Abstract
Objective
To determine if the extent of lymphadenectomy (number of recovered lymph nodes) was associated with long-term outcome in patients operated on for stage B and C colon cancer.
Summary Background Data
Lymphatic spreading is the main prognostic indicator in colon cancer patients, although the optimal extent of lymphadenectomy and its prognostic impact are still unknown.
Methods
In 3,648 patients (median follow-up 3.6 years) enrolled in two consecutive INTACC multicentric trials on adjuvant therapy for colon cancer, we studied the association of the number of recovered nodes with overall survival and relapse free survival by means of univariate and Cox regression analysis.
Results
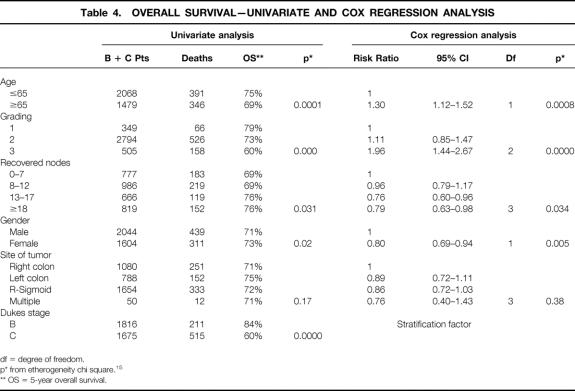
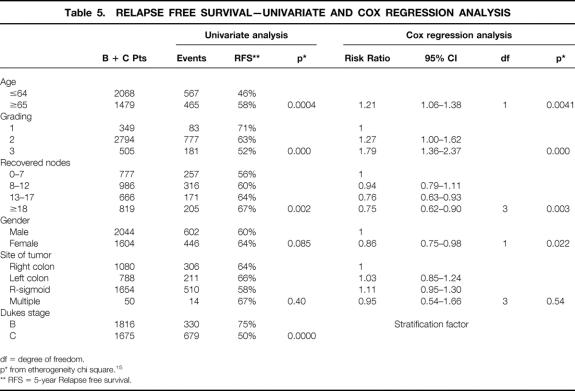
The worst overall survival was related to ages > 65 (risk ratio [RR] = 1.30), higher grading (RR = 1.96). Better overall survival was related to female gender (RR = 0.80) and to higher number of recovered nodes (8–12 nodes, RR = 0.46, 13–17 nodes, RR = 0.76, nodes >/= 18, RR = 0.79). The same pattern was observed for relapse free survival.
Longer overall and relapse free survival were related to a higher number of recovered nodes with P = .034 and P = .003 respectively (stratified analysis for absence or presence of positive nodes).
Stage B patients with fewer than 7 nodes in the specimen had both shorter overall survival (P = .0000) and relapse free survival (P = .0016) than the other B patients. Outcome of stage C patients was not related to the number of recovered nodes (P = .28 and 0.12 respectively). The interaction test between stage of disease and number of recovered nodes was statistically significant (P = .017).
Conclusions
Stage B patients with a small number of examined nodes may be understaged. Thus, these patients might be considered for adjuvant therapy because of their poorer life expectancy than other stage B patients. For stage C patients, the number of recovered nodes does not seem to affect long-term outcome.
The classification of colon cancer proposed by Dukes, 1 and subsequently modified by Astler and Coller 2 is considered prognostically reliable and has acquired wide clinical use since its formulation. Nevertheless, during the last two decades, interest has been directed to additional variables to predict the survival of patients and to improve case selection for adjuvant chemotherapy. 3 Despite these efforts, however, the presence of nodal metastasis is still the most important prognostic indicator of survival, and the quality of both surgical and pathologic procedures has been recently enhanced to provide the best information about the lymphatic spreading of colorectal cancer. 4–8
The exact number of lymph nodes to be dissected by the surgeon and the modality of the pathologic exam are still a matter of debate. 6–8 Since 1990, when it was first proposed to examine at least 12 lymph nodes to properly stage colorectal cancer as N0, 9 the suggested number of nodes to be examined has varied from 6 to 17, 4–8 until Goldstein et al. 6 suggested to pick up as many nodes as possible during curative resections for colon cancer. In the recent Guidelines for therapy of colon cancer 10 it is stressed that, “For adjuvant trial, a minimum of one lymph node must be examined for entry into a trial. For surgical trials or for entry into a colon adjuvant trial in which the lymph node are negative for disease, a minimum of 12 nodes must be examined. The TNM staging system should be used for all colorectal cancer trials. Level of evidence: III-IV. Grade of recommendation: C.”
