Abstract
Objective
To analyze the potential variability in rates of circumferential resection margin (CRM) involvement between different surgeons and time periods and to determine the suitability of using CRM status as an immediate predictor of outcome after rectal cancer surgery.
Summary Background Data
After disease stage has been taken into account, survival in rectal cancer has been shown to be very variable between surgeons and institutions. One of the major factors influencing survival is local recurrence, and this in turn is strongly related to inadequate tumor excision, particularly at the CRM.
Methods
In a study involving 608 patients who underwent surgery for rectal cancer in Leeds during the 12-year period 1986 to 1997, the authors examined the role of CRM status as an immediate predictor of likely outcome, paying particular attention to its relationships with different surgeons and time periods.
Results
Of 586 patients on whom full clinical follow-up was obtained, 165 (28.2%) had CRM involvement by carcinoma on pathologic examination. Up to the end of 1998, 105 (17.9%) patients had developed local recurrence. A significantly higher proportion (38.2%) of CRM-positive patients developed local recurrence than CRM-negative ones (10.0%). Kaplan-Meier survival analysis showed significant improvements in survival for CRM-negative patients over CRM-positive patients. Survival analysis in relation to two gastrointestinal surgeons and a group of other surgeons showed survival improvements that paralleled a reduction in the rates of CRM involvement for the two gastrointestinal surgeons during the period of the study. No improvement in survival or reduction in rates of CRM involvement was seen in the group of other surgeons.
Conclusions
These results show that CRM status may be used as an immediate predictor of survival after rectal cancer surgery and serves as a useful indicator of the quality of surgery. The frequency of CRM involvement can be used both for overall surgical audit and for monitoring the value of training programs in improving rectal surgery by individual surgeons. Its use in the current MRC CR07 study is valid and the best indicator of a requirement for further local therapy.
Recent studies have revealed continuing high rates of local recurrence after surgery for rectal cancer. 1,2 If local recurrence develops, the prognosis is poor, with a 90% chance of subsequent death from the disease. It has become apparent in recent years that not only do the pathologic characteristics of rectal cancer influence the long-term outcome in terms of local recurrence and survival, but also that the surgeon is an important variable. This point has been highlighted by the work of Heald et al, 3 McArdle and Hole, 4 Enker et al, 5 the German Study Group for Colorectal Carcinoma, 6 and others. 7,8
Arbman et al 9 have shown that adopting the technique of total mesorectal excision (TME) can result in a 20% improvement in the 4-year survival rate, and the Norwegian Rectal Cancer Group 2 showed a greater than 20% reduction in local recurrence after the introduction of TME in a study involving more than 3,000 patients, although marked variations between different surgical units still existed at the end of the study. Recently the Stockholm group 10 have reported equivalent data, with a further study highlighting the importance of assessing the circumferential resection margin (CRM) before surgery. 11 Such variations in surgery clearly show the need to define a parameter that may be used as an immediate indicator of quality of surgery, and to assess the benefit of intervention using videos, tuition, or other methods. This assumes even greater importance if we consider that many surgeons performing rectal cancer surgery, particularly those carrying out only a few operations per year, do not have the time or resources to audit their local recurrence and survival figures. Many of those who do use different denominators in the expression of their results (e.g., curative cases, R0 resections, cancer-specific survival, overall survival), some of which are open to criticism because of their subjective nature. Involvement of the CRM by tumor has been shown to be closely related to local recurrence and survival rates 12–15 and is the indicator for postoperative chemoradiotherapy in the current international MRC CR07 trial. The aim of this study was to examine whether, in an era of changing surgical practice, CRM involvement is a useful clinical governance tool as an immediate indicator of changing quality of surgery, ultimately leading to improved survival rates.
METHODS
Between January 1, 1986, and December 31, 1997, 608 rectal adenocarcinoma resection specimens from Leeds hospitals were received in the Department of Histopathology at Leeds General Infirmary. Pathologic data were collected on all 608. Clinical follow-up data recorded up to the end of 1998 or death of the patient were obtained on 586 of the 608 (96.4%). For the remaining 22, follow-up data could not be recorded because the case notes were untraceable.
