Abstract
Objective
This article reviews the current results of various locoregional therapies for hepatocellular carcinoma (HCC), with special reference to the implications for surgeons.
Summary Background Data
Resection or transplantation is the treatment of choice for HCC, but most patients are not suitable candidates. The past decade has witnessed the development of a variety of locoregional therapies for HCC. Surgeons are faced with the challenge of adopting these therapies in the management of patients with resectable or unresectable HCC.
Methods
A review of relevant English-language articles was undertaken based on a Medline search from January 1990 to August 2001.
Results
Retrospective studies suggested that transarterial chemoembolization is an effective treatment for inoperable HCC, but its perceived benefit for survival has not been substantiated in randomized trials, presumably because its antitumor effect is offset by its adverse effect on liver function. Nonetheless, it remains a widely used palliative treatment for HCC not amenable to resection or ablative therapies, and it also plays an important role as a treatment of postresection recurrence and as a pretransplant therapy for transplantable HCC. Better patient selection, selective segmental chemoembolization, and treatment repetition tailored to tumor response and patient tolerance may improve its benefit-risk ratio. Transarterial radiotherapy is a less available alternative that produces results similar to those of chemoembolization. Percutaneous ethanol injection has gained wide acceptance as a safe and effective treatment for HCCs 3 cm or smaller. Uncertainty in tumor necrosis limits its potential as a curative treatment, but its repeatability allows treatment of recurrence after ablation or resection of HCC that is crucial to prolongation of survival. Cryotherapy affords a better chance of cure because of predictable necrosis even for HCCs larger than 3 cm, but its use is limited by a high complication rate. There has been recent enthusiasm for heat ablation by microwave, radiofrequency, or laser, which provides predictable necrosis with a low complication rate. Preliminary data indicated that radiofrequency ablation is superior to ethanol injection in the radicality of tumor ablation. The advent of more versatile radiofrequency probes has allowed ablation of HCCs larger than 5 cm. Recent studies have suggested that combined transarterial embolization and heat ablation is a promising strategy for large HCCs. Thus far, no randomized trials comparing various thermoablative therapies have been reported. It is also uncertain whether a percutaneous route, laparoscopy, or open surgery affords the best approach for these therapies. Thermoablative therapies have been combined with resection or used to treat postresection recurrence, and they have also been used as a pretransplant therapy. However, the value of such strategies requires further evaluation.
Conclusions
Advances in locoregional therapies have led to a major breakthrough in the management of unresectable HCC, but the exact role of the various modalities needs to be defined by randomized studies. Novel thermoablative techniques provide the surgeon with an exciting opportunity to participate actively in the management of unresectable HCC. Locoregional therapies are also useful adjuncts in the management of patients with resectable or transplantable disease. Hence, surgeons must be equipped with the latest knowledge and techniques of ablative therapy to provide the most appropriate treatment for the wide spectrum of patients with HCC.
Hepatocellular carcinoma (HCC) is one of the most common malignancies, ranking fifth in frequency in the world. 1 Although it is more prevalent in Asia and Africa, its incidence is on the rise in Western countries. 2,3 Surgical resection is considered the treatment of choice for HCC in both cirrhotic and noncirrhotic patients, provided that the liver function reserve is adequate. Recent advances in surgical management have markedly reduced the surgical death rate, and some centers have reported a near-zero hospital death rate after resection of HCC. 4,5 The long-term survival after resection of HCC has also improved during the past decade, with an overall 5-year survival rate of about 50% achieved in recent years. 6 However, the majority of patients with HCC are not candidates for resection because of advanced tumors, tumor location near major intrahepatic vessels precluding a negative-margin resection, multifocal tumors, or poor hepatic functional reserve. Even in centers with extensive experience in hepatic resection for HCC, the resection rate was only in the range of 10% to 37%. 4,7,8 Liver transplantation has been established as an alternative curative treatment for small HCCs associated with cirrhosis, offering excellent survival results in patients with solitary HCCs smaller than 5 cm or those with up to three nodules each smaller than 3 cm. 9,10 However, its use has been restricted by the severe shortage of organ donors. Because of the limited applicability of surgical treatment for HCC, during the past decade efforts have been directed toward the development of nonsurgical therapeutic modalities for HCC.
Systemic chemotherapy has provided dismal results, with a response rate of less than 20%, and no significant survival benefit has been shown compared with symptomatic management. 11,12 Other systemic treatments such as immunotherapy using interferon and hormonal therapy with tamoxifen have also proved ineffective in randomized trials. 13–15 Hence, locoregional therapy has become the focus of interest in recent years. There is a growing list of locoregional therapeutic options for HCC; they can be broadly categorized into transarterial therapies and local ablative therapies. The latter embrace various techniques of destroying a liver tumor by chemical or thermal means. With the increasing detection of small HCCs from screening programs for cirrhotic patients, it is foreseen that locoregional therapy will play an increasingly important role in the management of HCC.
Locoregional therapies are often offered by gastroenterologists or interventional radiologists for patients with inoperable HCC and sometimes even for those with operable tumors. Surgeons must keep themselves updated on the recent advances in these therapies for several reasons. First, knowledge of the current results of these therapies enables a rational choice of surgical or nonsurgical treatment. The survival results of locoregional therapies may be comparable to that of resection in selected patients, and some of these therapies have been proposed as acceptable alternatives to surgery for small HCCs. Second, local ablative therapies may be given laparoscopically or by open surgery, and hence these therapies are in the domain of surgeons. Third, locoregional therapy may be useful as a neoadjuvant or adjuvant therapy for patients undergoing resection of HCC. Some patients with otherwise unresectable HCC may be amenable to curative resection after local cytoreductive treatment, and intraoperative ablative therapy may be combined with partial hepatectomy to provide a chance of cure for patients with multiple HCCs. Postoperative transarterial therapy has been used as an adjuvant treatment to reduce the risk of recurrence in those who have undergone a curative resection of HCC. Locoregional therapies are also widely used to treat postresection recurrent tumors, and they are used as “bridge” therapies for controlling tumor growth while waiting for a graft in patients with transplantable HCC.
This article aims to provide surgeons with a comprehensive review of the various locoregional therapies currently available for HCC. The surgical implications of the recent developments in these therapies are highlighted. A Medline literature search from January 1990 to August 2001 was undertaken, with additional pertinent references extracted from the bibliographies of the articles.
TRANSARTERIAL REGIONAL THERAPIES
Transarterial Chemoembolization
Transarterial chemoembolization (TACE) is a regional therapy widely used for unresectable HCC since the 1980s. During the procedure, iodized poppyseed oil (Lipiodol) and chemotherapeutic agents (doxorubicin, cisplatin, or mitomycin C) are administered through the feeding artery of the tumor, followed by arterial embolization with gelatin sponge particles. Transarterial chemotherapy and transarterial embolization are variations of the treatment that have been used by some authors.
Intraarterial injection of cytotoxic agents aims to achieve a higher local concentration of the agents with lower systemic toxicity. However, it has not been found to yield better results than intravenous chemotherapy for HCC in randomized trials. 16,17 Because the blood supply to HCCs is predominantly derived from the hepatic artery, 18 transarterial embolization can induce tumor necrosis in HCCs. 19 In a study of 100 patients with HCCs smaller than 4 cm treated by transarterial embolization, a complete necrosis rate of 64% and a 5-year survival rate of 53% were reported. 20 Lin et al 21 showed in a randomized trial that transarterial embolization improved the survival outcome of patients with HCC compared with chemotherapy with intravenous 5-fluorouracil (1-year survival rate 42% vs. 13%). However, a more recent randomized trial comparing transarterial embolization with symptomatic treatment in 80 patients with inoperable HCC found no difference in the survival outcome (2-year survival rate 49% vs. 50%), despite a high tumor response rate of 55% in the treated group. 22 The latter study has been criticized for more favorable baseline conditions and the surprisingly high survival rate in the untreated control group. 23 Currently, most clinicians consider chemoembolization to be a more rational therapy than embolization alone, although there is no definite evidence from randomized studies. Transarterial embolization does play an important role in controlling hemorrhage from ruptured HCC. It is considered the first-choice emergency treatment for patients with tumor rupture, and in selected patients effective hemostasis by embolization may allow subsequent elective resection of the tumor. 24
The combined use of a Lipiodol–cytotoxic drug emulsion and embolization has some theoretical advantages over chemotherapy or embolization alone. Lipiodol is selectively retained in the tumor for weeks and therefore helps to concentrate the cytotoxic agents into the tumor. 25 The exact mechanism responsible for the retention of Lipiodol in HCC is unknown, but it is likely to be related to the abnormal vasculature of HCC. Yoshikawa et al 26 showed in a randomized trial that infusion of Lipiodol–cytotoxic drug emulsion produced a significantly better response rate and survival than the cytotoxic drug alone. The Lipiodol retention results in intense staining of the tumor, which helps in monitoring the tumor’s response to the treatment (Fig. 1). The necrotizing effect of the Lipiodol–drug emulsion is further enhanced by arterial embolization. In a prospective trial, the 1-year survival rate after TACE was significantly better than the survival after transarterial chemotherapy with a Lipiodol–drug emulsion alone (86.3% vs. 65.9%). 27 Selective segmental or subsegmental treatment induces better tumor response and less injury to nontumorous liver compared with injection of Lipiodol–emulsion and embolizing particles in the common hepatic artery. 28 The treatment can be repeated every 8 to 12 weeks, which is considered important in prolonging the patient’s survival. 29 However, the benefit of repetition of TACE must be balanced against the progressive liver damage associated with the treatment. A recent trial comparing planned repetition of TACE for three cycles and repetition based on tumor response and patient tolerance showed fewer complications and better survival with the latter strategy. 30
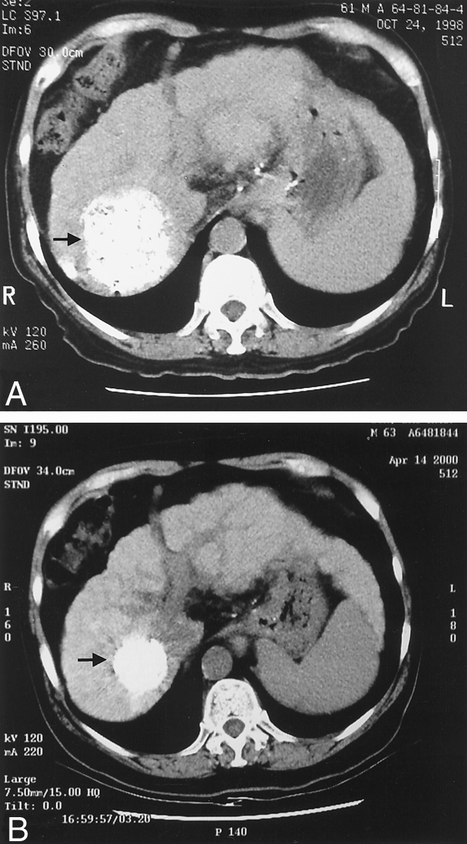
Figure 1. Reduction in size of a hepatocellular cancer after transarterial chemoembolization as indicated by the Lipiodol stain in the computed tomography scan before treatment (A, arrow) and after repeated chemoembolization treatments (B, arrow). The cross-section in A and B represents a similar anatomic location. There was also a reduction in size of the liver, suggesting advancement of cirrhosis.
Although TACE has been used for two decades, there is not yet a consensus on the patient selection criteria. The presence of main portal vein thrombosis, extrahepatic metastasis, Child C liver function, and severe arteriovenous shunting are generally accepted as contraindications for TACE, although some authors recommend TACE for treating nodular HCC with main portal vein thrombosis, provided there is good hepatic function and collateral circulation. 31 Careful patient selection is important to avoid serious complications. In a recent analysis of 484 patients who underwent TACE for inoperable or recurrent HCC in our institution, the overall treatment complication and death rates were 23% and 4.3%, respectively. 32 Complications included liver failure, liver abscess, rupture of tumor, peptic ulcer, acute cholecystitis, acute pancreatitis, and renal failure. Apart from the aforementioned complications, a self-limiting postembolization syndrome consisting of nausea, fever, and abdominal pain is frequently observed.
Several large retrospective studies from Asian and Western centers have shown favorable results after TACE for inoperable HCC, with tumor response rates (reduction in size or complete disappearance) of 29% to 62% and 1-year survival rates of 50% to 76%. 29,33–38 Three nonrandomized studies with matched or unmatched control groups showed that TACE significantly improved survival compared with symptomatic treatment (Table 1). 39–41 However, three randomized controlled trials from Western centers did not find significant differences in the survival results between patients treated with TACE and those managed with conservative treatment. 42–44 These randomized trials all showed that TACE had a marked antitumor effect, with a tumor response rate of 24% to 53%. However, the potential benefit of TACE was counteracted by its deleterious effect on liver function, with post-TACE liver failure rates of more than 50% in two of the randomized trials. 43,44 Further, these trials have been criticized for suboptimal techniques of TACE, an insufficient statistical power due to the small numbers of patients enrolled, and exclusion of some patients from the arm of the assigned treatment. 45 A prospective randomized trial of TACE versus conservative management in 80 Chinese patients with inoperable HCC was recently completed in our institution. Our study revealed significantly improved survival results with TACE treatment (3-year survival 26% vs. 3%, P = .002, unpublished data). The difference in outcome between our study and the Western trials may be partly attributable to the different clinicopathologic characteristics of the patient populations, but it is also likely to be related to different TACE techniques. In our institution, the TACE regimen used a lower dose of cytotoxic drug compared with that used in the previous randomized trials, and the dose was based on tumor size rather than a fixed dose as in the previous trials. 46 Further, chemoembolization was given by selective injection into the feeding artery of the tumor whenever possible, whereas in the previous trials chemoembolization was performed in the hepatic artery proper or its main branches. Unlike the previous randomized trials that used planned repetition of TACE, repetition of chemoembolization was based on tumor response and patient tolerance in our trial. These strategies help to reduce liver damage and may be crucial factors for the positive result observed in our trial.