The quality and the extent of lymphadenectomy, besides the diagnostic importance, have rarely been related to long-term outcome and a clear demonstration of their impact on survival has not yet been given. There are limitations to these studies. First, they are retrospective and based on small series of patients; and second, they seldom analyze the extent of lymphadenectomy in relationship to the long-term outcome. So far, only one large-scale trial (published in abstract form 11) is available and has concluded that aggressive lymphadenectomy may improve overall survival.
In this study we present the surgical data of two INTACC (National Intergroup for Adjuvant Therapy on Colon Cancer) trials on the adjuvant treatment of resected colon cancer. The aim of this study was to determine, in patients operated on for stage B and C colon cancer, if the extent of lymphadenectomy (number of recovered lymph nodes) is associated with long-term outcome.
MATERIALS AND METHODS
Patients
Patients considered here are from two large scale clinical trials conducted in Italy by INTACC (National Intergroup for Adjuvant Therapy on Colon Cancer). INTACC was created from the merge of 5 Italian cooperative groups: Genova, Gruppo Oncologico Italiano Ricerca Clinica (GOIRC), Gruppo Oncologico Nord Ovest (GONO), Gruppo Oncologico Piemontese Tumori Apparato Digerente (GOPTAD), and Instituo Oncologico Romagnolo (IOR), with the aim of assessing new adjuvant treatment modalities in colon cancer.
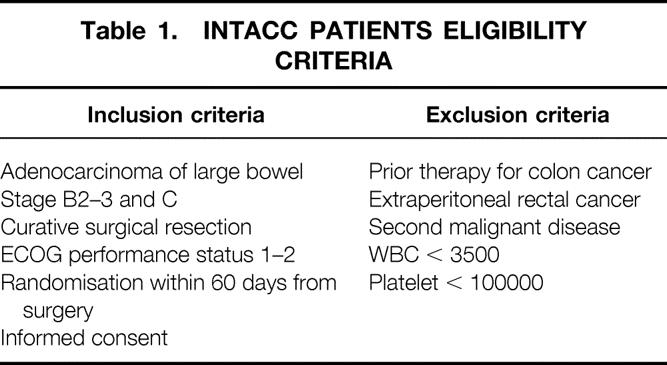
We analyzed data of surgical interest among data collected within the large scale randomized trials of adjuvant chemotherapy INTACC 01 and INTACC 02, which began in 1992 and in 1995 began enrolling patients radically resected for colorectal cancer. In these studies, patients were randomized to receive either 5-FU + Levamisole + 6-S-Leucovorin or 5-FU + Levamisole (INTACC 01), or 5-FU + Levamisole + Methotrexate (INTACC 02). Overall survival and disease-free survival were the primary outcomes in both trials. Eligibility criteria were the same in the two trials and are listed in table 1.
Table 1. INTACC PATIENTS ELIGIBILITY CRITERIA
An interim analysis of both trials was published at the 1999 Annual Congress of the American Society of Clinical Oncology. 12,13 No statistical difference among the treatments in term of disease-free survival or overall survival was demonstrated. Therefore, in this study we considered the two arms of the studies as a single cohort of patients, regardless of the adjuvant scheme.
There were 3,648 patients enrolled by INTACC during a 6-year period but, because of missing data on the characteristics of the patients, not all of them were included in the Cox regression analyses.
Patients’ characteristics are listed in table 2. The proportion of patients with negative nodes in INTACC studies is higher than in other studies 5,8 because of the eligibility criteria of both protocols, and because an international competitive study started for only stage C patients during the second study.