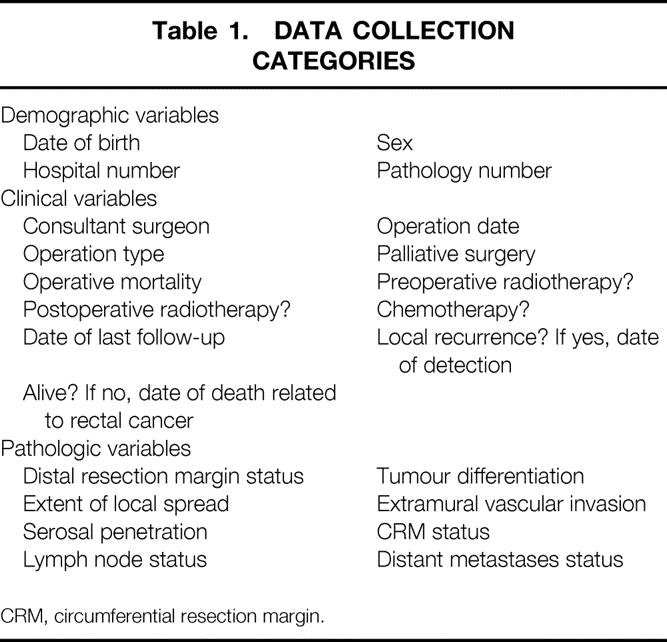
Pathologic dissection of each specimen was carried out in a standardized fashion according to a technique based on that originally described by Quirke et al 12 in 1986, and later in modified forms. 16–18 Briefly, this involved complete transverse slicing of the tumor and the segments above and below it at approximately 3- to 5-mm intervals, looking for continuous spread of tumor up to the CRM, or the presence of discontinuous tumor deposits or lymph nodes involved by tumor at the CRM. Departmental guidelines relating to the content of colorectal cancer pathology reports were referred to by all pathologists when reporting the cases, and from 1995 onward a reporting pro-forma was also filled in on each case to ensure clarity and completeness of data. Particular attention was given to measuring the minimum distance between the tumor and CRM, with a distance of 1 mm or less being taken as CRM involvement by tumor, as defined previously. 12,14 Because CRM involvement and local recurrence are strongly related, this definition of CRM involvement was validated by analyzing the effect of distance from the CRM on local recurrence rates. In addition, the prognostic significance of different modes of CRM involvement (e.g., direct, vascular) was also investigated. One researcher (K.F.B.) collected the majority of the pathologic data on the cases by reference to the original pathology reports, with cross-checking against the related pro-formas where available. Missing data were collected by reviewing the original histology slides or macroscopic photographs of the case. Pathologic data specifically relating to the mode of CRM involvement by tumor were collected by a second researcher (N.J.T.). Clinical data as recorded up to the end of 1998 or death of the patient were collected by a surgical research fellow (C.P.M.). The notes were reviewed and, in situations where there was inadequate hospital follow-up before 1998, the cancer registry or patient’s general practitioner was contacted for additional information. Table 1 summarizes the data recorded on each case.
Table 1. DATA COLLECTION CATEGORIES
CRM, circumferential resection margin.
Statistical Analysis
Analysis of the entire data set was performed in the first instance, with further analysis then being carried out after division of the data into three 4-year time periods (1986–89, 1990–93, and 1994–97). Within each period, cases were then allocated to one of three patient groups: the patients operated on by either of two high-volume consultant gastrointestinal surgeons (groups A and B), and a third group (C) consisting of an amalgamation of patients operated on by other surgeons. In all instances surgery was performed by or directly supervised by a consultant surgeon. Of the two gastrointestinal surgeons, one, during the study, became completely specialized in lower gastrointestinal tract surgery (surgeon A); the other continued to perform both upper and lower gastrointestinal tract surgery (surgeon B). It was necessary to amalgamate patients operated on by the remaining surgeons to make up the third grouping because of the low numbers of patients operated on by each of these other individuals.
A potentially curative procedure was defined as one in which the surgeon believed that all tumor (both primary and metastatic if present) had been removed at the time of surgery.
Computer analysis of the data, for both local recurrence and survival, was performed using SPSS for Windows (SPSS Inc., Chicago, IL). Local recurrence was defined as any recurrence within the pelvis and was recorded providing it was supported by one of the following: positive histology; diagnostic imaging evidence in conjunction with an increased carcinoembryonic antigen level; and macroscopic evidence of tumor recurrence at relaparotomy.
Patients who died of no-cancer-related illness were censored from further analysis from the time of death. The log-rank test was used to determine the significance of survival or cumulative risk of local recurrence differences between the different patient groups and for groups between different time periods.