Table 1. CONTROLLED STUDIES OF TRANSARTERIAL CHEMOEMBOLIZATION FOR HEPATOCELLULAR CARCINOMA
Some authors have compared the survival results of TACE with those of hepatic resection for HCC. Yoshimi et al 47 found that the survival after TACE was comparable to that after hepatectomy, despite a higher incidence of multiple tumors and more advanced HCC in the TACE group. Bronowicki et al 48 compared TACE for resectable HCC in 42 patients with resection in 30 patients and found similar survival results. However, there are no randomized trials comparing the two treatments, which is probably impossible to conduct today because resection has become widely accepted as the treatment of choice for HCC. TACE is unlikely to afford a cure because cancer tissue may survive at the periphery of the tumor, and it should be used only as a palliative treatment for unresectable HCC.
In many centers, TACE remains a standard therapy for advanced inoperable HCC despite the negative results of the three reported randomized trials. It is believed that better patient selection to optimize tumor response and decrease complications will enhance the benefit of the treatment for HCC. Hence, recent studies have focused on the prognostic classification of patients to provide better guidance in patient selection for TACE. Several studies have shown that poorer outcomes can be expected with large tumors, portal vein invasion, and poor liver function. 29,33–38 In a recent study from our department, a simple staging based on tumor size (≤10 or >10 cm) and serum albumin level (>35 or ≤35 g/L) was found to differentiate patients with favorable, intermediate, and unfavorable survival outcome after TACE. 32 Our study suggested that patients with HCCs larger than 10 cm and a serum albumin level of 35 g/L or less may not be appropriate candidates for TACE. Another group has proposed a prognostic index based on the serum alpha-fetoprotein level, tumor size, and Child-Pugh score to classify patients into three categories with different prognoses after TACE. 49 Further randomized trials should focus on subpopulations of patients with better prognoses to define the most appropriate role of TACE in the management of HCC. Because liver failure is the main limitation of the survival benefit, it is important to identify a test that can accurately select patients with the lowest risk of liver failure after TACE. Such a test has not yet been determined as it has been for hepatic resection. A recent study suggested that the tumor-to-liver volume assessed by computed tomographic (CT) volumetry can predict survival after TACE for HCC and thus may be useful in selecting the best candidates for TACE. 50 With careful patient selection to reduce the risk of liver failure, TACE should continue to be an important palliative treatment for patients with unresectable and nontransplantable HCC, especially those with tumors larger than 5 cm or multiple tumors that are not favorable for local ablative therapy.
Apart from its role in the management of primary inoperable HCC, TACE is used to treat intrahepatic recurrence after resection of HCC. 51 The result of TACE treatment for postresection recurrent tumors is better than that for primary inoperable HCC because of the small size of recurrent tumors when detected by postoperative surveillance. 32 Several retrospective studies on TACE for postresection recurrence have shown favorable survival results, with 1-year survival rates of 72% to 88%, 3-year survival rates of 38% to 48%, and 5-year survival rates of 21% to 27% after recurrence. 51–54 Preoperative TACE before hepatic resection for HCC has been reported to reduce the incidence of postoperative recurrence in nonrandomized studies, 55,56 but others have argued against its use because of its associated complications and damage to the liver. 57,58 Two prospective randomized trials failed to find any significant difference in surgical complications and recurrence with preoperative TACE compared with untreated control groups, but the number of patients in these studies may not be sufficient to show a statistically significant difference. 59,60 Despite these two negative trials, preoperative TACE continues to be used by some centers in an attempt to reduce postoperative recurrence. 61,62 Similar to the case of TACE for inoperable HCC, it seems that the benefit of preoperative TACE depends on a balance between its antitumor effect and its adverse effect on liver function reserve. In a prospective but nonrandomized study, the 5-year disease-free survival rate of patients who received preoperative TACE was significantly better than that of patients with resection alone (51% vs. 21%), but the overall 5-year survival rate was not significantly different (43% vs. 38%) because of a higher incidence of postoperative liver failure in the former group. 62 Hence, more refined selection of patients in terms of liver function reserve may be crucial to the use of TACE before hepatic resection. A recent study has shown that liver scintigraphy using technetium-99m diethylenetriamine pentaacetic acid-galactosyl human serum albumin can provide accurate assessment of liver function reserve before and after TACE. 63 It therefore may be used to select patients who will benefit most from TACE before resection of HCC. The routine use of preoperative TACE cannot be substantiated with the current evidence, but TACE may be recommended to downstage tumors to increase the chance of curative resection for patients with HCC of borderline resectability. 56,58 Postoperative transarterial chemotherapy has also been used to reduce recurrence after resection of HCC, but conflicting results have been reported from randomized trials. One randomized trial found that postoperative transarterial chemotherapy improved disease-free survival, 64 but others failed to show its benefit. 65,66
Frequently, TACE is used to control tumor growth in transplant candidates before a graft is available. 56,67 The efficacy of pretransplant TACE remains uncertain because of a lack of randomized trials. A study comparing patients with pretransplant TACE and a historical control group without TACE found that the treatment induced marked tumor necrosis but no improvement in survival, and patients with pretransplant TACE appeared to have an increased risk of early posttransplant infective complications. 68 Some centers have used TACE before transplant followed by postoperative chemotherapy for patients with HCCs larger than 5 cm, who are conventionally regarded as unsuitable candidates for transplantation. Preliminary reports from small series have documented favorable survival results with such an approach, 69,70 but further studies are needed to clarify its benefit.
Transarterial Radiotherapy
Conventional external radiotherapy has a very limited role in the treatment of HCC because of the severe damage to the nontumorous liver at the dose required to destroy tumor cells. Modern three-dimensional conformal radiotherapy can minimize beam scatter and deliver the dose of radiation to the tumor more specifically. A pilot study showed a tumor response rate of 58% and good liver tolerance with three-dimensional conformal radiotherapy in unresectable HCC. 71 Proton beam radiotherapy is another new technique that has produced a tumor response rate of more than 50% and minimal side effects. 72 Experience with these new modalities of external radiotherapy is still limited. More data are available regarding the efficacy of transarterial internal radiotherapy for HCC, which is a targeted therapy with a radioactive isotope carried in an agent that is selectively retained by the tumor.
Intraarterial iodine-131 injected with Lipiodol produced a tumor response rate ranging from 17% to 92% in various studies, and it appears to be well tolerated. 73–76 Complete tumor necrosis has been shown with superselective high-dose therapy in patients with HCCs smaller than 5 cm. 77 A recent prospective randomized trial comparing transarterial iodine-131 (n = 65) and TACE (n = 64) revealed no significant difference in tumor response rate (24% vs. 25%) or survival results (1-year survival rate 38% vs. 42%), but the former treatment was better tolerated. 78 Another randomized trial compared transarterial iodine-131 (n = 14) and symptomatic treatment (n = 13) for HCC associated with portal vein thrombosis, and the study found significantly better survival among patients treated with transarterial radiotherapy (6-month survival rate 48% vs. 0%, P < .01). 79 However, a more recent study of transarterial iodine-131 for 24 patients with HCC associated with portal vein thrombosis found only a 12% partial response rate but a 42% incidence of liver failure, indicating a limited role of the treatment for this group of patients. 80
Transarterial iodine-131 has also been investigated as an adjuvant therapy after curative resection of HCC. A phase 2 pilot study suggested that it is well tolerated in patients after resection of HCC and may reduce postoperative recurrence. 81 A randomized study in 43 patients reported a lower incidence of recurrence after curative resection of HCC in patients with adjuvant transarterial iodine-131 therapy compared with a control group who underwent surgery alone. 82 In that study, however, the majority of patients had early-stage disease and only one patient in each group had venous invasion. The value of adjuvant transarterial radiotherapy in patients with venous invasion or advanced-stage disease who are at higher risk of recurrence and thus are in greater need of adjuvant therapy remains unknown. The efficacy of adjuvant transarterial radiotherapy needs further evaluation by studies with a larger number of patients with advanced disease.
Yttrium-90 delivered in glass microspheres is another form of transarterial radiotherapy that has been used for HCC. It has a greater energy and cytotoxic effect than iodine-131. In a study of 71 patients with unresectable HCC treated with transarterial yttrium-90 microspheres, an overall response rate of 89% in terms of reduction in the serum alpha-fetoprotein level was reported, and the medium survival was 9.4 months. 83 It remains unclear whether yttrium-90 has any advantage over iodine-131 treatment because no comparative study has been reported yet. The use of transarterial radiotherapy for HCC has been confined to few centers because of its limited availability.
LOCAL ABLATIVE THERAPIES
Percutaneous Ethanol Injection
Percutaneous ethanol injection (PEI) induces tumor necrosis by cellular dehydration, protein denaturation, and thrombosis of small vessels. HCC is softer than the surrounding cirrhotic liver and is often encapsulated, thus allowing selective diffusion of ethanol within the tumor mass. The hypervascularization of HCC also favors ethanol injection therapy by enhancing the distribution of ethanol within the network of the tumor vessels. PEI can be done as an outpatient procedure under local anesthesia. A fine needle is inserted into the tumor under ultrasonographic guidance, and absolute ethanol is then injected slowly into the tumor until the whole area of tumor appears hyperechogenic on the ultrasound. PEI can also be performed under CT guidance for tumors not visualized by ultrasound. 84 The injection is repeated once or twice a week for up to six to eight sessions, depending on the tumor size. The therapeutic effect of PEI can be evaluated by contrast CT scan. The demonstration of a uniform low density without contrast enhancement is considered a reliable indication of tumor necrosis. 85 Color Doppler ultrasonography and magnetic resonance imaging (MRI) are alternative imaging techniques for assessing the response to PEI. 86,87
It is generally agreed that patients with HCCs 3 cm or smaller and three or fewer in number are the best candidates for PEI, although many centers perform PEI for HCCs up to 5 cm. 88–90 PEI is contraindicated in the presence of gross ascites, severe thrombocytopenia, or coagulopathy because of a high risk of bleeding. Large infiltrative tumors, thrombosis in the main portal or hepatic vein, and extrahepatic metastasis are also considered contraindications in most centers. Patients with tumors on the surface of the liver are not favorable candidates for PEI because the injected ethanol can leak back into the peritoneal cavity, and there is also a higher risk of tumor implantation into the peritoneal cavity. 91
PEI is a minimally invasive therapy with a good safety record. In a study of 746 patients with HCC treated by PEI, the treatment-related death rate was only 0.1%, and the rate of severe complications was 1.7%. 92 A similar death rate (0.09%) and complication rate (3.2%) have been reported in a multicenter survey of 1,066 patients after PEI for HCC. 90 Minor adverse effects of PEI such as pain, fever, and transient drunkenness are self-limiting, but more serious complications such as liver abscess, liver failure, cholangitis, hemobilia, and intraperitoneal hemorrhage can occur. 90,92 Tumor seeding along the needle track after PEI for HCC has been reported, with an incidence of 1% in a recent study of 348 patients. 93 Partial tumor necrosis induced by PEI may also enhance embolization of tumor cells and thus metastasis in extrahepatic sites such as the lung, 94 but its exact incidence is unknown.
Histopathologic studies have shown that PEI can induce complete tumor necrosis in about 70% of patients with HCCs smaller than 3 cm. 91,92,95 The extent of necrosis is closely related to the tumor size, with an almost 100% rate of complete necrosis in HCCs smaller than 2 cm. 95 The long-term survival results after PEI for HCC have been reported in several series (Table 2). The reported 5-year survival rates after PEI in patients with HCCs 5 cm or smaller was in the range of 24% to 40%. The survival results of PEI for HCC are influenced by the liver function status. In one large series of PEI for HCCs smaller than 5 cm, the 5-year survival rate was 47% for 293 Child A patients, 29% for 149 patients with Child B cirrhosis, and 0% for 20 patients with Child C cirrhosis. 92 Other adverse prognostic factors include tumor size larger than 3 cm, pretreatment serum alpha-fetoprotein level greater than 200 ng/mL, and multiple tumor nodules. 96–101
Table 2. SURVIVAL RESULTS AFTER PERCUTANEOUS ETHANOL THERAPY FOR HEPATOCELLULAR CARCINOMA
Retrospective studies have shown superior survival results with PEI therapy compared with symptomatic treatment, 100–103 but no randomized trial has ever been performed to confirm its survival benefit. Nor are there any randomized trials comparing PEI with hepatic resection, although a few retrospective studies showed that PEI may produce a survival outcome similar to that after resection for small HCCs (<3 cm). 96,103,104 Based on these nonrandomized studies, it has been suggested that PEI is an alternative treatment that can compete with surgical resection for patients with small HCCs. 105 However, a recent nationwide survey in Japan comparing resection in 8,010 patients and PEI in 4,037 patients showed that hepatectomy produced superior survival results for solitary tumors less than 2 cm in patients with normal liver function, and also for solitary tumors greater than 2 cm in patients with all stages of liver function. 106 Recent studies have shown a 5-year survival rate of around 60% after resection of HCCs smaller than 5 cm in patients with cirrhosis. 107,108 It is difficult to draw a definite conclusion regarding the relative role of PEI and resection for small HCCs without a randomized comparison. However, given the uncertainty of tumor necrosis after PEI, it is logical to recommend resection as the first option for cirrhotic patients with HCCs smaller than 5 cm provided the liver function is satisfactory, and to reserve PEI for those whose liver function reserve is inadequate for hepatic resection.
Tumor size larger than 5 cm has been traditionally regarded as a contraindication for ethanol injection therapy. A recent development of PEI is its use for HCCs of 5 to 10 cm by a single-session treatment under general anesthesia, which allows injection of a large volume of ethanol. 109–111 This appears to be a safe treatment, with a death rate of 0.7% in one series of 108 patients. 109 However, major complications such as peritoneal hemorrhage and liver failure are more common than after PEI for small HCCs. In the same series, the 4-year survival rate of 24 patients with single, encapsulated HCCs of 5 to 8.5 cm was 44%, but the 4-year survival rate of 21 patients with large HCCs associated with Child C cirrhosis or portal vein thrombosis was 0%, suggesting that PEI may not be an appropriate treatment for the latter group of patients. Another study reported a favorable 5-year survival rate of 59% in cirrhotic patients with a single HCC larger than 5 cm, and the authors suggested that single-session PEI under anesthesia may be a better option than surgical resection. 110,111 This needs to be substantiated by a randomized trial, but the likelihood of such a trial being conducted is low given the current acceptance of resection as the best treatment.
