroidal antiinflammatory drugs (NSAIDs) reduces the risk of colon cancer, 3–5 and other studies have suggested that aspirin may also reduce the incidence of lung cancer. 6 The best-known target of NSAIDs, including aspirin, is the enzyme cyclooxygenase (COX), a key enzyme involved in the production of prostaglandins and other eicosanoids from arachidonic acid. 7,8 Two COX isoforms, COX-1 and COX-2, have been identified. Whereas COX-1 is considered a constitutively expressed housekeeping gene, COX-2 is an inducible immediate-early gene associated with inflammation and carcinogenesis. 8–10 Overexpression of COX-2 has been reported in several human malignancies, including colorectal cancer, 11 gastric cancer, 12 breast cancer, 13 esophageal carcinoma, 14 and lung cancer. 15–19 However, the prognostic role of COX-2 protein expression in NSCLC remains controversial. Although Achiwa et al 17 reported an association between COX-2 overexpression and survival in a patients with stage 1 adenocarcinoma of the lung, Marrogi et al 19 were unable to detect any association between COX-2 expression and clinical outcome in patients with NSCLC. No studies concerning the prognostic role of COX-2 mRNA expression in NSCLC have been reported.
To determine the prognostic relevance of COX-2 mRNA expression in NSCLC, we performed quantitative real-time reverse transcriptase–polymerase chain reaction (RT-PCR; Taqman) 20,21 on surgically removed tumor specimens from 89 patients with curatively resected NSCLC.
PATIENTS AND METHODS
Patients and Specimens
Tumor specimens from 89 patients with NSCLC, available from a previous prospective clinical trial of 103 consecutive patients, 22 were included in this study. There were 67 (75%) men and 22 (25%) women, with a median age of 64 years (range 34–83). Forty-one (46%) patients had squamous cell carcinomas, 33 (37%) had adenocarcinomas, and 15 (17%) had large cell carcinomas. The primary tumors were graded histopathologically as well-differentiated (G1, one patient), moderately differentiated (G2, 19 patients), and poorly differentiated (G3, 69 patients). Tumor staging was performed according to the International Union Against Cancer (UICC) TNM classification:23 44 patients (50%) had stage 1 tumors, 18 (20%) had stage 2 tumors, and 27 (30%) had stage 3a tumors. All 89 patients underwent thoracic surgery. All tumors were radically removed (R0 resection) by lobectomy (n = 57), bilobectomy (n = 11), pneumonectomy (n = 11), and extended pneumonectomy (n = 10) including mediastinal lymphadenectomy. Patients with histopathologic stage 3a tumors received postoperative radiotherapy. Informed consent was obtained from each patient.
The median follow-up was 85.9 months (range 63–105), and no patient was lost to follow-up. Tissue for gene expression analysis was obtained during surgery immediately after lung resection and before starting mediastinal lymphadenectomy. The tissues were immediately frozen in liquid nitrogen and stored at −80°C. Six-micrometer frozen sections were taken from blocks of tumor tissue, and starting with the first section, every fifth section was routinely stained with hematoxylin and eosin and evaluated histopathologically. Sections were pooled for analysis from areas estimated to have at least 75% malignant cells.
mRNA Isolation
Total RNA was isolated by a single-step guanidinium isothiocyanate method using the QuickPrepMicro mRNA Purification Kit (Amersham Pharmacia Biotech Inc., Piscataway, NJ) according to the manufacturer’s instructions. After RNA isolation, cDNA was prepared from each sample as described previously. 24
Real-Time Polymerase Chain Reaction Quantification
Quantitation of cDNA was done using a fluorescence based real-time detection method (ABI PRISM 7700 Sequence Detection System [Taqman], Applied Biosystems, Foster City, CA) as previously described. 20,21 The PCR reaction mixture consisted of 600 nmol/L of each primer, 200 nmol/L probe, 2.5 U AmpliTaq Gold Polymerase, 200 μmol/L each dATP, dCTP, dGTP, 400 μmol/L dUTP, 5.5 mmol/L MgCl2, and 1 × Taqman Buffer A containing a reference dye, to a final volume of 25 μL (all reagents Applied Biosystems). Cycling conditions were 50°C for 10 seconds and 95°C for 10 minutes, followed by 46 cycles at 95°C for 15 seconds and 60°C for 1 minute.