Table 2. PATIENTS’ CHARACTERISTICS
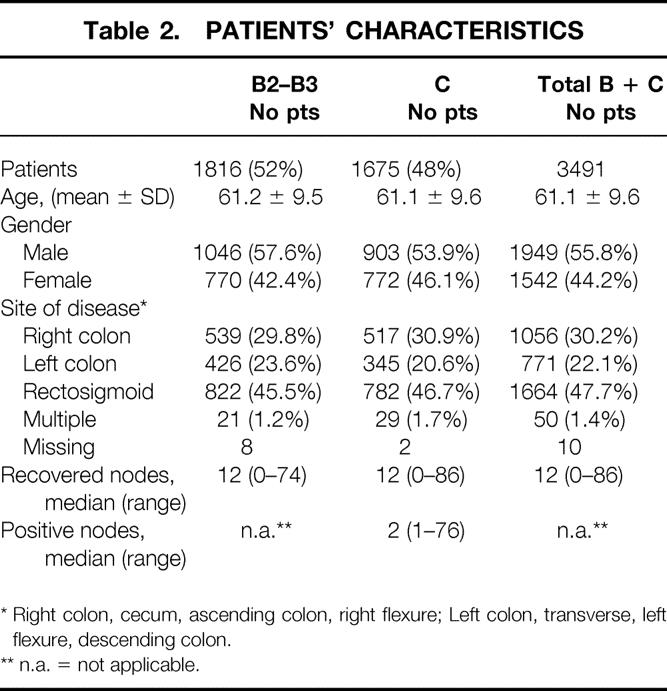
* Right colon, cecum, ascending colon, right flexure; Left colon, transverse, left flexure, descending colon.
** n.a. = not applicable.
Active follow-up is maintained for the studies; both INTACC 01 and INTACC 02 are mature trials and are going to be published. Median follow-up is 3.6 years (95% CI: 3.5–3.7 years).
Statistical Analysis
The association of recovered and positive lymph nodes with prognostic factors was analyzed using the χ-square test. Continuous variables were analyzed with the Student t-test for unpaired samples.
Relapse free survival was computed as the time from randomization in the main study to the first observation of disease recurrence or occurrence of a second primary cancer, or death due to any cause. Overall survival was computed as the time from randomization to death due to any cause. Kaplan-Meier estimate and log-rank test were used for comparisons.
All survival and relapse free survival analyses were stratified for presence or absence of positive nodes. Cox regression analysis was used to model overall survival and relapse free survival as a function of a set of independent variables. All the variables were categorical: age (< 65 and ≥65); histologic grading (1 = well, 2 = moderately well, 3 = poorly differentiated, 9 = intermediate category for missing); recovered lymph nodes (0–7, 8–12, 13–17, and ≥18 according to quartiles); gender and site of disease (1 = right colon: cecum, ascending colon, and hepatic flexure; 2 = left colon: transverse colon, splenic flexure, and descending colon; 3 = intraperitoneal rectum and sigmoid; 4 = multiple locations). Both analyses for overall survival and relapse free survival were stratified for stage of disease (positive and negative nodes at the pathologic examination).
For Cox regression analyses a backward procedure, based on the likelihood ratio test, was used with P in = .05 and P out = .10. All P values are two-sided. Presence of interaction between stage of disease and categories of recovered nodes was evaluated only in overall survival analysis, using the likelihood ratio test. Because of missing values all the sums of patients considered in different analyses may be different.
SPSS statistical package software (Microsoft Corp., Redmond, WA) was used for all the procedures.
RESULTS
Among the 3,648 patients enrolled in the two INTACC trials, 3,248 had the necessary data to assess the lymphatic state (both exact number of recovered nodes and positive ones). Dukes’ B patients were 1,635 (50.3%) and 1,613 (49.7%) were stage C. Dukes’ B and C patients showed no statistically significant differences as regards age, sex, tumor location, and number of recovered lymph nodes. The median follow-up of all patients is 3.6 years.
First, we looked for any association between the number of recovered lymph nodes and the other variables (data not shown in tables). Tumor location was significantly related to the number of recovered lymph nodes (P = .000; degree of freedom = 9): a higher number of nodes was recovered from right colon specimens than from left colon and rectosigmoid. This difference was statistically significant even considering Dukes’ B and C patients separately (P = .000). Older age (≥ 65 years) was associated to a lower number of recovered nodes (P = .041). The same analysis was then performed considering Dukes’ B and C patients separately. Grading (P = .19) and gender (P = .27) showed no association with the number of recovered nodes.