One major concern of PEI for HCC is the high incidence of recurrence. The cumulative intrahepatic recurrence rates at 1, 3, and 5 years after PEI for small HCCs (<5 cm) were in the range of 26% to 32%, 51% to 81%, and 60% to 83%, respectively, in reported series. 97,101,112–115 The majority of recurrences are new lesions at different portions of the liver, but local recurrence at the site of the initial lesion treated by PEI accounted for 16% to 38% of the recurrent tumors. 97,113,115 The substantial local recurrence rate is reminiscent of the difficulty in ascertaining complete necrosis after PEI resulting from inhomogeneous diffusion of ethanol in the tumor. A high intrahepatic recurrence rate is also a major problem after resection of HCC. 116 Few studies have compared the recurrence rate after PEI and hepatic resection for small HCCs. In a retrospective study, Okuda 117 found similar rates of new lesions after PEI and resection in two comparable groups of patients, which probably reflects the multicentric nature of hepatocarcinogenesis in the cirrhotic liver. However, with the additional local recurrences from incomplete necrosis after PEI, the overall recurrence rate after PEI is likely to be higher than that after resection. In a study comparing two cohorts of patients with solitary HCCs 4 cm or smaller treated by PEI and hepatic resection, the 2-year recurrence rates after PEI and hepatic resection were 66% and 45%, respectively, and the difference was most obvious among patients with tumors 3 to 4 cm in size. 96 One advantage of PEI is its easy repeatability, which allows further treatment of both local and distant intrahepatic recurrences. In fact, PEI is widely used for treating recurrence in the liver remnant after resection of HCC. 116
Percutaneous Acetic Acid Injection
The efficacy of PEI is limited by the presence of septa in the tumor nodule, which prevents uniform diffusion of ethanol and necessitates repeated treatment sessions. Further, capsular invasion cannot be ablated because ethanol cannot dissolve the fibrous capsule. Percutaneous acetic acid injection (PAI) has been used as an alternative. 118 Acetic acid has a stronger necrotizing power than ethanol because it can dissolve lipids and extract collagen. Its low pH induces swelling of the fibers and promotes dissociation of intermolecular collagen cross-links. One randomized controlled trial conducted in 60 patients with HCCs smaller than 3 cm showed that the survival of patients treated with PAI was significantly better than those treated with PEI (2-year survival rate, 92% vs. 63%). 119 The local recurrence rate was also significantly lower in the PAI group compared with the PEI group (2-year local recurrence rate, 10% vs. 44%). In that study, major treatment-related complications were rare with both modalities. However, no further data are available to confirm the advantages of PAI over PEI.
Cryotherapy
Cryotherapy has been used for the treatment of liver tumors since the 1980s, with the initial experience mainly in patients with metastatic malignancies. 120 Rapid freezing to subzero temperature leads to ice formation in the extracellular space and drawing of water from the cells, causing cellular damage by dehydration and destruction of the normal cellular structures. 120 The procedure is usually performed during surgery with insertion of a cryoprobe cooled with liquid nitrogen or liquid argon into the tumor mass (Fig. 2). Intraoperative ultrasound provides guidance to the placement of the cryoprobe in the liver mass, and it also provides a means of monitoring the growth of the freeze zone to ensure an adequate margin. The growing ice ball can be seen as an expanding hyperechoic lesion, and a margin of at least 1 cm of liver tissue around the tumor should be frozen to ensure compete tumor ablation. 121 The ability to monitor the ablation process precisely by ultrasound is an advantage of cryotherapy over the other currently available local ablative therapies. Although cryotherapy for liver tumor is usually performed through laparotomy, percutaneous or laparoscopic cryotherapy has also been reported. 121–123 Conversion to open surgery may be required with the latter approaches for control of bleeding from the cracking of surface parenchyma. 122 Cryotherapy is most effective for tumors smaller than 5 cm, although larger tumors can be treated by multiple probes inserted simultaneously. 121 One theoretical limitation of cryotherapy is the “heat-sink” effect of flowing blood in adjacent vessels, which may reduce the freezing effect on tumors that abut a major vessel. However, it has been shown in an animal study that complete necrosis of perivascular tissue can be achieved by cryoablation without damage to the vessel wall. 124
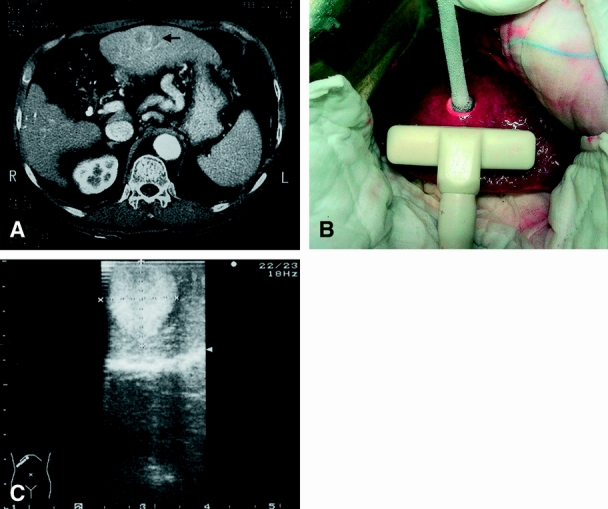
Figure 2. A 2-cm hepatocellular carcinoma in a severely cirrhotic liver (A, arrow) treated by intraoperative cryotherapy (B). The ice ball can be clearly visualized by intraoperative ultrasound (C).
Several studies have found effective ablation of unresectable HCC by cryotherapy, even for large HCCs (>5 cm). 125–132 The main concern of cryotherapy is its associated complications. The complication rate in reported series of cryoablation for HCC ranged from 8% to 41%, and the death rate ranged from 0% to 17%. 126–132 Specific complications of cryoablation include bleeding from cracking of liver parenchyma, freezing injury to adjacent structures such as the colon, intrahepatic abscess, and bile duct damage resulting in the formation of biloma or biliary fistula. 126–132 A syndrome of thrombocytopenia, disseminated intravascular coagulation, adult respiratory distress syndrome, and acute renal failure after hepatic cryotherapy has been described and termed “cryoshock phenomenon”; this has been observed most frequently after ablation of large tumors. 133 Although the exact cause of this phenomenon is not certain, it may be related to the release of cytokines such as interleukin-6 and tumor necrosis factor-alpha after large-volume freezing or repeated cycles of freezing and thawing. 133 A survey of 2,173 patients undergoing cryotherapy for liver tumors in 72 centers around the world revealed a 1.5% death rate after hepatic cryotherapy. 133 Cryoshock phenomenon occurred in only 1% of patients, but it was associated with a 28% risk of death. The authors did not specify the type of liver tumors in the patients, and hence these figures cannot be extrapolated directly to patients with HCC.
Only limited data are available on the survival results after cryotherapy for HCC, mostly from small series of 8 to 12 patients with a short duration of follow-up. 126–128,130,132 The reported 2-year survival rate after cryoablation of HCC was 30% to 60%. 125,127,130,132 The largest series of cryotherapy for HCC was reported by a Chinese group, who found a 5-year survival rate of 37.9% among 191 patients, and a 5-year survival rate of 53.1% in a subgroup of 56 patients with tumors smaller than 5 cm. 129 These survival results appear to be comparable with that after hepatic resection. So far no study has directly compared the results of cryoablation and hepatic resection for HCC.
Apart from the survival results, the local recurrence rate is another important outcome to be considered in evaluating any local ablative therapy. Pearson et al 131 reported local recurrence in 12 (13.6%) of 88 primary or metastatic liver tumors treated by cryoablation after a median follow-up of 15 months. Interestingly, in 8 of the 12 local recurrences, the original tumor was on or near a major intrahepatic vessel. Cha et al 132 found a similar local recurrence rate of 12% in 38 patients with primary or metastatic liver malignancies treated by cryoablation with or without combined resection after a median follow-up of 28 months. These two studies included both HCCs and metastatic liver tumors in the analysis. Adam et al 127 observed that local recurrence occurred more frequently after cryoablation for metastasis than for HCC: the local recurrence rate was 44% in 25 patients with colorectal metastasis and 0% in 9 patients with HCC after a mean follow-up of 16 months. Overall, current data suggest that cryotherapy is an effective local ablative therapy for unresectable HCC, although it is associated with a relatively high complication rate. We have also reported satisfactory preliminary results with the use of cryotherapy for ablation of recurrent HCC after previous hepatic resection. 134
Microwave Coagulation Therapy
Microwave coagulation therapy (MCT) is a form of thermoablative treatment in which tissue necrosis is induced by the heating effect of microwaves of frequency 2,450 ± 50 MHz emitted from a needle electrode inserted into the tumor. The microwaves act mainly on the watery component of tissues, producing dielectric heat and tissue coagulation. Irreversible cellular damage from protein coagulation occurs at temperatures above 50°C. Compared with PEI, MCT creates a more predictable and reproducible area of tissue necrosis, and it can ablate the tumor capsule as well as surrounding extracapsular invasion. The extent of necrosis can be checked by using CT scan, MRI, or color Doppler ultrasound. 135,136
MCT can be performed percutaneously, laparoscopically, or through laparotomy. The percutaneous approach has the advantages of applicability to high-risk patients and repeatability, but its use is restricted to patients with HCCs smaller than 2 to 3 cm because of the greater chance of incomplete tumor ablation in larger tumors. 137–139 Ohmoto et al 138 studied the results of percutaneous MCT in 17 tumor nodules and found complete remission in 80% of tumors 2 cm or smaller, whereas 71% of tumors larger than 2 cm developed local recurrence. In another study of 20 HCCs treated with percutaneous MCT, 70% of tumors 3 cm or smaller had reduction in tumor size or complete disappearance of the tumor on follow-up CT scan, whereas the response rate for tumors larger than 3 cm was only 55%. 139 Laparoscopic MCT is particularly suited for superficial tumors that can be visualized, and it can be used for tumors up to 5 cm. 140–144 Seki et al 141 reported a complete ablation rate of 87.5% after laparoscopic MCT in 26 HCC tumors 1.5 to 4.5 cm in size. The open approach by laparotomy offers liberal maneuverability and potentially superior radicality compared with laparoscopic MCT. 145 The open approach may be useful for tumors larger than 5 cm and tumors whose location is unfavorable for percutaneous or laparoscopic ablation. 146,147 However, MCT is not recommended for lesions near the hepatic hilum because the procedure may injure hilar structures. 143,146 For large tumors, multiple needle electrode insertion may be needed for complete tumor ablation. 145 Intraoperative MCT has also been used for ablating smaller tumor nodules in combination with resection. 143
In cirrhotic patients, MCT appears to be well tolerated. A few series of MCT for HCC reported no serious complications, 137,138,140 whereas others reported a complication rate of 11% to 14%. 142,144,147 Complications after MCT for liver tumors include pneumothorax, liver abscess, biloma, portal vein thrombosis, subcapsular hematoma, intraabdominal bleeding, and dissemination of cancer cells into the peritoneal cavity. 142,144,147,148 One study found that the complication rate was significantly higher after MCT for HCCs larger than 4 cm compared with MCT for HCCs 4 cm or smaller. 147
With a favorable safety profile and tumor ablation rate, MCT appears to be a promising therapy for patients with unresectable HCC, especially those with small tumors associated with poor liver function. However, there are few data on the long-term survival or recurrence rate after MCT for HCC. Seki et al 141 reported a 3-year survival rate of 92% and a 1-year local recurrence rate of 12.5% among 24 patients with HCCs 1.5 to 4.5 cm in size treated by laparoscopic MCT. Another study of 27 patients with HCC of mean size 3.3 cm treated by laparoscopic or open MCT reported a 3-year crude survival rate of 86% and a disease-free survival rate of 44%. 144 In the latter study, the survival results of patients undergoing MCT were comparable to those of 23 patients with HCC of similar size treated by wedge excision, but MCT resulted in a lower complication rate (11.1% vs. 34.8%). Midorikawa et al 149 found that the death, complication, recurrence, and survival rates among 38 patients who underwent MCT and 51 patients who underwent hepatic resection for HCC were comparable, despite significantly poorer pretreatment liver function in the former group. A recent retrospective study compared the efficacy of percutaneous MCT in 48 patients and PEI in 42 patients with cirrhosis and solitary HCCs 2 cm or smaller. 150 The overall 5-year survival rate was not significantly different (70% vs. 78%), but among patients with moderately or poorly differentiated HCC, MCT resulted in significantly better survival (5-year survival rate, 78% vs. 35%) and a lower recurrence rate in the original liver subsegment (8% vs. 41%) than PEI. There have been no randomized trials comparing MCT with resection or other local ablative therapies for HCC.