The primers and probe sequences used were as follows: COX-2 primers: GCTCAAACATGATGTTTGCATTC and GCTGGCCCTCGCTTATGA; probe 6FAM (carboxyfluorescein) 5′-TGCCCAGCACTTCACGCATCAGTT-3′TAMRA(N,N,N′,N′-tetramethyl-6carboxyrhodamine); and β-actin primers: TGAGCGCGGCTACAGCTT and TCCTTAAT-GTCACGCACGATTT; probe: 6FAM5′-ACCACCACG-GCCGAGCG G-3′TAMRA.
Statistical Analysis
Taqman analyses yield values that are expressed as ratios between two absolute measurements (gene of interest/internal reference gene). The chi-square test was used to analyze the association between categorical clinicopathologic data and COX-2 expression status. Hazard ratios were used to calculate the relative risks of death. These calculations were based on the Pike estimate, with the use of the observed and expected number of events as calculated in the log-rank test statistic. 25 The maximal chi-square method of Miller and Siegmund 26 and Halpern 27 was adapted to determine which expression value best segregated patients into poor- and good-prognosis subgroups (in terms of likelihood of surviving), with the log-rank test and the stratified log-rank test used to measure the strength of the grouping. To determine a P value that would be interpreted as a measure of the strength of the association based on the maximal chi-square analysis, 1.000 boot-straplike simulations were used to estimate the distribution of the maximal chi-square statistics under the hypothesis of no association. 27 Multivariate analysis was performed with the Cox proportional hazards regression model. The level of significance was set to P < .05. All P values reported were based on two-sided tests.
RESULTS
COX-2 mRNA expression was detectable by quantitative real-time PCR (Taqman) in all 89 (100%) tumor specimens. The median COX-2 mRNA expression, expressed as a ratio to the internal reference gene β-actin, was 0.85 (range 0.02–15.77).
With a median follow-up of 85.9 months for the 89 patients analyzed in this study, the median survival was 59.7 months (range 3.8–105.3). The maximal chi-square statistic of Miller and Siegmund 26 and Halpern 27 was adapted to determine which COX-2 mRNA expression value best segregated patients into poor- and good-prognosis subgroups. This method found that segregation for COX-2 mRNA levels was best achieved with a relative expression level of 0.6. By this criterion, 47 (53%) patients had a low COX-2 expression status and 42 (47%) patients had a high COX-2 expression status. Table 1 shows associations between clinicopathologic data and COX-2 expression status. No statistically significant associations were detectable.
Median and 5-year survival rates depending on various clinical variables and COX-2 expression status are summarized in Table 2. The median survival for patients with a high COX-2 mRNA expression status was 31.1 months (95% confidence interval 8.72–53.48), whereas the median survival for those with a low COX-2 mRNA expression status was not reached (P = .0032, log-rank test). The respective survival curves are presented in Figure 1 and show 5-year survival rates of 31.2% ± 7.7 for patients with high COX-2 expression levels and 61.7% ± 7.1 for those with low COX-2 expression levels (P = .0025). Further, by stratifying patients by stage (stage 1 vs. stage 2 vs. stage 3a), COX-2 mRNA expression was shown to be an even stronger predictor of survival for curatively resected NSCLC (P = .0002, stratified log-rank test).