The association among number of positive nodes and clinical variables is reported in table 3: the association is statistically significant for grading (P = .0000) and for gender (P = .028).
Table 3. ASSOCIATION OF POSITIVE NODES WITH CLINICAL COVARIATES
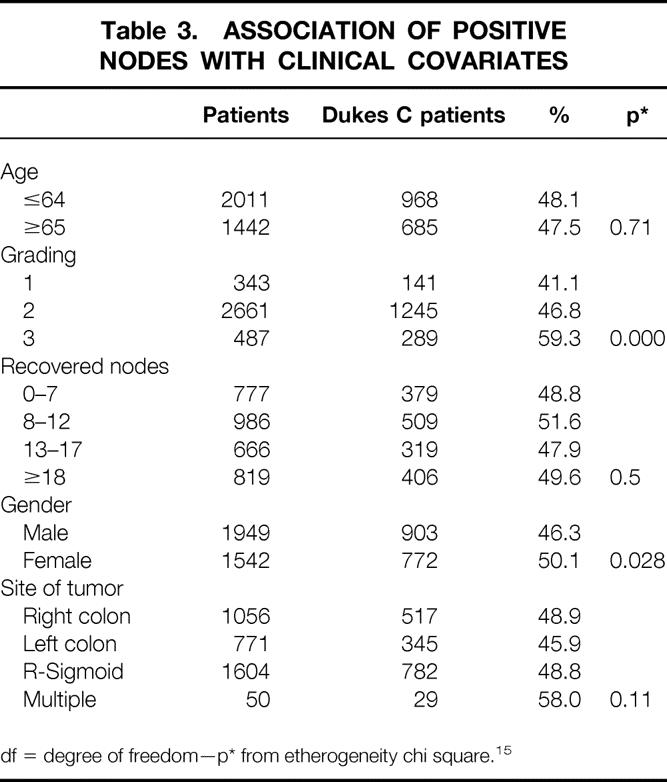
df = degree of freedom—p* from etherogeneity chi square. 15
Second,the association between independent variables and outcome is reported in tables 4 and 5: we found that age < 65, lower grading, higher number of recovered nodesm, and female gender were significantly related to both better overall survival and better relapse free survival.
Table 4. OVERALL SURVIVAL—UNIVARIATE AND COX REGRESSION ANALYSIS
Table 5. RELAPSE FREE SURVIVAL—UNIVARIATE AND COX REGRESSION ANALYSIS
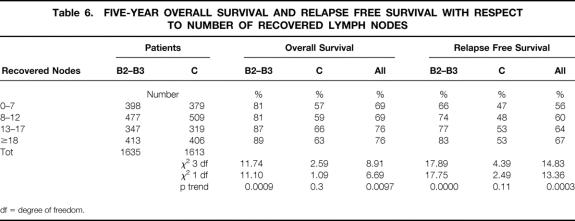
Third, we focused on the different survival with respect to the number of recovered nodes (P = .03 for overall survival [table 4], and P = .002 for relapse free survival [table 5]). See tables 4 and 5 for risk ratios at various levels of recovered nodes. We divided B and C patients into quartiles according to the number of recovered nodes; the 5-years overall survival and relapse free survival for each quartile are shown in table 6. Dukes’ B patients in the subgroups analysis explain all the difference, with most of the χ-square explained by the trend (P = .0009 and P = .0000 respectively). This is due to the worse outcome of Dukes’ B patients with a lower number of recovered nodes. In Dukes’ C patients the association between the number of recovered nodes and survival and relapse free survival did not achieve statistical significance.
Table 6. FIVE-YEAR OVERALL SURVIVAL AND RELAPSE FREE SURVIVAL WITH RESPECT TO NUMBER OF RECOVERED LYMPH NODES
df = degree of freedom.
The test for interaction between stage of disease and number of recovered nodes is statistically significant (P = .017).