Laser Therapy
Laser is another method of interstitial therapy for liver tumors that causes tissue destruction by hyperthermic coagulative necrosis. Using a single conventional neo-dymium-yttrium-aluminum-garnet (Nd-YAG) laser fiber, the maximum diameter of the ablated lesion is 2 cm, although larger lesions can be ablated by laser splitting with simultaneous heating of multiple probes or diffuse-tip fibers that emit the laser light in a more diffuse fashion. 151 Even with these newly designed laser fibers, the limiting diameter for adequate tissue destruction using laser currently is approximately 5 cm. 152 The cooling effect of blood flow in nearby vessels is also a problem with laser ablation, but the size of ablation can be significantly increased by eliminating portal flow using the Pringle maneuver. 151
Laser ablation can be performed percutaneously, laparoscopically, or during surgery. The procedure can be performed under local anesthesia for high-risk patients. A recent study of percutaneous laser ablation of 676 patients with liver secondaries or HCC revealed no clinically relevant complications. 153 However, careful patient selection in terms of liver function is important, because severe liver failure and death after laser ablation of HCC have been reported in patients with Child C cirrhosis. 154 Most of the studies on laser therapy for liver tumors involved patients with metastatic disease, and few data exist on the clinical efficacy of laser ablation for HCC. A recent study showed that percutaneous laser hyperthermia induced complete necrosis in 70 (82%) of 85 HCC nodules, 154 but there were no long-term data on survival or recurrence. In a small series of eight patients with HCC treated by percutaneous laser therapy, follow-up CT scan showed complete necrosis in all patients with HCCs smaller than 4 cm but not in tumors larger than 5 cm despite repeated treatments, and histologic examination of two large HCCs revealed a peripheral rim of viable cells after laser therapy. 152
One potential advantage of laser therapy over other hyperthermic ablative therapies is that the area of tumor ablation can be monitored with real-time ultrasound by following a change from a hypoechoic to hyperechoic pattern after coagulative necrosis. 152 Real-time MRI monitoring may allow even better visualization of the volume of laser-induced changes and their relation to the neighboring structures. 153 The current limitation of laser therapy is the size of lesions that can be ablated, although future technological developments may allow ablation of larger tumors. No study has yet compared laser with other locoregional therapies.
Radiofrequency Ablation
Radiofrequency ablation (RFA) uses the energy of 450- to 500-KHz radiowaves for hyperthermic ablation of liver tumors. During the procedure, a needle electrode with an uninsulated tip and an insulated needle shaft is inserted into the tumor. A flux of high-frequency alternating current passes through the uninsulated needle tip into the surrounding tissue, generating rapid vibration of the ions in the tissue and frictional heat. The heat created around the electrode is subsequently conducted into the surrounding tissue in a predictable manner, causing coagulative necrosis at a temperature between 50°C and 100°C. 155 The size of the ablated area is determined largely by the current’s intensity and length, the gauge of the electrode tip, and the duration of energy applied. 156 The current intensity that can be used is limited by tissue carbonization around the needle tip, which can result in a sharp rise in tissue impedance and thus interruption of the radiofrequency wave flow. This effectively limits the area of tissue that can be ablated by a single probe. Tissue vascularization is also an important factor that determines the volume of tissue ablated. 155 Like other heat ablation methods, complete necrosis of highly vascular tumors or tumors adjacent to large vessels may be impeded by the cooling effect of blood flow. The Pringle maneuver during RFA is an effective measure to reduce the cooling effect of blood flow. 157 RFA with the conventional single needle electrode can ablate tumors smaller than 2 cm, but ablation of larger tumors is possible with recent technical improvements. 158 The use of a cooled-tip electrode avoids charring of tissue immediately around the electrode by cooling the internal chamber of the needle via cold saline infusion, thus allowing the use of a higher power than the conventional needle. Delivering RFA energy in pulses is another way to prevent charring around the needle tip. The use of multiple-prong (clustered) electrodes or an expandable electrode with multiple retractable J hooks to create overlapping ablation fields can be used to ablate tumors up to 7 cm. 158,159 Because of these versatile probe designs that allow ablation of large tumors, the enthusiasm for RFA has far exceeded that for either microwave or laser ablation in recent years.
Similar to MCT and laser therapy, RFA can be performed percutaneously, laparoscopically, or through laparotomy. 160–171 Percutaneous RFA is done with ultrasound guidance under local anesthesia and may be performed as a day procedure. However, the laparoscopic or open approach may be necessary in patients with a high risk of bleeding from severe coagulopathy, large HCCs (>5 cm), superficial nodules adjacent to other visceral organs at risk of thermal injury, or deeply located lesions not accessible to percutaneous puncture (Fig. 3). The ablative process can be monitored with real-time ultrasound, which shows an intense area of hyperechogenicity in the ablated area around the electrode caused by thermal tissue changes. However, unlike cryoablation, in which the evolving ablated area can be monitored by ultrasound to obtain an exact margin, the hyperechoic area seen in RFA does not correspond exactly to the area of tumor ablation, nor does it indicate whether tumor ablation is complete. This can be particularly problematic in large tumors, when multiple overlapping zones of ablation are needed for the destruction of the tumor and a surrounding rim of nontumorous liver. The extent of coagulative necrosis can be more accurately assessed by CT scan, MRI scan, or color or power Doppler scan performed after the procedure. 172–174 Using contrast CT scan, several studies have shown complete tumor necrosis in 80% to 90% of HCCs smaller than 3 to 5 cm after a single session of RFA. 166,167,175 The compete ablation rate for larger tumors is less favorable: a study of RFA for 126 HCCs 3.1 to 9.5 cm (mean 5.4 cm) reported a complete necrosis rate of 48% even with the use of a clustered electrode. 159
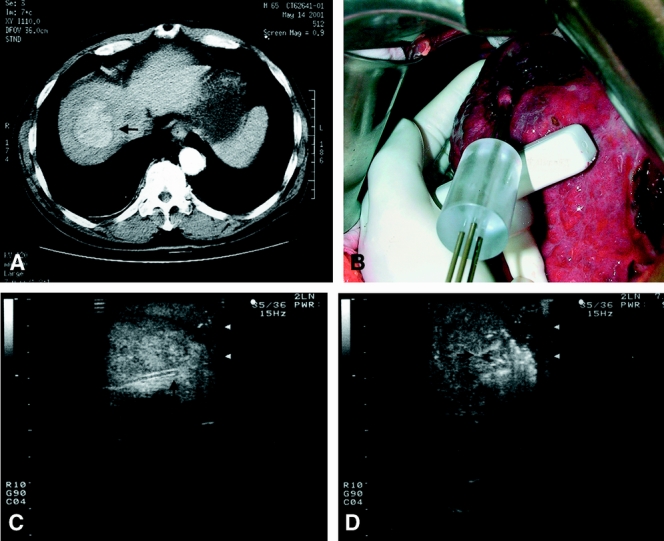
Figure 3. A 5-cm hepatocellular carcinoma at the dome of the liver (A, arrow) treated by intraoperative radiofrequency ablation using a clustered probe (B). Intraoperative ultrasound provides guidance to positioning of the probe (C, arrow shows the tip of the probe) in the tumor before starting radiofrequency ablation, but the exact margin of ablation is obscured by hyperechoic shadow resulting from thermal changes in the tissue after starting the ablation (D, arrows).
Like other hyperthermic ablative therapies, RFA appears to be a safe procedure for treatment of HCC in patients with cirrhosis. Reported complications include liver abscess, intraperitoneal hemorrhage, subcapsular hematoma, hemobilia, biliary strictures, pneumothorax, pleural effusion, injury to adjacent organs, and liver failure. 159,170,171,175 The complication rate ranged from 0% to 12% in various series, and the treatment-related death rate ranged from 0% to 1%. 159,165,167–171,175 Tumor cell seeding along the needle track has not been reported in early series of RFA treatment for HCC, although the follow-up in most of these series was relatively short. 160–171 However, a recent study reported the occurrence of biopsy-proven needle track tumor seeding 4 to 18 months after RFA in 4 of 32 (12.5%) patients with solitary HCCs 5 cm or smaller. 176 The risk of tumor cell seeding was found to be associated with subcapsular location, poorly differentiated tumor, and a high baseline serum alpha-fetoprotein level. This incidence of needle track tumor seeding was much higher than that of 0.6% to 1% reported after PEI for HCC, 90,93 and the authors suggested that it may be related to the larger needles (15–18G) of the RFA electrode compared with the fine needle used for PEI, or release of tumor cells associated with intratumoral explosion resulting from the increase in temperature during the ablation process.
Because RFA is a relatively new treatment modality for HCC, the data in the literature are mostly preliminary observations with a short duration of follow-up. Recently, survival results have become available from centers that pioneered RFA therapy for HCC. Table 3 summarizes reported studies of RFA for HCC with well-documented follow-up results. Complete necrosis was achieved in 88% to 100%, although in some situations a second session of RFA was required to ablate residual tumor tissue after the first session. Like other local ablative therapies, intrahepatic recurrence of tumor is common, ranging from 20% to 49% after a mean or median follow-up of 9 to 34 months. 160,161,163,164,170 However, the majority of recurrent tumors are new lesions that are probably related to multicentric hepatocarcinogenesis of cirrhosis. In the largest series of RFA for HCC reported so far, the overall recurrence rate was 49% after a median follow-up of 19 months among 110 patients, but local recurrence at the RFA site occurred in only 4 (3.6%) patients, all with original tumors larger than 4 cm. 170 This low recurrence rate compared favorably with that of 16% to 38% reported after PEI. 97,113,115 However, another study of percutaneous RFA for small HCCs (≤3.5 cm) reported a local recurrence rate of 20% among 88 patients after a mean follow-up of 34 months. 177 In the latter study, the authors also found that the local recurrence rate was lower in patients treated with an expandable probe than those treated with a conventional probe (14% vs. 29%). Thus far, only two studies have provided a long-term survival rate. The 5-year survival rate after RFA was 40% for HCCs 3 cm or smaller in one report, 160 and 33% for HCC 3.5 cm or smaller in another report. 177
Table 3. FOLLOW-UP RESULTS AFTER RADIOFREQUENCY ABLATION (RFA) THERAPY FOR HCC
P, percutaneous; L, laparoscopic; I, intraoperative.
* Figures indicate median follow-up.
† Figures in parenthesis indicate the local recurrence rate.
Recently, RFA has been compared with other local ablative therapies, and the preliminary data seem to suggest that RFA may be a superior option. Compared with PEI, necrosis induced by RFA is more predictable, and treatment by a single session is sufficient in most patients with small HCCs. A prospective nonrandomized study comparing RFA in 42 patients and PEI in 44 patients with HCCs 3 cm or smaller showed that RAF achieved a higher complete necrosis rate (90% vs. 80%) with fewer treatment sessions (mean 1.2 vs. 4.8 sessions), but RFA was associated with a higher complication rate (12% vs. 0%). 175 Preliminary data from two randomized trials comparing RFA and PEI for small HCCs also indicated that the treatment time was significantly shorter and the radicality of tumor ablation was superior with RFA. 178,179 RFA was compared with cryotherapy in a prospective nonrandomized study involving 146 patients with unresectable HCC or other hepatic malignancies. 131 The study showed that RFA resulted in a significantly lower complication rate (3.3% vs. 40.7%) and local recurrence rate (2.2% vs. 13.6%) than cryoablation after a median follow-up of 15 months in two groups of patients with comparable tumor size (median 3.8 cm vs. 3.6 cm). A retrospective study reported significantly reduced bleeding and thrombocytopenia and a shorter hospital stay after RFA compared with cryoablation, but the local recurrence rate was significantly higher after RFA than cryoablation for tumors larger than 3 cm (38% vs. 17%). 180 However, in both studies the majority of patients had metastatic tumors rather than HCC. The clinical efficacy of RFA has not been compared with MCT, but an experimental study showed that RFA may be superior to MCT in its capacity to produce a larger area of coagulative necrosis. 181 Randomized controlled studies are needed to evaluate the potential benefit of RFA over other local ablative therapies in patients with HCC.
COMBINED TRANSARTERIAL AND LOCAL ABLATIVE THERAPIES
Apart from the technologic developments aiming to increase the efficacy of ablative therapy for large HCCs, recent research interest has also been directed toward combined transarterial and local ablative therapies. A combination of TACE and PEI has been used to overcome the limitations of each for the treatment of large HCCs. TACE causes tumor necrosis and disruption of intratumoral septa, which facilitates ethanol diffusion during the subsequent PEI to destroy the residual viable tissue. The washout of ethanol is delayed after arterial embolization, resulting in longer retention and tumoricidal effect. Compared with repeated TACE, PEI after single TACE avoids problems such as the development of collateral blood supply to the tumor, resistance to cytotoxic drugs, and progressive liver damage that can reduce the effectiveness of repeated TACE. Several retrospective studies comparing combined TACE and PEI with TACE alone for HCCs larger than 3 cm have shown an improved therapeutic response and long-term survival with combination therapy, and no major treatment-related complications have been reported. 182–184 A randomized trial comparing combined TACE and PEI with repeated TACE alone in 53 patients with HCCs 3.1 to 8 cm showed that the combination therapy resulted in a significantly higher complete response rate and a better recurrence-free survival. 185 In a more recent prospective study of combined TACE and PEI treatment for 85 patients with HCCs 3 to 8 cm, a complete response was obtained in 82% of patients. 186 The 1-, 3-, and 5-year survival rates were 92%, 69%, and 47%, respectively, which appear to be comparable to what can be achieved with hepatic resection for such patients. No randomized trial comparing combined TACE and PEI with PEI alone has been published, but a retrospective study showed that the survival result of the combination therapy was superior to that of PEI alone. 184 Retrospective studies have also suggested that combined TACE and PEI gives better survival results than TACE alone even for postresection small recurrent HCCs. 187,188
Another novel approach of combination locoregional therapy is transarterial embolization before local hyperthermic ablation, because the cooling effect of blood flow is one of the main limiting factors for heat ablation. The main blood supply of HCC is derived from the artery. Hence, transarterial occlusion of its blood supply before heat ablation may significantly increase the size of the ablation lesion. Buscarini et al 189 and Rossi et al 190 have recently reported the use of RFA to ablate large HCCs (>3.5 cm) after interruption of the tumor’s arterial blood supply by segmental embolization or balloon occlusion of the hepatic artery. In a study of 62 patients with HCCs 3.5 to 8.5 cm treated by this approach, Rossi et al 190 reported no major complications and a 90% complete response rate. A 1-year survival rate of 87% was reported, but the 1-year local recurrence and overall intrahepatic recurrence rates were 19% and 45%, respectively. Seki et al 191 performed percutaneous MCT after TACE in 18 patients with HCCs 2 to 3 cm and found complete necrosis of tumors in 17 (94%) patients with no local recurrence after a mean follow-up of 21.5 months. Alternatively, TACE may be used after thermoablation of large HCCs to eradicate the peripheral viable tissue. Pacella et al 192 reported a complete tumor necrosis rate of 90% and a 3-year local recurrence rate of 7% among 30 patients with large HCCs (3.5–9.6 cm) treated by interstitial laser ablation followed by TACE. Large clinical trials and long-term outcome data are needed to verify the efficacy of this promising strategy for large HCCs. Apart from transarterial occlusion of blood supply, transarterial chemotherapy may also augment the benefit of thermal ablation of malignant liver tumors. Kainuma et al 193 reported combined RFA and transarterial infusion chemotherapy to treat hepatic metastasis from colorectal cancer. The role of combined chemotherapy and thermal ablation for patients with unresectable HCC also deserves evaluation.