RESULTS
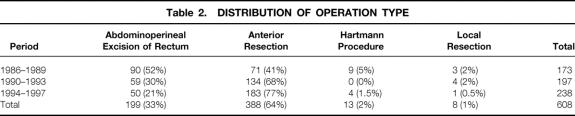
Before the follow-up analysis, the initial study group of 608 patients (342 men, 266 women) was reduced to 586 by the exclusion of the 22 patients (10 men, 12 women) for whom follow-up data could not be obtained. The median age of this group of 586 was 69.6 years (range 27.9–96.6). The distribution of operation type for each of the 4-year periods for the original group of 608 patients is shown in Table 2. Twenty-five (4.3%) of the 586 patients for whom follow-up details were obtained received preoperative radiotherapy and 70 (11.9%) received adjuvant chemotherapy, but there were no significant associations between these therapies and any particular surgical subgroup (chi-square tests, P = .631 and P = .954, respectively). Similarly, within the follow-up group, there were no associations between any particular surgical subgroup and particular Dukes stages, T stages, or N stages (chi-square tests, P = .921, P = .791, and P = .714 respectively), or between proportions of palliative cases and surgical subgroup (chi-square, P = .782). Overall, 488 (83.3%) of these operations were considered to be potentially curative resections and 98 (16.7%) palliative.
Table 2. DISTRIBUTION OF OPERATION TYPE
Tumor Involvement of the Circumferential Resection Margin
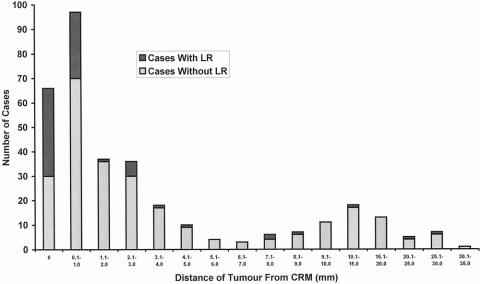
One hundred sixty-five of the 586 patients with follow-up (28.2% [95% confidence interval, 24.5–31.9%]) showed CRM involvement by tumor. Of these 165, 66 were involved by way of tumor extending right up to the CRM (i.e., with zero clearance) and 97 by tumor approaching to within 1 mm of the margin but not actually extending up to it. For the remaining two patients the exact distance could not be determined. There was a significant association between tumor being present at the margin, or being between 0 and 1 mm from it, and development of local recurrence (54.5% [36/66] and 27.8% [27/97] of patients, respectively; chi-square, P < .001). The local recurrence rate of those with tumor more than 1 mm from the margin was 10% (42/421). Figure 1 illustrates the significant differences in risk of local recurrence for different distances of tumor from the CRM. When grouped according to Dukes stage, CRM involvement reflected the stage of the disease (i.e., the more advanced the stage, the greater the chance of CRM involvement). The circumferential margin positivity rates were 1.1%, 21.2%, 38.6%, 50.0%, and 47.9% for disease stages A (i.e., CRM in the muscularis propria when positive), B, C1, C2, and D, respectively.
Figure 1. Variation in proportion of patients developing local recurrence with increasing distance of tumor from the circumferential resection margin.
Local Recurrence
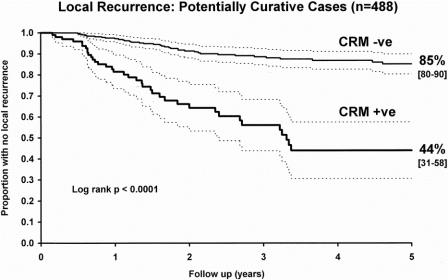
Local recurrence was diagnosed in 105 (17.9%) of the 586 patients who were followed up. Of 165 patients with CRM involvement by tumor, 63 (38.2%) developed local recurrence, whereas only 42 (10%) of the 421 patients deemed to have a clear CRM at histologic examination developed recurrence. The accuracy of CRM status in predicting the likelihood of local recurrence was 75%. The proportion of patients free of local recurrence at 5 years, as estimated by the Kaplan-Meier method, when comparing groups of patients with and without CRM involvement, was 84% (95% confidence interval, 79–89%) for patients without CRM involvement compared with 38% (95% confidence interval, 26–49%) for those with involvement (log-rank, P < .0001) when considering both potentially curative and palliative operations (n = 586). For potentially curative operations alone (n = 488), the figures were 85% (95% confidence interval, 80–90%) and 44% (95% confidence interval, 31–58%), respectively (log-rank, P < .0001, Fig. 2). The risk of developing local recurrence was significantly greater when there was histologic involvement of the CRM by tumor.