DISCUSSION
We studied a very large cohort of homogeneously treated patients who were selected by means of strict protocol entry criteria. Stages of the disease were comparable and treatment and follow-up were carried out according to a uniform standard. All our patients underwent chemotherapy in stable clinical conditions; therefore the long-term statistics had no initial bias due to operative morbidity. Furthermore, the mainstays of clinico-pathologic evaluation (gender, age, stage, grading, lymphatic spreading, tumor location) were collected and considered in the statistical analysis. As far as median follow-up and number of events are concerned, INTACC studies are mature trial and are going to be published. The largest series of patients we were able to find in the surgical literature 3–8,14 comprised between 103 and 750 patients. Only one large-scale trial (from the medical oncology literature addressing these surgical issues) is available but published in abstract form only. 11
The first task in our study was to look for an association among the number of collected nodes, the number of positive nodes, and the clinico-pathologic variables.
The number of positive nodes seems not to be related to the number of recovered nodes (P = .5). As we will consider below, this data are not in agreement with other studies. 7,11
The variables related to a higher number of recovered nodes are tumor location (right colon tumors were associated to a higher number of nodes in the specimen) and patients’ age (patients younger than 65 had more nodes collected). Our results are in keeping with those of other studies. In fact, the former difference was also noted by Hernanz et al., 3 and can be explained on the basis of a largerpiece of mesenteric lymphatic stations exciseable during right colectomy rather than during left colectomy. The latter finding (age > 65) may be hypothetically explained as a rough index of the surgeon’s approach in high-risk patients not tolerating invasive resections. We cannot explain this issue otherwise because other important comorbidities were not considered within the entry form.
The principle aim of this study was to determine whether different outcomes might be associated with the extent of lymphadenectomy.
We considered the survival and relapse free survival curves for patients divided in quartiles according to the number of recovered nodes. The smaller the number of recovered lymph nodes, the smaller the overall survival and relapse-free survival in stage B patients. Such information is contradictory with the one discussed above (positive nodes not related to number of recovered nodes), but seems to have statistical evidence as far as survival is concerned, because it is sustained in the univariate analysis and confirmed by Cox regression analysis and by the interaction analysis. This last information is clinically more important because it directly relates a surgical/pathologic staging to precise outcomes data, giving the clear evidence of the impact of surgery on survival.
These data are similar to those ones published by Caplin 7 and Le Voyer. 11 Caplin studied 211 stage B patients, showing that B patients with fewer than 6 nodes had poorer overall survival and their survival curve had the tendency to behave like the one seen in stage C patients. Le Voyers showed data regarding 648 stage B patients, with statistically significant worsening of overall survival and cancer specific survival of B patients with fewer collected nodes. More than 1,500 stage B patients compose our series and this larger sample size may allow us to draw more precise indications from the follow-up data. Our stage B patients with fewer than 7 collected nodes had worse outcome than the other stage B patients, but always better than all stage C patients. This result seems to demonstrate the risk of understaging some of these patients when fewer than 7 nodes were collected. The number of inadequately (< 7 collected nodes) staged patients in our series was high: 24.3% of N0 patients. Our data, however, do not allow us to investigate if this result is due to a limited lymphadenectomy (which might be caused by intraoperative reasons, such as occlusion, hypo-tension, adhesions, or others) or if it is due to an ineffective research of nodes during the pathologic examination. In fact, once stabilized and within 60 days after surgery, all patients entered the INTACC trials and received chemotherapy without distinction based on the number of collected nodes, because it was not an entry criteria. Outside any randomized adjuvant trial, these understaged patients could have not been evaluated for chemotherapy.
Our results, in keeping with those of other studies, 4–8 emphasize the need to report the number of collected nodes in the pathologic report as quality criterion for the diagnostic value of colonic oncological surgery. This minimum number must be surely greater than 7, and it could be even greater for right hemicolectomies. Thus, we agree with Goldstein 6 about the indication of performing aggressive colectomy to give the pathologist a more complete specimen to collect as many nodes as possible. 15
According to our data and given that the classification as stage C is pivoting in the adjuvant treatment, the N0 patients with fewer than 7 recovered nodes should be evaluated in a different way for adjuvant chemotherapy, especially if other risk factor are present. Also, these patients have recently been considered not eligible for surgical trials or for entry into colon adjuvant trials for N0 stage. 10 Thus, the surgeon must clearly describe in his report the characteristics of the performed lymphadenectomy. If any intraoperative situation has led to a scarce lymphadenectomy, it should be mentioned to justify a failure in staging the disease.