DISCUSSION
The recent development of a wide array of locoregional therapeutic options provides patients with HCC the opportunity for more effective management, but it also poses major challenges to hepatic surgeons. Surgeons are increasingly confronted with the dilemma of selecting the best option among resection, transplantation, transarterial, and local ablative therapies for a patient with HCC localized to the liver. Surgeons are also faced with the challenge of adopting new ablative therapies in the surgical management of HCC. During the past decade, research interest in locoregional therapy for HCC has led to the publication of numerous studies on the various therapeutic modalities, each with its own advocates. The vast amount of fragmented data in the literature are difficult to interpret, especially with the diverse opinions regarding the advantages of one treatment modality over the others. A critical appraisal of the data not only aids in the appropriate choice of therapy, but also provides insights into the research opportunities for surgeons in this exciting area of development. To this end, we have presented a systematic review of the current results of transarterial and local ablative therapies for HCC.
Although a few retrospective studies have suggested that the survival results after TACE or PEI may be comparable to that after hepatectomy for resectable HCCs, 48,96,103,104 the uncertainty of tumor necrosis with such techniques renders them a second choice after resection. It is unlikely that randomized trials comparing TACE or PEI with resection will ever be performed, especially with the availability of newer ablative therapies that appear to be more effective in inducing tumor necrosis. Cryotherapy or hyperthermic ablation using MCT, laser, or RFA produces a more predictable area of necrosis that encompasses not only the tumor tissue but also the capsule and a margin of surrounding liver tissue. In a sense, this is equivalent to a limited hepatic resection, and hence these therapies have been proposed to be curative for small HCCs. 143,169 Even though these new modalities can produce complete necrosis in 80% to 90% of HCCs less than 3 to 5 cm, it is difficult to ensure complete ablation. Further, satellite nodules are frequently present around the main tumor and can be cleared only by an anatomic resection. The possibility of needle track tumor seeding with percutaneous ablative therapies further jeopardizes the chance of cure. Hence, it is unlikely that these thermoablative therapies can replace resection as the curative treatment for HCC.
A comparison of long-term survival results will ultimately be required to define the role of ablative therapies and hepatic resection in the management of small HCCs. Currently, there are limited data on the long-term survival with the new ablative modalities. A study of cryoablation for patients with HCCs smaller than 5 cm reported a 5-year survival rate of 53%, 129 and another study documented a 5-year survival rate of 40% after RFA for HCCs smaller than 3 cm. 160 Now that a near-zero death rate and a 5-year survival rate of about 60% can be achieved with hepatectomy for HCCs 5 cm or smaller, 107,108 it is hard to conceive that any ablative therapy will be superior to resection. Liver transplantation produces similar or even better survival results for HCCs 5 cm or smaller than resection and hence should be the treatment of choice for patients with small HCCs (≤5 cm) and Child C cirrhosis. 9,10 Two retrospective studies showed similar survival results with MCT and resection for HCC, 144,149 but no other studies have compared cryotherapy or hyperthermic ablative therapies with surgical resection. When more favorable long-term survival data from these local ablative treatments become available, it may be justified to conduct randomized trials comparing local ablative therapies and hepatic resection for small HCCs (<3–5 cm) in cirrhotic patients. A recent study from our department showed that hepatic resection improved the quality of life of patients with HCC. 194 Thus far, none of the studies on locoregional therapies for HCC have alluded to the quality of life outcome. It would be important to evaluate quality of life as an outcome in future clinical trials, in addition to survival and disease recurrence rates, because a perceived benefit of locoregional therapy is its minimal invasiveness.
Although it is less arguable that surgical resection or transplantation remains the first-choice treatment for HCC, the choice of locoregional treatment for patients who are not candidates for resection or transplantation is a matter of controversy. The proper selection of locoregional therapy depends on careful evaluation of the tumor and liver function status as well as an updated knowledge of the results of various treatment modalities. For patients with large or multifocal HCCs and reasonable liver function, TACE is still considered an effective treatment despite the negative results of randomized trials. One lesson to learn from these trials is the need for more careful selection of patients with a better chance of tumor response and a lower risk of liver failure to achieve a favorable benefit–risk ratio. The optimal TACE technique and regimen are also crucial for a favorable outcome. In particular, it is important to tailor repetition of TACE based on tumor response. Contrast-enhanced CT scan or MRI should be performed regularly, preferably after each TACE treatment, to monitor the size of the tumor and any new tumor nodules. TACE is not recommended for patients with massive HCCs, main portal vein thrombosis, or Child C cirrhosis, who should be considered for new clinical trial protocols. Transarterial internal radiotherapy appears to produce survival results similar to TACE for advanced HCC, but its application is limited by high cost and restricted availability.
For patients with solitary HCCs smaller than 5 cm or multiple small tumors up to three in number, local ablation should be offered when surgical resection or transplantation is not possible. PEI has been the standard therapy for small HCCs, but preliminary data from recent studies suggest that hyperthermic ablation by MCT or RFA is superior in the radicality of tumor ablation and has the additional advantage of requiring only one or two sessions for complete tumor ablation. It is likely that hyperthermic ablation will replace PEI as the treatment of choice for unresectable small HCCs, but randomized trials are required to support this contention. PEI will remain a useful treatment for lesions located in areas unsafe for hyperthermic ablation, such as those near major bile duct or hilar structures. Further, PEI will continue to be a treatment option in places where the more expensive heat ablation techniques are not available. Cryotherapy offers effective necrosis of large HCCs and has the advantage that the ablation process can be monitored precisely by real-time ultrasound. However, the daunting complication of cryoshock phenomenon and troublesome bleeding from the cracking of liver parenchyma have rendered it a less popular choice than heat ablation.
Figure 4 summarizes the roles of various locoregional therapies in the management of HCC based on the current evidence. The choice of therapy in a particular center may be influenced by the availability of treatment modalities and local expertise. The roles of the different locoregional treatment modalities may change with further development of technology and availability of data from future prospective randomized trials.
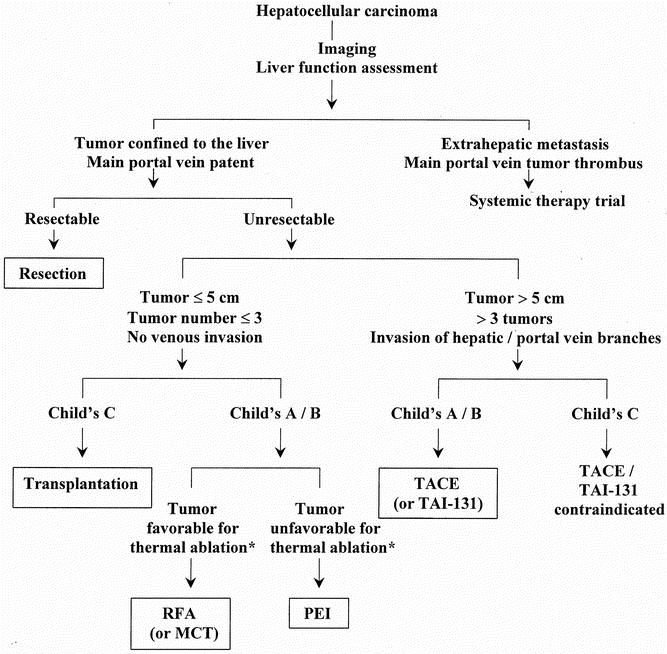
Figure 4. Algorithm for management of hepatocellular carcinoma. MCT, microwave coagulation therapy; PEI, percutaneous ethanol injection; RFA, radiofrequency ablation; TACE, transarterial chemoembolization; TAI-131, transarterial iodine-131. *Current evidence suggests that thermal ablation is superior to ethanol injection, but tumors near hilar vessels or major bile ducts that are not suitable for thermal ablation may be treated with ethanol injection.
The advent of the new ablative therapies for HCC is bound to change the surgeon’s role in the management of HCC. These novel techniques provide surgeons with a challenge, or rather an opportunity, to become more actively involved in the management of patients with unresectable HCC who are traditionally treated by gastroenterologists or interventional radiologists. Although the hyperthermic ablative therapies can be performed percutaneously, these techniques are useful in the hands of surgeons. Laparoscopic or surgical ablation may be necessary in patients with a high risk of bleeding from coagulopathy, deeply located lesions not accessible to percutaneous puncture, or superficial nodules adjacent to diaphragm or bowel. Apart from these specific indications, a laparoscopic or surgical approach has some general advantages over percutaneous ablation of HCC. First, laparoscopy or laparotomy allows detection of peritoneal metastasis and extrahepatic invasion that may not be diagnosed even with extensive preoperative imaging. 195 In a recent study, evidence of extrahepatic disease was identified in 7 of 59 (12%) patients undergoing laparoscopy before RFA for unresectable primary or secondary liver tumors, and such a finding logically led to abortion of the ablative procedure. 180 Second, intraoperative or laparoscopic ultrasound using a high-frequency transducer placed directly over the liver surface allows detection of small tumor nodules not identified on preoperative imaging. The ablation of these additional tumor nodules is important if the goal of the treatment is potential cure. Such additional nodules are fairly common, as shown in a recent study that identified new tumor lesions in 5 (18.5%) of 27 patients with HCCs 5 cm or smaller undergoing laparoscopic ultrasonography before RFA. 167 Third, intraoperative ultrasound provides better visualization of the tumor and allows a more precise placement of the ablation probe, thus optimizing the chance of complete ablation with a clear margin. This is further enhanced by the freedom of probe insertion at different angles with laparoscopic or open approaches, with mobilization of the liver if necessary. Curley et al 170 found a 100% complete ablation rate in 65 HCCs treated by RFA during laparotomy or laparoscopy, whereas incomplete ablation was observed in 7.1% (6/84) of HCCs treated by percutaneous RFA. One probable explanation was the better resolution of the tumors and RFA treatment provided by intraoperative ultrasound. Finally, with the surgical approach, the Pringle maneuver can be used to temporarily interrupt the portal vein and hepatic artery blood flow to facilitate heat ablation of large hypervascular tumors and tumors near major blood vessels. For tumors near a major bile duct, intraductal cooling by cold perfusion via a choledochal incision has been reported to allow intraoperative RFA without bile duct damage. 196 The laparoscopic approach is particularly appealing because it combines the advantages of the surgical approach with those of the percutaneous approach, namely minimal invasiveness and a low complication rate. The Pringle maneuver can be applied laparoscopically by snaring a loop around the hepatoduodenal ligament. 197 A current limitation of the laparoscopic approach is the restricted maneuverability of the ultrasound and ablation probes, which may limit the angle and accuracy of needle probe insertion into the tumor. However, this problem may be circumvented with further technological developments. No direct comparison of percutaneous and laparoscopic approaches for ablation of HCC has been reported. There are sufficient grounds to suggest that the laparoscopic approach should be used more often in heat ablation for HCC by RFA, MCT, or laser, especially for tumors being treated with a curative intent. This underscores the need for surgeons to acquire the techniques of local ablation for HCC. The percutaneous approach will continue to have an important role in ablation of small HCCs in patients in whom general anesthesia would pose a high risk. The easy repeatability of percutaneous ablation also makes it an attractive approach for recurrent tumors after previous local ablation or resection of HCC. Early detection of recurrent tumors while they are small and few in number is important to allow repeat ablative treatment. Hence, regular surveillance for local or new intrahepatic recurrences should be performed every 3 months for patients after ablation of HCC, as for those after hepatic resection. Contrast-enhanced CT or MRI scans are currently the most sensitive imaging modalities in detecting recurrence after thermoablative therapy of HCC. 198
Apart from their role in the management of unresectable HCC, the diverse options of locoregional therapy provide useful adjuncts in the management of patients with resectable or transplantable disease. The role of neoadjuvant or adjuvant TACE and transarterial radiotherapy in preventing recurrence after resection of HCC deserves further investigation by properly designed randomized trials. Intraoperative local ablation of small tumor nodules can be combined with resection of a large tumor to increase the chance of curative treatment. 132,143 Thermoablative techniques also provide novel options for the control of postresection intrahepatic recurrence. TACE and PEI have been the mainstay of treatment for unresectable intrahepatic recurrent HCC. 116 It is likely that thermoablative therapies will be used with increasing frequency for recurrent tumors after hepatic resection. There are few data on the use of thermoablative therapies for recurrent HCC; this is an important area of future research for surgeons. TACE has been the bridging therapy used by many centers for patients waiting for liver transplantation. 9,10 However, with the advent of novel hyperthermic ablation therapies for HCC, some centers have recently switched to percutaneous MCT or RFA as a pretransplant treatment. 199,200 As stated elsewhere, local ablative therapies carry a risk of tumor cell seeding along the needle track, which may render the disease incurable. A recent report of an alarmingly high incidence of needle track tumor seeding after percutaneous RFA should prompt caution in the use of such therapies in transplant candidates. 176 Further studies with sufficient long-term follow-up are needed on the risk of needle track tumor seeding associated with these new thermoablative therapies.