Figure 2. Comparison of proportions of patients undergoing potentially curative surgery with no local recurrence for groups with either uninvolved or involved circumferential resection margins (Kaplan-Meier plot with 95% confidence intervals).
Survival
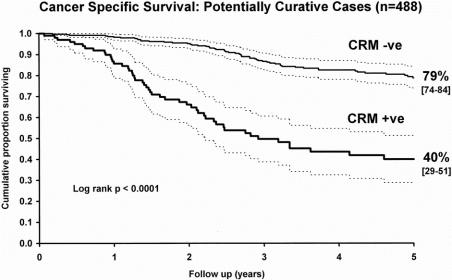
In a similar comparison of survival, Kaplan-Meier estimates of 5-year survival rates were 72% (95% confidence interval, 67–78%) for patients with an uninvolved margin and 29% (95% confidence interval, 21–38%) for patients with an involved margin (log-rank, P < .0001) when considering both potentially curative and palliative operations. For potentially curative operations only, the equivalent survival estimates were 79% (95% confidence interval, 74–84%) and 40% (95% confidence interval, 29–51%), respectively (log-rank, P < .0001, Fig. 3). Patients with CRM involvement showed significantly poorer survival than those without involvement.
Figure 3. Comparison of cancer-specific survival in patients undergoing potentially curative surgery for groups with either uninvolved or involved circumferential resection margins (Kaplan-Meier plot with 95% confidence intervals).
Univariate and Multivariate Analysis
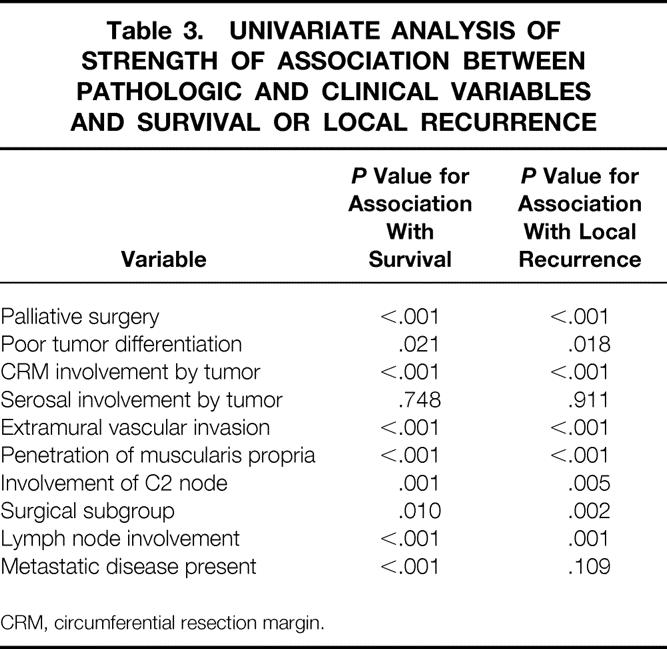
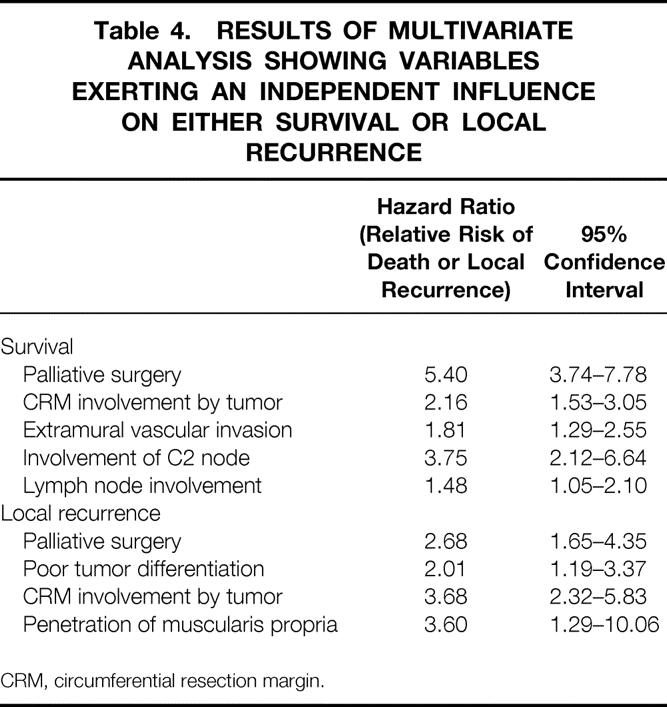
Univariate analysis was performed to test the strength of association between each of the pathologic variables and local recurrence or survival, the only exception being distal resection margin status, which was positive in only 1% of patients. Two further variables that were taken into consideration were surgical subgroup and whether the operation was thought to be palliative. Table 3 shows the results of this analysis. Multivariate analysis was then performed using the variables that showed a significant result in the univariate analysis. The variables that emerged as having an independent influence on either survival or local recurrence are shown in Table 4, together with the relative risks of death or local recurrence associated with them. Two variables, namely whether surgery was considered to be palliative or not and whether the CRM was involved by tumor or not, were noted to have an independent influence in terms of conferring both poor survival and an increased likelihood of local recurrence. In respect to survival, extramural vascular invasion and lymph node involvement, particularly C2 node involvement, were also found to independently confer a poorer prognosis. Poor tumor differentiation and penetration of the muscularis propria were the other variables independently conferring a greater risk of local recurrence. A positive CRM conveyed more than twice as great a risk of death and more than 3.5 times the risk of local recurrence compared with no CRM involvement.