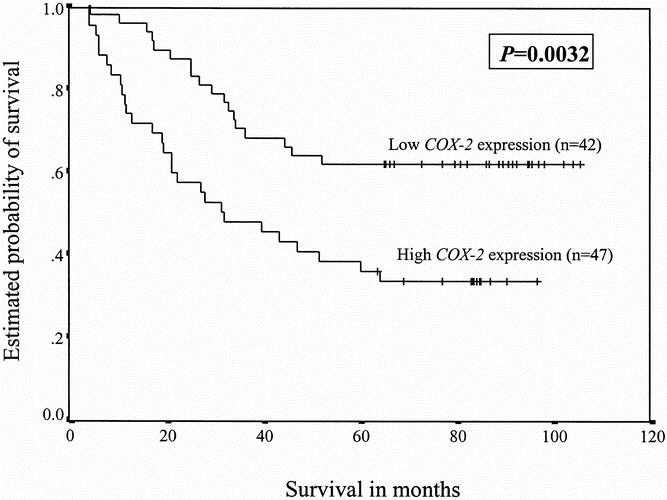
Figure 1. Survival of patients with non-small cell lung cancer as a function of the amount of COX-2 mRNA expression (the ratio between COX-2 and the internal reference gene β-actin) in specimens of primary non-small cell lung cancer. High COX-2 mRNA levels constituted ratios greater than 0.6; low levels were ratios equal to or less than 0.6.
The importance of COX-2 as a prognostic factor was next determined by the Cox proportional hazards model analysis. Two logistic regression models were tested. Model A included the parameters age, gender, histopathologic type, UICC TNM tumor stage, and COX-2 expression status. Model B included the pT and pN categories instead of histopathologic tumor stage. Significant independent prognostic factors were shown to be UICC TNM tumor stage (P < .001) and COX-2 (P < .001) expression status in model A, and the pN categories (P < .001) and COX-2 expression status (P = .013) in model B. Table 3 shows the statistically significant parameters in the two regression models.
DISCUSSION
To test whether the level of COX-2 expression is of prognostic importance in NSCLC, we used a quantitative real-time RT-PCR (Taqman) method to analyze COX-2 mRNA expression in tumor specimens of 89 patients with curatively resected NSCLC. Expression of COX-2 was detectable in all (100%) specimens analyzed; however, the intratumoral content of COX-2 mRNA expression varied over a 788-fold range. This observation of seemingly variable amounts of mRNA implies heterogeneity of expression patterns within individual tumor cells and may reflect the biologic behavior of these tumors.
Overexpression of COX-2 has been reported in human NSCLC, 16,18,19 and previous studies have shown that COX-2 overexpression may alter the biologic behavior of tumor cells in a number of ways. 28–30 Tsujii et al 29 have shown that constitutive expression of COX-2 could result in phenotypic changes that enhance the metastatic potential of colorectal cancer cells, leading to an increased invasiveness. In analogy to colorectal carcinomas, Hida et al 15 reported that a marked increase in COX-2 immunoreactivity in NSCLC, specifically adenocarcinomas, was associated with tumor-invasive lesions and lymph node metastasis, suggesting that increased COX-2 expression may be associated with an invasive and more aggressive phenotype in this disease. Indeed, Achiwa et al 17 were the first to show a prognostic significance of elevated COX-2 expression in human cancer. Although no association between elevated COX-2 protein expression and clinical outcome in stage 1 to 3 adenocarcinomas of the lung was detectable, high COX-2 expression correlated with inferior survival in a subgroup of adenocarcinomas, namely stage 1 disease. In contrast to our findings, Marrogi et al 19 recently reported no association between COX-2 protein expression and clinical outcome in patients with NSCLC. There are several possible explanations for these discordant findings. First, Marrogi et al investigated COX-2 protein expression, whereas we examined COX-2 expression at the mRNA level. Second, immunohistochemical methods are semiquantitative compared with the quantitative RT-PCR technique used in our study. Third, the study population analyzed by Marrogi et al 19 consisted of patients with stage 1 to 4 NSCLC, whereas only patients with curatively resected NSCLC stage 1 to 3a were included in our study.
The most striking finding in our study is the association between high COX-2 mRNA expression levels and worse survival in patients with curatively resected NSCLC. This finding may be clinically important, particularly because our study population represents a large number of patients with different histopathologic subtypes of lung cancer, in all of whom curative surgery was achieved. Our observation adds another step toward the development of a molecular classification of NSCLC and suggests that quantitation of COX-2 mRNA expression might help identify NSCLC patients at higher risk of early disease recurrence who will benefit from additional therapy to control their disease.