In this era of rapidly evolving technologies, it is of paramount importance for surgeons to keep abreast of the latest development in local ablative techniques for HCC. Locoregional therapies should be considered complementary rather than competitive with surgical treatment because they are useful adjuncts for the preoperative or postoperative management of patients with resectable or transplantable HCC. Similarly, transarterial and local ablative therapies should not be regarded as mutually exclusive, because combinations of the two may produce a synergistic effect. Further new technologies for ablation of liver tumors and monitoring of the process are on the horizon. For example, high-intensity focused ultrasound has been shown to be effective in ablating experimental liver tumors, 201 and recently it has been applied in the management of patients with HCC. 202 Interstitial photon beam has been shown to generate sharply demarcated and completely necrosed hepatic lesions not affected by the blood flow of nearby major vessels. 203 Real-time MRI 153 or three-dimensional ultrasound 204 may provide more precise guidance of probe placement and monitoring of the ablative process. However, the judgment of surgeons is probably more important than the technology in determining patient outcomes. Careful consideration is required not only in selecting the best treatment modality for patients, but also in choosing the best approach for the treatment. Randomized controlled trials must be performed to resolve such critical issues as the best treatment modalities for different groups of patients with different tumor and liver function status; the relative benefits of laparoscopic versus percutaneous approach in local ablative therapies; the role of neoadjuvant or adjuvant locoregional therapy in patients with resectable HCC; and the optimal pretransplant therapy of HCC in transplant candidates. Surgeons should play a key role in the multidisciplinary management of patients with HCC. Hence, surgeons must master the techniques and knowledge of novel local ablative therapies for HCC and actively pursue research in this area in conjunction with gastroenterologists and radiologists.
Footnotes
Correspondence: Ronnie Tung-Ping Poon, MS, FRCS, Department of Surgery, University of Hong Kong Medical Centre, Queen Mary Hospital, 102 Pokfulam Road, Hong Kong, China.
E-mail: poontp@hkucc.hku.hk
Accepted for publication December 10, 2001.
References
- 1.Bosch FX, Ribes J, Borras J. Epidemiology of primary liver cancer. Semin Liver Dis 1999; 19: 271–285. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med 1999; 340: 745–750. [DOI] [PubMed] [Google Scholar]
- 3.Taylor-Robinson SD, Foster GR, Arora S, et al. Increase in primary liver cancer in the UK, 1979–94. Lancet 1997; 350: 1142–1143. [DOI] [PubMed] [Google Scholar]
- 4.Fan ST, Lo CM, Liu CL, et al. Hepatectomy for hepatocellular carcinoma: toward zero hospital deaths. Ann Surg 1999; 229: 322–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Torzilli G, Makuuchi M, Inoue K, et al. No-mortality liver resection for hepatocellular carcinoma in cirrhotic and noncirrhotic patients. Is there a way? A prospective analysis of our approach. Arch Surg 1999; 134: 984–992. [DOI] [PubMed] [Google Scholar]
- 6.Poon RTP, Fan ST, Lo CM, et al. Improving survival results after resection of hepatocellular carcinoma. A prospective study of 377 patients over 10 years. Ann Surg 2001; 234: 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colella G, Bottelli R, De Carlis L, et al. Hepatocellular carcinoma: Comparison between liver transplantation, resective surgery, ethanol injection and chemoembolization. Transplant Int 1998; 11 (suppl 1): S193–196. [DOI] [PubMed] [Google Scholar]
- 8.Fong Y, Sun RL, Jarnagin W, et al. An analysis of 412 cases of hepatocellular carcinoma at a Western center. Ann Surg 1999; 229: 790–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mor E, Kaspa RT, Sheiner P, Schwartz M. Treatment of hepatocellular carcinoma associated with cirrhosis in the era of liver transplantation. Ann Intern Med 1998; 129: 643–653. [DOI] [PubMed] [Google Scholar]
- 10.Bismuth H, Majno PE, Adam R. Liver transplantation for hepatocellular carcinoma. Semin Liver Dis 1999; 19: 311–322. [DOI] [PubMed] [Google Scholar]
- 11.Nerenstone SR, Ihde DC, Friedman MA. Clinical trials in primary hepatocellular carcinoma: current status and future directions. Cancer Treat Rev 1988; 15: 1–31. [DOI] [PubMed] [Google Scholar]
- 12.Mathurin P, Rixe O, Carbonell N, et al. Review article: Overview of medical treatments in unresectable hepatocellular carcinoma—an impossible meta-analysis? Aliment Pharmacol Ther 1998; 12: 111–126. [DOI] [PubMed] [Google Scholar]
- 13.Llovet JM, Sala M, Castells L, et al. Randomized controlled trial of interferon treatment for advanced hepatocellular carcinoma. Hepatology 2000; 31: 54–58. [DOI] [PubMed] [Google Scholar]
- 14.Castells A, Bruix J, Bru C, et al. Treatment of hepatocellular carcinoma with tamoxifen: a double-blind placebo-controlled trial in 120 patients. Gastroenterology 1995; 109: 917–922. [DOI] [PubMed] [Google Scholar]
- 15.Liu CL, Fan ST, Ng IOL, et al. Treatment of advanced hepatocellular carcinoma with tamoxifen and the correlation with expression of hormone receptors: a prospective randomized study. Am J Gastroenterol 2000; 95: 218–222. [DOI] [PubMed] [Google Scholar]
- 16.Kajanti M, Pyrhonen S, Mantyla M, et al. Intra-arterial and intravenous use of 4-epidoxorubicin combined with 5-fluorouracil in primary hepatocellular carcinoma. A randomized comparison. Am J Clin Oncol 1992; 15: 37–40. [DOI] [PubMed] [Google Scholar]
- 17.Tzoracoleftherakis EE, Spiliotis JD, Kyriakopoulou T, et al. Intra-arterial versus systemic chemotherapy for non-operable hepatocellular carcinoma. Hepato-Gastroenterology 1999; 46: 1122–1125. [PubMed] [Google Scholar]
- 18.Bhattacharya S, Davidson B, Dhillon AP. Blood supply of early hepatocellular carcinoma. Semin Liver Dis 1995; 15: 390–401. [DOI] [PubMed] [Google Scholar]
- 19.Bruix J, Castells A, Montanya X, et al. Phase II study of transarterial embolization in European patients with hepatocellular carcinoma: need for controlled trials. Hepatology 1994; 20: 643–650. [PubMed] [Google Scholar]
- 20.Matsui O, Kadoya M, Yoshikawa J, et al. Subsegmental transcatheter arterial embolization for small hepatocellular carcinomas: local therapeutic effect and 5-year survival rate. Cancer Chemother Pharmacol 1994; 33 (suppl): S84–88. [DOI] [PubMed] [Google Scholar]
- 21.Lin DY, Liaw YF, Lee TY, et al. Hepatic arterial embolization in patients with unresectable hepatocellular carcinoma. A randomized controlled trial. Gastroenterology 1988; 94: 453–456. [DOI] [PubMed] [Google Scholar]
- 22.Bruix J, Llovet JM, Castells A, et al. Transarterial embolization versus symptomatic treatment in patients with advanced hepatocellular carcinoma: results of a randomized, controlled trial in a single institution. Hepatology 1998; 27: 1578–1583. [DOI] [PubMed] [Google Scholar]
- 23.Farinati F, Rinaldi M, Gianni S, et al. Transcatheter arterial embolization in hepatocellular carcinoma [letter]. Hepatology 1998; 28: 1441–1443. [DOI] [PubMed] [Google Scholar]
- 24.Zhu LX, Wang GS, Fan ST. Spontaneous rupture of hepatocellular carcinoma. Br J Surg 1996; 83: 602–607. [DOI] [PubMed] [Google Scholar]
- 25.Bhattacharya S, Novell JR, Winslet MC, et al. Iodized oil in the treatment of hepatocellular carcinoma. Br J Surg 1994; 81: 1563–1571. [DOI] [PubMed] [Google Scholar]
- 26.Yoshikawa M, Saisho H, Ebara M, et al. A randomized trial of intrahepatic arterial infusion of 4′-epidoxorubicin with Lipiodol versus 4′-epidoxorubicin alone in the treatment of hepatocellular carcinoma. Cancer Chemother Pharmacol 1994; 33 (suppl): S149–152. [DOI] [PubMed] [Google Scholar]
- 27.Hatanaka Y, Yamashita Y, Takahashi M, et al. Unresectable hepatocellular carcinoma: analysis of prognostic factors in transcatheter management. Radiology 1995; 195: 747–752. [DOI] [PubMed] [Google Scholar]
- 28.Nishimine K, Uchida H, Matsuo N, et al. Segmental transarterial chemoembolization with Lipiodol mixed with anticancer drugs for nonresectable hepatocellular carcinoma: follow-up CT and therapeutic results. Cancer Chemother Pharmacol 1994; 33 (suppl): S60–68. [DOI] [PubMed] [Google Scholar]
- 29.Ikeda K, Kumada H, Saitoh S, et al. Effect of repeated transcatheter arterial embolization on the survival time in patients with hepatocellular carcinoma. An analysis by the Cox proportional hazard model. Cancer 1991; 68: 2150–2154. [DOI] [PubMed] [Google Scholar]
- 30.Ernst O, Sergent G, Mizrahi D, et al. Treatment of hepatocellular carcinoma by transcatheter arterial chemoembolization: comparison of planned periodic chemoembolization and chemoembolization based on tumor response. AJR Am J Roentgenol 1999; 172: 59–64. [DOI] [PubMed] [Google Scholar]
- 31.Lee HS, Kim JS, Choi IJ, et al. The safety and efficacy of transcatheter arterial chemoembolization in the treatment of patients with hepatocellular carcinoma and main portal vein obstruction: a prospective controlled study. Cancer 1997; 79: 2087–2094. [PubMed] [Google Scholar]
- 32.Poon RTP, Ngan H, Lo CM, et al. Transarterial chemoembolization for inoperable hepatocellular carcinoma and postresection intrahepatic recurrence. J Surg Oncol 2000; 73: 109–114. [DOI] [PubMed] [Google Scholar]
- 33.Hsieh MY, Chang WY, Wang LY, et al. Treatment of hepatocellular carcinoma by transcatheter arterial chemoembolization and analysis of prognostic factors. Cancer Chemother Pharmacol 1992; 31 (suppl): S82–85. [DOI] [PubMed] [Google Scholar]
- 34.Yang CF, Ho YJ. Transcatheter arterial chemoembolization for hepatocellular carcinoma. Cancer Chemother Pharmacol 1992; 31 (suppl): S86–88. [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto K, Masuzawa M, Kato M, et al. Analysis of prognostic factors in patients with hepatocellular carcinoma treated by transcatheter arterial embolization. Cancer Chemother Pharmacol 1992; 31 (suppl): S77–81. [DOI] [PubMed] [Google Scholar]
- 36.Bismuth H, Morino M, Sherlock D, et al. Primary treatment of hepatocellular carcinoma by arterial chemoembolization. Am J Surg 1992; 163: 387–394. [DOI] [PubMed] [Google Scholar]
- 37.Ngan H, Lai CL, Fan ST, et al. Treatment of inoperable hepatocellular carcinoma by transcatheter arterial chemoembolization using an emulsion of cisplatin in iodized oil and gelfoam. Clin Radiol 1993; 47: 315–320. [DOI] [PubMed] [Google Scholar]
- 38.Mondazzi L, Bottelli R, Brambilla G, et al. Transarterial oily chemoembolization for the treatment of hepatocellular carcinoma: a multivariate analysis of prognostic factors. Hepatology 1994; 19: 1115–1123. [PubMed] [Google Scholar]
- 39.Vetter D, Wenger JJ, Bergier JM, et al. Transcatheter oily chemoembolization in the management of advanced hepatocellular carcinoma in cirrhosis: results of a Western comparative study in 60 patients. Hepatology 1991; 13: 427–433. [PubMed] [Google Scholar]
- 40.Bronowicki JP, Vetter D, Dumas F, et al. Transcatheter oily chemoembolization for hepatocellular carcinoma. A 4-year study of 127 French patients. Cancer 1994; 74: 16–24. [DOI] [PubMed] [Google Scholar]
- 41.Stefanini GF, Amorati P, Biselli M, et al. Efficacy of transarterial targeted treatments on survival of patients with hepatocellular carcinoma: an Italian experience. Cancer 1995; 75: 2427–2434. [DOI] [PubMed] [Google Scholar]
- 42.Pelletier G, Roche A, Ink O, et al. A randomized trial of hepatic arterial chemoembolization in patients with unresectable hepatocellular carcinoma. J Hepatol 1990; 11: 181–184. [DOI] [PubMed] [Google Scholar]
- 43.Groupe d’Etude et de Traitment du Carcinome Hepatocellulaire. A comparison of Lipiodol chemoembolization and conservative treatment for unresectable hepatocellular carcinoma. N Engl J Med 1995; 332: 1256–1261. [DOI] [PubMed] [Google Scholar]
- 44.Pelletier G, Ducreux M, Gay F, et al. Treatment of unresectable hepatocellular carcinoma with Lipiodol chemoembolization: a multicenter randomized trial. Groupe CHC. J Hepatol 1998; 29: 129–134. [DOI] [PubMed] [Google Scholar]
- 45.Okada S. Transcatheter arterial embolization for advanced hepatocellular carcinoma: the controversy continues. Hepatology 1998; 27: 1743–1744. [DOI] [PubMed] [Google Scholar]