Table 3. UNIVARIATE ANALYSIS OF STRENGTH OF ASSOCIATION BETWEEN PATHOLOGIC AND CLINICAL VARIABLES AND SURVIVAL OR LOCAL RECURRENCE
CRM, circumferential resection margin.
Table 4. RESULTS OF MULTIVARIATE ANALYSIS SHOWING VARIABLES EXERTING AN INDEPENDENT INFLUENCE ON EITHER SURVIVAL OR LOCAL RECURRENCE
CRM, circumferential resection margin.
Relationship Between Mode of Circumferential Resection Margin Involvement, Local Recurrence, and Survival
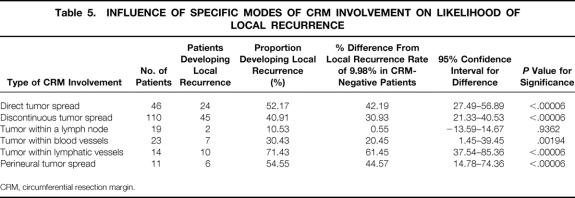
For 163 of the 165 patients showing CRM involvement by tumor, the histology slides were closely reviewed with a view to identifying the mode of involvement in each. Six distinct types of tumor involvement of the CRM were identified, with many of the patients displaying more than one mode of involvement (Table 5).
Table 5. INFLUENCE OF SPECIFIC MODES OF CRM INVOLVEMENT ON LIKELIHOOD OF LOCAL RECURRENCE
CRM, circumferential resection margin.
We found that cancer-specific survival was not significantly influenced by the mode of CRM involvement. However, there was a significant association with an increased rate of local recurrence when CRM involvement was found to be present by way of either direct tumor spread or by the presence of tumor with lymphatic vessels at the margin. Conversely, CRM involvement as a result of tumor within a lymph node less than 1 mm from the margin was associated with a significantly lower-than-expected rate of local recurrence, although the number of patients falling into this category was small (n = 19).
Variation Between Surgeons and Over Time
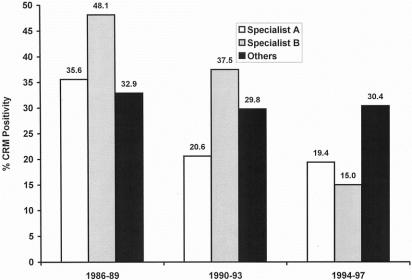
The two gastrointestinal surgeons, A and B, were responsible for 416 of the 608 resections (68.4%). Figure 4 shows the rates of CRM involvement by tumor in each surgical subgroup for each of the three time periods. Both of the gastrointestinal surgeons progressively reduced their rates of CRM involvement over the period of the study, both having involvement rates of less than 20% in the final period. In contrast, there was no such decrease in the group of other surgeons, with rates of involvement being consistently around 30% over the three time periods. In the most recent period of the study (1994–97), the rates of CRM involvement by surgeons A and B decreased to levels lower than that of the other surgeons, with surgeon A’s CRM involvement rate being 11% less than that seen in the other group, whereas that of surgeon B was 15% less.
Figure 4. Circumferential resection margin involvement rates according to surgeon over time.