Previous studies have shown that treatment with NSAIDs decreases cell growth and induces apoptosis in NSCLC in vitro. 31,32 Of particular interest is that the responsiveness to selective COX-2 inhibitors was influenced by the degree of COX-2 expression. 31 The availability of selective COX-2 inhibitors gives our results additional importance because increased COX-2 expression might represent a direct therapeutic target in patients with NSCLC. Further studies investigating selective COX-2 inhibition in patients with NSCLC are warranted to gain more insight into the potential clinical usefulness of this therapeutic approach.
Footnotes
Supported by Hubert Burda Foundation for Cancer Research, Germany (J.B.), and NCI grant RO1 CA 71716 (P.V.D.).
Correspondence: Jan Brabender, MD, Department of Surgery, University of Cologne, Joseph-Stelzmann Str. 9, 50931 Cologne, Germany.
E-mail: drbrabender@cs.com
The first two authors contributed equally to the preparation of the manuscript.
Accepted for publication August 6, 2001.
References
- 1.Greenlee RT, Murray T, Bolden A, et al. Cancer statistics 2000. CA Cancer J Clin 2000; 50: 7–30. [DOI] [PubMed] [Google Scholar]
- 2.Ginsberg RJ, Vokes EE, Raben A. Non-small cell lung cancer. In: DeVita VT Jr, Hellmann S, Rosenberg SA, eds. Cancer: principles in practice of oncology, 5th ed. Philadelphia: Lipincott-Raven, 1997: 858–910.
- 3.Thun MJ, Mohan M, Namboodiri BS, et al. Aspirin use and risk of fatal colon cancer. N Engl J Med 1991; 325: 1953–1956. [DOI] [PubMed] [Google Scholar]
- 4.Thun MJ. Aspirin, NSAIDs, digestive tract cancers. Cancer Metastasis Rev 1994; 13: 269–277. [DOI] [PubMed] [Google Scholar]
- 5.Giardello FM, Offerhaus GJA, DuBois RN. The role of nonsteroidal anti-inflammatory drugs in cancer prevention. Eur J Cancer 1995; 31A: 1071–1076. [DOI] [PubMed] [Google Scholar]
- 6.Schreinemachers DM, Everson RB. Aspirin use and lung, colon, and breast cancer incidence in a prospective study. Epidemiology 1994; 5: 138–146. [DOI] [PubMed] [Google Scholar]
- 7.Vane JR, Botting RM. Mechanism of action of aspirin-like drugs. Semin Arthritis Rheum 1997; 27: 2–10. [DOI] [PubMed] [Google Scholar]
- 8.Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annu Rev Pharmcol Toxical 1998; 38: 97–120. [DOI] [PubMed] [Google Scholar]
- 9.Eberhart CE, Coffey RJ, Radhika A, et al. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology 1994; 107: 1184–1188. [DOI] [PubMed] [Google Scholar]
- 10.Oshima M, Dinchuk JE, Kargman SL, et al. Suppression of intestinal polyposis in Apc 54 716 knockout mice by inhibition of cyclooxygenase 2 (COX-2). Cell 1996; 87: 803–809. [DOI] [PubMed] [Google Scholar]
- 11.Sano H, Kuwahito Y, Wilder RL, et al. Expression of cyclooxygenase-1 and -2 in human colorectal cancer. Cancer Res 1997; 55: 3785–3789. [PubMed] [Google Scholar]
- 12.Ristimaki A, Honkanen N, Jankala H, et al. Expression of cyclooxygenase-2 in human gastric carcinoma. Cancer Res 1997; 57: 1276–1280. [PubMed] [Google Scholar]
- 13.Hwang D, Scollard D, Byrne J, et al. Expression of cyclooxygense-1 and cyclooxygenase-2 in human breast cancer. J Natl Cancer Inst 1998; 90: 455–460. [DOI] [PubMed] [Google Scholar]
- 14.Zimmermann KC, Sarbia M, Weber AA, et al. Cyclooxygenase expression in human esophageal carcinoma. Cancer Res 1999; 59: 198–204. [PubMed] [Google Scholar]