Acknowledgments
The authors thank Carla Curti, Elisabetta Mauri, Monica Mestriner, MD, Elda Montanaro, and Daniela Rossi, MD, for active data management and technical support.
Footnotes
Supported by C.N.R. ACRO 93.02333.PF.39 and A.I.R.C. 134 AGV 65.
Correspondence: Mario Prandi, MD, Chirurgia Generale - Ospedale Infermi, Via L. Settembrini 2, 47900 Rimini, Italy
E-mail: m.prandi@libero.it
Accepted for publication December 21, 2001.
References
- 1.Dukes CE. The classification of cancer of the rectum. J Pathol Bact 1932; 35: 323–32. [Google Scholar]
- 2.Astler VB, Coller FA. The prognostic significance of direct extension of carcinoma of the colon and rectum. Ann Surg 1954; 139: 846–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chapuis PH, Dent OF, Fisher R, et al. A multivariate analysis of clinical and pathological variables in prognosis after resection of large bowel cancer. Br J Surg 1985 Sept; 72:698–702. [DOI] [PubMed]
- 4.Scott KW, Grace RH. Detection of lymph node metastases in colorectal carcinoma before and after fat clearance. Br J Surg 1989 Nov; 76(11):1165–7. [DOI] [PubMed]
- 5.Hernanz F, Revuelta S, Redondo C, et al. Colorectal adenocarcinoma: quality of the assessment of lymph node metastases. Dis Colon Rectum 1994 Apr; 37(4):373–6; discussion 376–7. [DOI] [PubMed]
- 6.Goldstein NS, Sanford W, Coffey M, et al. Lymph node recovery from colorectal resection specimens removed for adenocarcinoma. Trends over time and a recommendation for a minimum number of lymph nodes to be recovered. Am J Clin Pathol 1996 Aug; 106(2):209–16. [DOI] [PubMed]
- 7.Caplin S, Cerottini JP, Bosman FT, et al. For patients with Dukes’ B (TNM Stage II) colorectal carcinoma, examination of six or fewer lymph nodes is related to poor prognosis. Cancer 1998 Aug 15; 83(4):666–72. [PubMed]
- 8.Wong JH, Severino R, Honnebier MB, et al. Number of nodes examined and staging accuracy in colorectal carcinoma. J Clin Oncol 1999 Sep; 17(9):2896–900. [DOI] [PubMed]
- 9.Fielding LP, Arsenault PA, Chapuis PH, et al. Working party report to the world congress of gastroenterology, Sidney 1990. J Gastroenterol Hepatol 1991; 6: 325–44. [DOI] [PubMed] [Google Scholar]
- 10.Nelson H, Petrelli N, Carlin A, et al. Guidelines 2000 for Colon and Rectal Cancer Surgery. J Natl Cancer Inst 2001; 93: 583–96. [DOI] [PubMed] [Google Scholar]
- 11.Le Voyer TE, Sigurdson ER, Hanlon AR, et al. Colon cancer survival is associated with increasing number of lymph nodes removed. A secondary analysis of INT-0089. ASCO 2000; 19: 239a. [DOI] [PubMed] [Google Scholar]
- 12.Di Costanzo F for INTACC Italy. INTACC 01: 5 fluorouracil (5FU) + Levamisole (LEVA) vs. 5FU + 6-S-Leucovorin (6-S-LV) + LEVA: an Italian intergroup study of adiuvant therapy for resected colon cancer. ASCO 1999; 18:266a.
- 13.Lionetto R for INTACC Italy. INTACC 02: 5 fluorouracil (5FU) + 6-S-Leucovorin (6-S-LV) + Levamisole (LEVA) vs. methotrexate(MTX) + 5FU + LEVA: an Italian intergroup study of adiuvant therapy for resected colon cancer. ASCO 1999; 18: 291a. [Google Scholar]
- 14.Shida H, Ban K, Matsumoto M, et al. Prognostic significance of location of lymph node metastases in colorectal cancer. Dis Colon Rectum 1992; 35: 1046–50. [DOI] [PubMed] [Google Scholar]
- 15.Buyse ME, Staquet MJ, Sylvester RJ. In Cancer clinical trials - Methods and practice, Chapter 24: Identification of prognostic factors. Oxford University Press 1984.