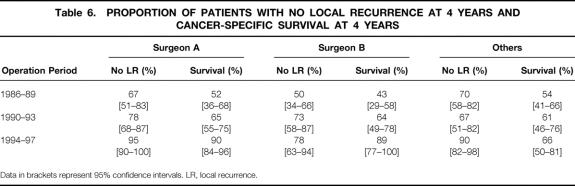
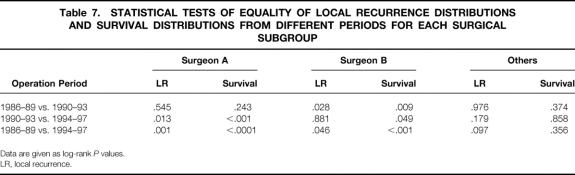
Tables 6, 7, and 8 show the differences in proportion of patients remaining free of local recurrence for each surgical subgroup for each time periods. For surgeons A and B there was a significant increase in the proportion of patients remaining free of local recurrence over the period of the study (log-rank, P = .001 for surgeon A, P = .046 for surgeon B). An increase was also seen for the “other” group, but this did not reach statistical significance (log-rank, P = .097). Despite showing a clear relationship between CRM involvement and local recurrence and a significant difference in CRM involvement rates between surgeons A and B and the other surgeons during the 1994–1997 period, we did not find any significant increase in the proportion of surgeon A and B’s patients remaining free of local recurrence compared with those operated on by the other surgeons.
Table 6. PROPORTION OF PATIENTS WITH NO LOCAL RECURRENCE AT 4 YEARS AND CANCER-SPECIFIC SURVIVAL AT 4 YEARS
Data in brackets represent 95% confidence intervals. LR, local recurrence.
Table 7. STATISTICAL TESTS OF EQUALITY OF LOCAL RECURRENCE DISTRIBUTIONS AND SURVIVAL DISTRIBUTIONS FROM DIFFERENT PERIODS FOR EACH SURGICAL SUBGROUP
Data are given as log-rank P values.
LR, local recurrence.
Table 8. STATISTICAL TESTS OF EQUALITY OF LOCAL RECURRENCE DISTRIBUTIONS AND SURVIVAL DISTRIBUTIONS FOR DIFFERENT SURGICAL SUBGROUPS FOR THE PERIOD 1994–97
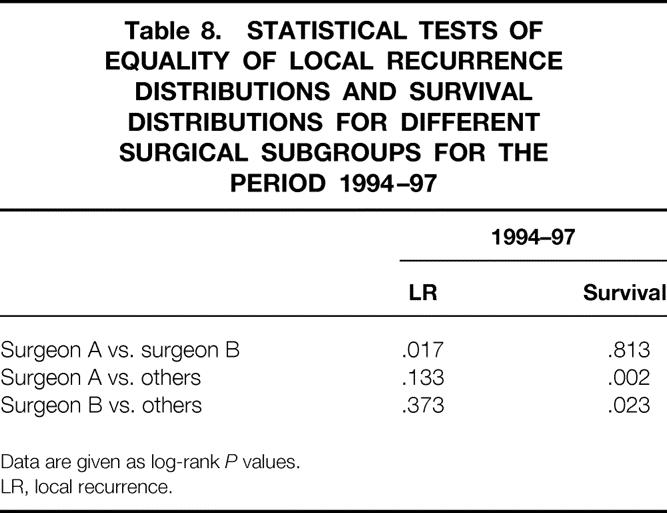
Data are given as log-rank P values.
LR, local recurrence.
Survival
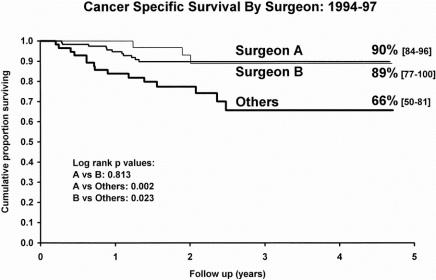
Tables 6, 7, and 8 also show a comparison of cancer-specific survival for each study period in relation to the surgical subgroups. The decrease in surgeon A’s rates of CRM involvement coincided with significantly improved survival, particularly between the second and final periods. Similarly, significant improvements in surgeon B’s survival figures were seen between each of the three periods. The other surgeons showed no such improvements in survival between the periods. Figure 5 shows a Kaplan-Meier survival plot focusing on the final period (where the greatest differences between the CRM involvement rates of surgeons A and B and of the “other” group were seen). This shows significantly better survival for patients of surgeons A and B compared with those in the “other” group (log-rank;P = .002, A vs. others;P = .023, B vs. others).
Figure 5. Comparison of cancer-specific survival for patients in the different surgical subgroups during the final period of the study (Kaplan-Meier plot with 95% confidence intervals).