- 46.Ngan H, Lai CL, Fan ST, et al. Transcatheter arterial chemoembolization in inoperable hepatocellular carcinoma: four-year follow-up. J Vasc Interv Radiol 1996; 7: 419–425. [DOI] [PubMed] [Google Scholar]
- 47.Yoshimi F, Nagao T, Inoue S, et al. Comparison of hepatectomy and transcatheter arterial chemoembolization for the treatment of hepatocellular carcinoma: necessity for prospective randomized trial. Hepatology 1992; 16: 702–706. [DOI] [PubMed] [Google Scholar]
- 48.Bronowicki JP, Boudjema K, Chone L, et al. Comparison of resection, liver transplantation and transcatheter oily chemoembolization in the treatment of hepatocellular carcinoma. J Hepatol 1996; 24: 293–300. [DOI] [PubMed] [Google Scholar]
- 49.Llado L, Virgili J, Figueras J, et al. A prognostic index of the survival of patients with unresectable hepatocellular carcinoma after transcatheter arterial chemoembolization. Cancer 2000; 88: 50–57. [DOI] [PubMed] [Google Scholar]
- 50.Vogl TJ, Trapp M, Schroeder H, et al. Transarterial chemoembolization for hepatocellular carcinoma: volumetric and morphologic CT criteria for assessment of prognosis and therapeutic success-results from a liver transplantation center. Radiology 2000; 214: 349–357. [DOI] [PubMed] [Google Scholar]
- 51.Poon RTP, Fan ST, Lo CM, et al. Intrahepatic recurrence after curative resection of hepatocellular carcinoma. Long-term results of treatment and prognostic factors. Ann Surg 1999; 229: 216–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakoa N, Kamino K, Miura K, et al. Recurrent hepatocellular carcinoma after partial hepatectomy: value of treatment with transcatheter arterial chemoembolization. AJR Am J Roentgenol 1991; 156: 1177–1179. [DOI] [PubMed] [Google Scholar]
- 53.Ouchi K, Matsubara S, Fukuhara K, et al. Recurrence of hepatocellular carcinoma in the liver remnant after hepatic resection. Am J Surg 1993; 166: 270–273. [DOI] [PubMed] [Google Scholar]
- 54.Shimada M, Takenaka K, Gion T, et al. Prognosis of recurrent hepatocellular carcinoma: a 10-year surgical experience in Japan. Gastroenterology 1996; 111: 720–726. [DOI] [PubMed] [Google Scholar]
- 55.Imaoka S, Sasaki Y, Shibata T, et al. A pre-operative chemoembolization therapy using Lipiodol, cisplatin and gelatin sponge for hepatocellular carcinoma. Cancer Chemother Pharmacol 1989; 23: S126–128. [DOI] [PubMed] [Google Scholar]
- 56.Majno PE, Adam R, Bismuth H, et al. Influence of preoperative transarterial Lipiodol chemoembolization on resection and transplantation for hepatocellular carcinoma in patients with cirrhosis. Ann Surg 1997; 226: 688–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Uchida M, Kohno H, Kubota H, et al. Role of preoperative transcatheter arterial oily chemoembolization for resectable hepatocellular carcinoma. World J Surg 1996; 20: 326–331. [DOI] [PubMed] [Google Scholar]
- 58.Harada T, Matsuo K, Inoue T, et al. Is preoperative hepatic arterial chemoembolization safe and effective for hepatocellular carcinoma? Ann Surg 1996; 224: 4–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu CC, Ho YZ, Ho WL, et al. Preoperative transcatheter arterial chemoembolization for resectable large hepatocellular carcinoma: a reappraisal. Br J Surg 1995; 82: 122–126. [DOI] [PubMed] [Google Scholar]
- 60.Yamasaki S, Hasegawa H, Kinoshita H, et al. A prospective randomized trial of the preventive effect of pre-operative transcatheter arterial embolization against recurrence of hepatocellular carcinoma. Jpn J Cancer Res 1996; 87: 206–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang Z, Liu Q, He J, et al. The effect of preoperative transcatheter hepatic arterial chemoembolization on disease-free survival after hepatectomy for hepatocellular carcinoma. Cancer 2000; 89: 2606–2612. [PubMed] [Google Scholar]
- 62.Gerunda GE, Neri D, Merenda R, et al. Role of transarterial chemoembolization before liver resection for hepatocarcinoma. Liver Transplant 2000; 6: 619–626. [DOI] [PubMed] [Google Scholar]
- 63.Nakano H, Yoshida K, Takeuchi S, et al. Liver scintigraphy is useful for selecting candidates for preoperative transarterial chemoembolization among patients with hepatocellular carcinoma and chronic liver disease. Am J Surg 1999; 178: 385–389. [DOI] [PubMed] [Google Scholar]
- 64.Izumi R, Shimizu K, Iyobe T, et al. Postoperative adjuvant hepatic arterial infusion of Lipiodol containing anticancer drugs in patients with hepatocellular carcinoma. Hepatology 1994; 20: 295–301. [PubMed] [Google Scholar]
- 65.Kohno H, Nagasue N, Hayashi T, et al. Postoperative adjuvant chemotherapy after radical resection for hepatocellular carcinoma (HCC). Hepato-Gastroenterology 1996; 43: 1405–1409. [PubMed] [Google Scholar]
- 66.Lai ECS, Lo CM, Fan ST, et al. Postoperative adjuvant chemotherapy after curative resection of hepatocellular carcinoma: a randomized controlled trial. Arch Surg 1998; 133: 183–188. [DOI] [PubMed] [Google Scholar]
- 67.Troisi R, Defreyne L, Hesse UJ, et al. Multimodality treatment for hepatocellular carcinoma on cirrhosis: the role of chemoembolization and alcoholization before liver transplantation. Clin Transplant 1998; 12: 313–319. [PubMed] [Google Scholar]
- 68.Oldhafer KJ, Chavan A, Fruhauf NR, et al. Arterial chemoembolization before liver transplantation in patients with hepatocellular carcinoma: Marked tumor necrosis but no survival benefit? J Hepatol 1998; 29: 953–959. [DOI] [PubMed] [Google Scholar]
- 69.Cherqui D, Piedbois P, Pierga JY, et al. Multimodal adjuvant treatment and liver transplantation for advanced hepatocellular carcinoma. A pilot study. Cancer 1994; 73: 2721–2726. [DOI] [PubMed] [Google Scholar]
- 70.Schwartz ME, Sung M, Mor E, et al. A multidisciplinary approach to hepatocellular carcinoma in patients with cirrhosis. J Am Coll Surg 1995; 180: 596–603. [PubMed] [Google Scholar]
- 71.Cheng SH, Lin YM, Chuang VP, et al. A pilot study of three-dimensional conformal radiotherapy in unresectable hepatocellular carcinoma. J Gastroenterol Hepatol 1999; 14: 1025–1033. [DOI] [PubMed] [Google Scholar]
- 72.Matsuzaki Y, Osuga T, Chiba T, et al. New effective treatment using proton irradiation for unresectable hepatocellular carcinoma. Intern Med 1995; 34: 302–304. [DOI] [PubMed] [Google Scholar]
- 73.Raoul JI, Bretagne JF, Caucanas JP, et al. Internal radiation therapy for hepatocellular carcinoma. Results of a French multicenter phase II trial of transarterial injection of iodine-131-labeled Lipiodol. Cancer 1992; 69: 346–352. [DOI] [PubMed] [Google Scholar]
- 74.Kajiya Y, Kobayashi H, Nakajo M. Transarterial internal radiation therapy with I-131 Lipiodol for multifocal hepatocellular carcinoma: immediate and long-term results. Cardiovasc Intervent Radiol 1993; 16: 150–157. [DOI] [PubMed] [Google Scholar]
- 75.Leung WT, Lau WY, Ho S, et al. Selective internal radiation therapy with intra-arterial iodine-131-Lipiodol in inoperable hepatocellular carcinoma. J Nucl Med 1994; 35: 1313–1318. [PubMed] [Google Scholar]
- 76.Maini CL, Scelsa MG, Fiumara C, et al. Superselective intra-arterial radiometabolic therapy with I-131 Lipiodol in hepatocellular carcinoma. Clin Nucl Med 1996; 21: 221–226. [DOI] [PubMed] [Google Scholar]
- 77.Yoo HS, Park CH, Lee JT, et al. Small hepatocellular carcinoma: high dose internal radiation therapy with superselective intra-arterial injection of I-131-labeled Lipiodol. Cancer Chemother Pharmacol 1994; 33 (suppl): S128–133. [DOI] [PubMed] [Google Scholar]
- 78.Raoul JL, Guyader D, Bretagne JF, et al. Prospective randomized trial of chemoembolization versus intra-arterial injection of 131I-labeled-iodized oil in the treatment of hepatocellular carcinoma. Hepatology 1997; 26: 1156–1161. [DOI] [PubMed] [Google Scholar]
- 79.Raoul JL, Guyader D, Bretagne JF, et al. Randomized controlled trial for hepatocellular carcinoma with portal vein thrombosis: intra-arterial iodine-131-iodized oil versus medical support. J Nucl Med 1994; 35: 1782–1787. [PubMed] [Google Scholar]
- 80.de Baere T, Taourel P, Tubiana JM, et al. Hepatic intraarterial 131I iodized oil for treatment of hepatocellular carcinoma in patients with impeded portal venous flow. Radiology 1999; 212: 665–668. [DOI] [PubMed] [Google Scholar]
- 81.Partensky C, Sassolas G, Henry L, et al. Intra-arterial iodine 131-labeled Lipiodol as adjuvant therapy after curative liver resection for hepatocellular carcinoma: a phase 2 clinical study. Arch Surg 2000; 135: 1298–1300. [DOI] [PubMed] [Google Scholar]
- 82.Lau WY, Leung TW, Ho SK, et al. Adjuvant intra-arterial iodine-131-labelled Lipiodol for resectable hepatocellular carcinoma: a prospective randomised trial. Lancet 1999; 353: 797–801. [DOI] [PubMed] [Google Scholar]
- 83.Lau WY, Leung WT, Ho S, et al. Treatment of inoperable hepatocellular carcinoma with intrahepatic arterial yttrium-90 microspheres: a phase I and II study. Br J Cancer 1994; 70: 994–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Furuse J, Satake M, Iwasaki M, et al. Percutaneous ethanol injection under interventional radiographic computed tomography-fluoroscopic guidance for the treatment of small hepatocellular carcinoma. Int J Clin Oncol 1998; 3: 102–107. [Google Scholar]
- 85.Ebara M, Kita K, Sugiura N, et al. Therapeutic effect of percutaneous ethanol injection on small hepatocellular carcinoma: evaluation with CT. Radiology 1995; 195: 371–377. [DOI] [PubMed] [Google Scholar]
- 86.Lencioni R, Caramella D, Bartolozzi C. Hepatocellular carcinoma: use of color Doppler US to evaluate response to treatment with percutaneous ethanol injection. Radiology 1995; 194: 113–118. [DOI] [PubMed] [Google Scholar]
- 87.Bartolozzi C, Lencioni R, Caramella D, et al. Treatment of hepatocellular carcinoma with percutaneous ethanol injection: evaluation with contrast-enhanced MR imaging. AJR Am J Roentgenol 1994; 162: 827–831. [DOI] [PubMed] [Google Scholar]
- 88.Ebara M, Ohto M, Sugiura N, et al. Percutaneous ethanol injection for treatment of small hepatocellular carcinoma. Study of 95 patients. J Gastroenterol Hepatol 1990; 5: 616–626. [DOI] [PubMed] [Google Scholar]
- 89.Livraghi T, Bolondi L, Lazzaroni S, et al. Percutaneous ethanol injection in the treatment of hepatocellular carcinoma in cirrhosis: A study on 207 patients. Cancer 1992; 69: 925–929. [DOI] [PubMed] [Google Scholar]
- 90.Di Stasi M, Buscarini L, Livraghi T, et al. Percutaneous ethanol injection in the treatment of hepatocellular carcinoma. A multicenter survey of evaluation practices and complication rates. Scand J Gastroenterol 1997; 32: 1168–1173. [DOI] [PubMed] [Google Scholar]
- 91.Shina S, Tagawa K, Unuma T, et al. Percutaneous ethanol injection therapy for hepatocellular carcinoma. A histopathologic study. Cancer 1991; 68: 1524–1530. [DOI] [PubMed] [Google Scholar]
- 92.Livraghi T, Giorgio A, Marin G, et al. Hepatocellular carcinoma and cirrhosis in 746 patients: long-term results of percutaneous ethanol injection. Radiology 1995; 197: 101–108. [DOI] [PubMed] [Google Scholar]
- 93.Ishii H, Okada S, Okusaka T, et al. Needle tract implantation of hepatocellular carcinoma after percutaneous ethanol injection. Cancer 1998; 82: 1638–1642. [PubMed] [Google Scholar]
- 94.Matsumata T, Okamoto M, Ishikawa H, et al. Necrosis of the hepatocellular carcinoma nodule can aggravate metastasis. Nippon Geka Gakkai Zasshi 1998; 99: 197–200. [PubMed] [Google Scholar]
- 95.Vilana R, Bruix J, Bru C, et al. Tumor size determines the efficacy of percutaneous ethanol injection for the treatment of small hepatocellular carcinoma. Hepatology 1992; 16: 353–357. [DOI] [PubMed] [Google Scholar]
- 96.Castells A, Bruix J, Bru C, et al. Treatment of small hepatocellular carcinoma in cirrhotic patients: a cohort study comparing surgical resection and percutaneous ethanol injection. Hepatology 1993; 18: 1121–1126. [PubMed] [Google Scholar]
- 97.Shiina S, Tagawa K, Niwa Y, et al. Percutaneous ethanol injection therapy for hepatocellular carcinoma: results in 146 patients. AJR Am J Roentgenol 1993; 160: 1023–1028. [DOI] [PubMed] [Google Scholar]
- 98.Isobe H, Sakai H, Imari Y, et al. Intratumor ethanol injection therapy for solitary minute hepatocellular carcinoma. A study of 37 patients. J Clin Gastroenterol 1994; 193: 747–752. [DOI] [PubMed] [Google Scholar]