- 15.Hida T, Yatabe Y, Achiwa H, et al. Increased expression of cyclooxygenase 2 occurs frequently in human lung cancer, specifically adenocarcinomas. Cancer Res 1998; 58: 3761–3764. [PubMed] [Google Scholar]
- 16.Wolff H, Saukkonen K, Antilla S, et al. Expression of Cyclooxygenase-2 in human lung carcinoma. Cancer Res 1998; 58: 4997–5001. [PubMed] [Google Scholar]
- 17.Achiwa H, Yatabe Y, Hida T, et al. Prognostic significance of elevated cyclooxygenase 2 expression in primary, resected lung adenocarcinomas. Clin Cancer Res 1999; 5: 1001–1005. [PubMed] [Google Scholar]
- 18.Ochiai M, Oguri T, Isobe T, et al. Cyclooxygenase-2 (COX-2) mRNA expression levels in normal lung tissues and non-small cell lung cancers. Jpn J Cancer Res 1999; 90: 1338–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marrogi AJ, Travis WD, Welsh JA, et al. Nitric oxide synthase, cyclooxygenase 2, and vascular endothelial growth factor in the angiogenesis of non-small cell lung cancer. Clin Cancer Res 2000; 6: 4739–4744. [PubMed] [Google Scholar]
- 20.Heid CA, Stevens J, Livak KJ, et al. Real-time quantitative PCR. Genome Res 1996; 6: 986–994. [DOI] [PubMed] [Google Scholar]
- 21.Gibson UE, Heid CA, Williams PM. A novel method for real-time quantitative RT-PCR. Genome Res 1996; 6: 995–1001. [DOI] [PubMed] [Google Scholar]
- 22.Schneider PM, Praeuer HW, Stoeltzing O, et al. Multiple molecular marker testing (p53,C-Ki-ras, c-erbB-2) improves estimation of prognosis in potentially curative resected non-small cell lung cancer. Br J Cancer 2000; 83: 473–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest 1997; 111: 1710–1717. [DOI] [PubMed] [Google Scholar]
- 24.Lord RV, Salonga D, Danenberg KD, et al. Telomerase reverse transcriptase expression is increased early in the Barrett’s metaplasia, dysplasia, carcinoma sequence. J Gastrointest Surg 2000; 4: 135–142. [DOI] [PubMed] [Google Scholar]
- 25.Pike MC. Asymptomatically efficient rank invariant procedures. J R Stat Soc Series A 1972; 135: 201–203. [Google Scholar]
- 26.Miller R, Siegmund D. Maximally selected chi-square statistics. Biometrics 1982; 38: 1011–1016. [Google Scholar]
- 27.Halpern J. Maximally selected chi-square statistics for small samples. Biometrics 1982; 38: 1017–1023. [Google Scholar]
- 28.Tsujii M, DuBois RN. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell 1995; 83: 493–501. [DOI] [PubMed] [Google Scholar]
- 29.Tsujii M, Kawano S, DuBois RN. Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. Proc Natl Acad Sci USA 1997; 94: 3336–3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DuBois RN, Shao J, Tsujii M, et al. G1 delay in cells overexpressing prostaglandin endoperoxide synthase-2. Cancer Res 1996; 56: 733–737. [PubMed] [Google Scholar]
- 31.Hida T, Leyton J, Makheja A, et al. Non-small cell lung cancer cyclooxygenase activity, proliferation are inhibited by nonsteroidal antiinflammatory drugs. Anticancer Res 1998; 18: 775–782. [PubMed] [Google Scholar]
- 32.Hida T, Kozaki K, Muramatsu H, et al. Cyclooxygenase-2 inhibitor induces apoptosis and enhances cytotoxicity of various anticancer agents in non-small cell lung cancer cell lines. Clin Cancer Res 2000; 6: 2006–2011. [PubMed] [Google Scholar]