DISCUSSION
In this study we have shown that involvement of the CRM by tumor in rectal cancer is the only pathologic variable that independently influences both survival and local recurrence. Ultimately, it confers a poorer prognosis, doubling the risk of death and increasing by 3.5 times the risk of local recurrence compared with patients without such involvement. Fortunately, this is one parameter that the operating surgeon has the power to control. We have shown that in our institution surgeons are attaining different levels of CRM involvement: those who carry out rectal cancer surgery most often are the surgeons with the lowest levels of CRM involvement. We believe that CRM status can be used as an immediate predictor of likely survival in rectal cancer. What appears to be more effective surgery at the pathologic level is also reflected at the clinical level in improved survival figures for the same surgeons. These results send a clear message that when factors such as disease stage and use of adjuvant therapy are equal, the quality of surgery—in particular the skill of resection of the mesorectum at the CRM—becomes one of the most important aspects of management. In many institutions, standard rectal cancer surgery has consisted of removal of the rectum by techniques involving a combination of blunt digital dissection and traction. This method has great potential for leaving substantial amounts of mesorectal tissue behind in the pelvis, and in such situations there is a significant risk of leaving lymph nodes or tumor deposits behind or creating a CRM involved by tumor. Heald et al 3 have shown that meticulous excision of the mesorectum along the appropriate fascial plane, either in total or in part, can result in markedly more favorable outcomes. Many other authors have also shown that training and specialization by surgeons in such techniques can result in remarkable reductions in the rates of local recurrence and improvements in survival;2,5,9 however, they have not used CRM status as an immediate predictor of likely outcome. The CRM should receive close attention from the surgeon at the time of surgery and subsequently from the pathologist by careful specimen dissection and histologic assessment. 19 The best way of achieving this would be a high level of specialization in rectal cancer work in both of these groups.
Although we could not show significant differences between local recurrence rates for two gastrointestinal surgeons and those obtained by a group of other surgeons in the most recent study period, one possible explanation may be that local recurrence is a relatively “soft” endpoint. Both CRM involvement by tumor and survival are “hard” endpoints for which the ultimate status is certain (involved or uninvolved, alive or dead). The recorded local recurrence status, on the other hand, may not be an accurate reflection of the true status because of failures in detection. For example, there may be differing levels of investigation, or simply the patient may have died before local recurrence has presented itself and there is no autopsy. Indeed, a failure on the part of the surgeon to actively look for local recurrence in the absence of symptoms may also lead to a falsely low rate. A relatively higher rate of detection of local recurrence by the gastrointestinal surgeons compared with the other surgeons could explain the apparent conflict between significant differences in CRM positivity rates but nonsignificant differences in local recurrence rates.
We believe that we have confirmed the validity of using the “tumor within 1 mm or less” rule for the definition of CRM involvement, this being both a logical and practically useful approach. Even if carcinoma does not directly encroach on the CRM, there is a strong relationship between increased rates of local recurrence and the finding of carcinoma at a distance of 1 mm or less from it. Equally, it becomes obvious that when tumor is more than 1 mm from the margin, the relationship with increased local recurrence no longer exists. This is important because this definition of CRM involvement is currently being used as the criterion for postoperative chemoradiotherapy in the MRC CR07 trial.
This study suggests that CRM involvement is an immediate indicator of likely outcome after rectal cancer surgery, that it varies between surgeons, and that it may be a good indicator of the overall quality of surgical practice. We have also confirmed that the current MRC CR07 study is using a robust measurement to define patients who would benefit from aggressive postoperative therapy. Failure of pathologists to report this feature therefore amounts to a disservice to both the clinical team and the patient.
Acknowledgments
Kevin Birbeck wrote the paper with the assistance, comments, and suggestions of all the individuals named as coauthors, and in particular with help from Wendy Parsons and Professors Mike Dixon and Phil Quirke. Pathologic data were collected by Kevin Birbeck and Nick Tiffin with contributions from Mike Dixon, Nic Mapstone, Cedric Abbott, Nigel Scott, and Phil Quirke. Chris Macklin collected the clinical and outcome data, which was extensively contributed to by Paul Finan and Professor David Johnston. Statistical analysis of the data was carried out by Kevin Birbeck and Wendy Parsons.
Footnotes
Supported by grants from the Special Trustees of Leeds General Infirmary (K.F.B.) and Yorkshire Cancer Research (P.Q.).
Correspondence: Philip Quirke, PhD, FRCPath, Professor of Pathology and Honorary Consultant in Histopathology, Academic Unit Of Pathology, School of Medicine, University of Leeds, Leeds, LS2 9JT, UK.
E-mail: philq@pathology.leeds.ac.uk
Accepted for publication November 29, 2001.
References
- 1.Medical Research Council Rectal Cancer Working Party. Randomised trial of surgery alone versus surgery followed by radiotherapy for mobile cancer of the rectum. Lancet 1996; 348: 1610–1614. [PubMed] [Google Scholar]
- 2.The Norwegian Rectal Cancer Group. Total mesorectal excision (TME) in Norway: A national rectal cancer project. Dis Colon Rectum 1999; 42: A26. [Google Scholar]
- 3.Heald RJ, Moran BJ, Ryall RD, et al. Rectal cancer: the Basingstoke experience of total mesorectal excision, 1978–1997. Arch Surg 1998; 133: 894–899. [DOI] [PubMed] [Google Scholar]
- 4.McArdle CS, Hole D. Impact of variability among surgeons on post-operative morbidity and mortality and ultimate survival. Br Med J 1991; 302: 1501–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Enker WE, Thaler HT, Cranor ML, et al. Total mesorectal excision in the operative treatment of carcinoma of the rectum. J Am Coll Surg 1995; 181: 335–346. [PubMed] [Google Scholar]
- 6.Hohenberger W. The effect of specialisation on organisation of rectal cancer surgery. In: Soreide O, Norstein J, eds. Rectal Cancer Surgery: Optimisation, Standardisation, Documentation. Berlin: Springer-Verlag; 1997: 353–363.
- 7.Porter GA, Soskolne CL, Yakimets WW, et al. Surgeon-related factors and outcome in rectal cancer. Ann Surg 1998; 227: 157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harmon JW, Tang DG, Gordon TA, et al. Hospital volume can serve as a surrogate for surgeon volume for achieving excellent outcomes in colorectal resection. Ann Surg 1999; 230: 404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arbman G, Nilsson E, Hallbröök O, et al. Local recurrence following total mesorectal excision for rectal cancer. Br J Surg 1996; 83: 375–379. [DOI] [PubMed] [Google Scholar]
- 10.Martling AL, Holm T, Rutqvist L-E, et al. Effect of a surgical training programme on outcome of rectal cancer in the County of Stockholm. Lancet 2000; 356: 93–96. [DOI] [PubMed] [Google Scholar]
- 11.Beets-Tan RGH, Beets GL, Vliegen RFA, et al. Accuracy of magnetic resonance imaging in prediction of tumour-free resection margin in rectal cancer surgery. Lancet 2001; 357: 497–504. [DOI] [PubMed] [Google Scholar]
- 12.Quirke P, Durdey P, Dixon MF, et al. Local recurrence of rectal adenocarcinoma due to inadequate surgical resection: histopathological study of lateral tumour spread and surgical excision. Lancet 1986; ii: 996–999. [DOI] [PubMed] [Google Scholar]
- 13.Ng IO, Luk IS, Yuen ST, et al. Surgical lateral clearance in resected rectal carcinomas. A multivariate analysis of clinicopathologic features. Cancer 1993; 71: 1972–1976. [DOI] [PubMed] [Google Scholar]
- 14.Adam IJ, Mohamdee MO, Martin IG, et al. Role of circumferential resection margin involvement in the local recurrence of rectal cancer. Lancet 1994; 344: 707–711. [DOI] [PubMed] [Google Scholar]
- 15.de Haas-Kock DF, Baeten CG, Jager JJ, et al. Prognostic significance of radial margins of clearance in rectal cancer. Br J Surg 1996; 83: 781–785. [DOI] [PubMed] [Google Scholar]
- 16.Quirke P, Dixon MF. The prediction of local recurrence in rectal adenocarcinoma by histopathological examination. Int J Colorectal Dis 1988; 3: 127–131. [DOI] [PubMed] [Google Scholar]
- 17.Quirke P, Scott N. The pathologist’s role in the assessment of local recurrence in rectal carcinoma. Surg Oncol Clin North Am 1992; 1: 1–17. [Google Scholar]
- 18.Quirke P. Limitations of existing systems of staging for rectal cancer: the forgotten margin. In: Soreide O, Norstein J, eds. Rectal Cancer Surgery: Optimisation, Standardisation, Documentation. Berlin: Springer-Verlag; 1997: 63–81.
- 19.The Royal College of Pathologists. Minimum Dataset for Colorectal Cancer Histopathology Reports. London: The Royal College of Pathologists; 1998.