- 99.Lencioni R, Bartolozzi C, Caramella D, et al. Treatment of small hepatocellular carcinoma with percutaneous ethanol injection. Analysis of prognostic factors in 105 Western patients. Cancer 1995; 76: 1737–1746. [DOI] [PubMed] [Google Scholar]
- 100.Orlando A, Cottone M, Virdone R, et al. Treatment of small hepatocellular carcinoma associated with cirrhosis by percutaneous ethanol injection. A trial with a comparison group. Scand J Gastroenterol 1997; 32: 598–603. [DOI] [PubMed] [Google Scholar]
- 101.Castellano L, Calandra M, Del Vecchio Blanco C, de Sio I. Predictive factors of survival and intrahepatic recurrence of hepatocellular carcinoma in cirrhosis after percutaneous ethanol injection: analysis of 71 patients. J Hepatol 1997; 27: 862–870. [DOI] [PubMed] [Google Scholar]
- 102.Lin SM, Lin DY, Lin CJ. Percutaneous ethanol injection treatment in 47 cirrhotic patients with hepatocellular carcinoma 5 cm or less: a long-term result. Int J Clin Pract 1999; 53: 257–262. [PubMed] [Google Scholar]
- 103.Livraghi T, Bolondi L, Buscarini L, et al. No treatment, resection and ethanol injection in hepatocellular carcinoma: a retrospective analysis of survival in 391 patients with cirrhosis. Italian Cooperative HCC Study Group. J Hepatol 1995; 22: 522–526. [DOI] [PubMed] [Google Scholar]
- 104.Ryu M, Shimamura Y, Kinoshita T, et al. Therapeutic results of resection, transcatheter arterial embolization and percutaneous transhepatic ethanol injection in 3225 patients with hepatocellular carcinoma: a retrospective multicenter study. Jpn J Clin Oncol 1997; 27: 251–257. [DOI] [PubMed] [Google Scholar]
- 105.Dalla Palma L. Diagnostic imaging and interventional therapy of hepatocellular carcinoma. Br J Radiol 1998; 71: 808–818. [DOI] [PubMed] [Google Scholar]
- 106.Arii S, Yamaoka Y, Futagawa S, et al. Results of surgical and nonsurgical treatment for small-sized hepatocellular carcinomas: a retrospective and nationwide survey in Japan. The Liver Cancer Study Group of Japan. Hepatology 2000; 32: 1224–1229. [DOI] [PubMed] [Google Scholar]
- 107.Fong Y, Sun RL, Jarnagin W, Blumgart LH. An analysis of 412 cases of hepatocellular carcinoma at a Western center. Ann Surg 1999; 229: 790–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Poon RTP, Fan ST, Lo CM, et al. Long-term prognosis after resection of hepatocellular carcinoma associated with hepatitis B-related cirrhosis. J Clin Oncol 2000; 18: 1094–1101. [DOI] [PubMed] [Google Scholar]
- 109.Livraghi T, Benedini V, Lazzaroni S, et al. Long-term results of single-session percutaneous ethanol injection in patients with large hepatocellular carcinoma. Cancer 1998; 83: 48–57. [PubMed] [Google Scholar]
- 110.Giorgio A, Tarantino L, Manniello N, et al. Percutaneous ethanol injection under general anaesthesia for hepatocellular carcinoma: 3-year survival in 112 patients. Eur J Ultrasound 1998; 8: 201–206. [DOI] [PubMed] [Google Scholar]
- 111.Giorgio A, Tarantino L, de Stefano G, et al. Ultrasound-guided percutaneous ethanol injection under general anesthesia for the treatment of hepatocellular carcinoma on cirrhosis: long-term results in 268 patients. Eur J Ultrasound 2000; 12: 145–154. [DOI] [PubMed] [Google Scholar]
- 112.Pompili M, Rapaccini GL, de Luca F, et al. Risk factors for intrahepatic recurrence of hepatocellular carcinoma in cirrhotic patients treated by percutaneous ethanol injection. Cancer 1997; 79: 1501–1508. [DOI] [PubMed] [Google Scholar]
- 113.Hasegawa S, Yamasaki N, Hiwaki T, et al. Factors that predict intrahepatic recurrence of hepatocellular carcinoma in 81 patients initially treated by percutaneous ethanol injection. Cancer 1999; 861682–1690. [DOI] [PubMed] [Google Scholar]
- 114.Khan KN, Yatsuhashi H, Yamasaki K, et al. Prospective analysis of risk factors for early intrahepatic recurrence of hepatocellular carcinoma following ethanol injection. J Hepatol 2000; 32: 269–278. [DOI] [PubMed] [Google Scholar]
- 115.Koda M, Murawaki Y, Mitsuda A, et al. Predictive factors for intrahepatic recurrence after percutaneous ethanol injection therapy for small hepatocellular carcinoma. Cancer 2000; 88: 529–537. [PubMed] [Google Scholar]
- 116.Poon RTP, Fan ST, Wong J. Risk factors, prevention and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg 2000; 232: 10–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Okuda K. Intratumor ethanol injection. J Surg Oncol 1993; 3 (suppl): 97–99. [DOI] [PubMed] [Google Scholar]
- 118.Ohnishi K, Ohyama N, Ito S, Fujiwara K. Small hepatocellular carcinoma: treatment with US-guided intratumoral injection of acetic acid. Radiology 1994; 193: 747–752. [DOI] [PubMed] [Google Scholar]
- 119.Ohnishi K, Yoshioka H, Ito S, Fujiwara K. Prospective randomized controlled trial comparing percutaneous acetic acid injection and percutaneous ethanol injection for small hepatocellular carcinoma. Hepatology 1998; 27: 67–72. [DOI] [PubMed] [Google Scholar]
- 120.Ravikumar TS, Steele GD Jr. Hepatic cryosurgery. Surg Clin North Am 1989; 69: 433–440. [DOI] [PubMed] [Google Scholar]
- 121.Cuschieri A, Crosthwaite G, Shimi S, et al. Hepatic cryotherapy for liver tumors. Development and clinical evaluation of a high-efficiency insulated multineedle probe system for open and laparoscopic use. Surg Endosc 1995; 9: 483–489. [DOI] [PubMed] [Google Scholar]
- 122.Lezoche E, Paganini AM, Feliciotti F, et al. Ultrasound-guided laparoscopic cryoablation of hepatic tumors: preliminary report. World J Surg 1998; 22: 829–836. [DOI] [PubMed] [Google Scholar]
- 123.Silverman SG, Tuncali K, Adams DF, et al. MR imaging-guided percutaneous cryotherapy of liver tumors: initial experience. Radiology 2000; 217: 657–664. [DOI] [PubMed] [Google Scholar]
- 124.Weber SM, Lee FT Jr, Chinn DO, et al. Perivascular and intralesional tissue necrosis after hepatic cryoablation: results in a porcine model. Surgery 1997; 122: 742–747. [DOI] [PubMed] [Google Scholar]
- 125.Zhou XD, Tang ZY, Yu YQ, et al. Clinical evaluation of cryosurgery in the treatment of primary liver cancer: report of 60 cases. Cancer 1988; 61: 1889–1892. [DOI] [PubMed] [Google Scholar]
- 126.Wren SM, Coburn MM, Tan M, et al. Is cryosurgical ablation appropriate for treating hepatocellular cancer? Arch Surg 1997; 132: 599–603. [DOI] [PubMed] [Google Scholar]
- 127.Adam R, Akpinar E, Johann M, et al. Place of cryosurgery in the treatment of malignant liver tumors. Ann Surg 1997; 225: 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Crews KA, Kuhn JA, McCarty TM, et al. Cryosurgical ablation of hepatic tumors. Am J Surg 1997; 174: 614–617. [DOI] [PubMed] [Google Scholar]
- 129.Zhou XD, Tang ZY. Management of hepatocellular carcinoma: long-term outcome in 2639 cases. Gan To Kagaku Ryoho 1997; 24 (suppl 1): 9–16. [PubMed] [Google Scholar]
- 130.Wong WS, Patel SC, Cruz FS, et al. Cryosurgery as a treatment for advanced-stage hepatocellular carcinoma: results, complications, and alcohol ablation. Cancer 1998; 82: 1268–1278. [DOI] [PubMed] [Google Scholar]
- 131.Pearson AS, Izzo F, Fleming RY, et al. Intraoperative radiofrequency ablation or cryoablation for hepatic malignancies. Am J Surg 1999; 178: 592–599. [DOI] [PubMed] [Google Scholar]
- 132.Cha C, Lee FT, Rikkers LF, et al. Rationale for the combination of cryoablation with surgical resection of hepatic tumors. J Gastrointest Surg 2001; 5: 206–213. [DOI] [PubMed] [Google Scholar]
- 133.Seifert JK, Morris DL. World survey on the complications of hepatic and prostate cryotherapy. World J Surg 1999; 23: 109–114. [DOI] [PubMed] [Google Scholar]
- 134.Lam CM, Yuen WK, Fan ST. Hepatic cryosurgery for recurrent hepatocellular carcinoma after hepatectomy: a preliminary report. J Surg Oncol 1998; 68: 104–106. [DOI] [PubMed] [Google Scholar]
- 135.Hyodoh H, Hyodoh K, Takahashi K, et al. Microwave coagulation therapy on hepatomas: CT and MR appearance after therapy. J Magn Reson Imaging 1998; 2: 451–458. [DOI] [PubMed] [Google Scholar]
- 136.Takeuchi H, Tamura R, Baba T, et al. Real-time evaluation of the effectiveness of microwave coagulation therapy for hepatocellular carcinoma using color Doppler imaging. Acta Med Okayama 1998; 52: 255–260. [DOI] [PubMed] [Google Scholar]
- 137.Seki T, Wakabayashi M, Nakagawa T, et al. Ultrasonically guided percutaneous microwave coagulation therapy for small hepatocellular carcinoma. Cancer 1994; 74: 817–825. [DOI] [PubMed] [Google Scholar]
- 138.Ohmoto K, Miyake I, Tsuduki M, et al. Percutaneous microwave coagulation therapy for unresectable hepatocellular carcinoma. Hepato-Gastroenterology 1999; 46: 2894–2900. [PubMed] [Google Scholar]
- 139.Matsukawa T, Yamashita Y, Arakawa A, et al. Percutaneous microwave coagulation therapy in liver tumors. A 3-year experience. Acta Radiol 1997; 38: 410–415. [DOI] [PubMed] [Google Scholar]
- 140.Yamanaka N, Okamoto E, Tanaka T, et al. Laparoscopic microwave coagulonecrotic therapy for hepatocellular carcinoma. Surg Laparosc Endosc 1995; 5: 444–449. [PubMed] [Google Scholar]
- 141.Seki S, Sakaguchi H, Kadoya H, et al. Laparoscopic microwave coagulation therapy for hepatocellular carcinoma. Endoscopy 2000; 32: 591–597. [DOI] [PubMed] [Google Scholar]
- 142.Abe T, Shinzawa H, Wakabayashi H, et al. Value of laparoscopic microwave coagulation therapy for hepatocellular carcinoma in relation to tumor size and location. Endoscopy 2000; 32: 598–603. [DOI] [PubMed] [Google Scholar]
- 143.Sato M, Watanabe Y, Ueda S, et al. Microwave coagulation therapy for hepatocellular carcinoma. Gastroenterology 1996; 110: 1507–1514. [DOI] [PubMed] [Google Scholar]
- 144.Yamanaka N, Tanaka T, Oriyama T, et al. Microwave coagulonecrotic therapy for hepatocellular carcinoma. World J Surg 1996; 20: 1076–1081. [DOI] [PubMed] [Google Scholar]
- 145.Ishikawa M, Ikeyama S, Sasaki K, et al. Intraoperative microwave coagulation therapy for large hepatic tumors. J Hepatobiliary Pancreat Surg 2000; 7: 587–591. [DOI] [PubMed] [Google Scholar]
- 146.Hamazoe R, Hirooka Y, Ohtani S, et al. Intra-operative microwave tissue coagulation as treatment for patients with non-resectable hepatocellular carcinoma. Cancer 1995; 75: 794–800. [DOI] [PubMed] [Google Scholar]
- 147.Shimada S, Hirota M, Beppu T, et al. Complications and management of microwave coagulation therapy for primary and metastatic liver tumors. Surg Today 1998; 28: 1130–1137. [DOI] [PubMed] [Google Scholar]
- 148.Mitsuzaki K, Yamashita Y, Nishiharu T, et al. CT appearance of hepatic tumors after microwave coagulation therapy. AJR Am J Roentgenol 1998; 171: 1397–1403. [DOI] [PubMed] [Google Scholar]
- 149.Midorikawa T, Kumada K, Kikuchi H, et al. Microwave coagulation therapy for hepatocellular carcinoma. J Hepatobiliary Pancreat Surg 2000; 7: 252–259. [DOI] [PubMed] [Google Scholar]
- 150.Seki T, Wakabayashi M, Nakagawa T, et al. Percutaneous microwave coagulation therapy for patients with small hepatocellular carcinoma. Comparison with percutaneous ethanol injection therapy. Cancer 1999; 85: 1694–1702. [PubMed] [Google Scholar]
- 151.Heisterkamp J, van Hillegersberg R, Mulder PG, et al. Importance of eliminating portal flow to produce larger intrahepatic lesions with interstitial laser coagulation. Br J Surg 1997; 84: 1245–1248. [PubMed] [Google Scholar]
- 152.Christophi C, Muralidharan V. Treatment of hepatocellular carcinoma by percutaneous laser hyperthermia. J Gastroenterol Hepatol 2001; 16: 548–552. [DOI] [PubMed] [Google Scholar]
- 153.Vogl TJ, Eichler K, Straub R, et al. Laser-induced thermotherapy of malignant liver tumors: general principles, equipment, procedure, side effects, complications and results. Eur J Ultrasound 2001; 13: 117–127. [DOI] [PubMed] [Google Scholar]
- 154.Giorgio A, Tarantino L, de Stefano G, et al. Interstitial laser photocoagulation under ultrasound guidance of liver tumors: results in 104 treated patients. Eur J Ultrasound 2000; 11: 181–188. [DOI] [PubMed] [Google Scholar]
- 155.Buscarini L, Rossi S. Technology for radiofrequency thermal ablation of liver tumors. Semin Laparosc Surg 1997; 4: 96–101. [DOI] [PubMed] [Google Scholar]
- 156.Goldberg SN, Gazelle GS, Dawson SL, et al. Tissue ablation with radiofrequency: effect of probe size, gauge, duration, and temperature on lesion volume. Acad Radiol 1995; 2: 399–404. [DOI] [PubMed] [Google Scholar]
- 157.Patterson EJ, Scudamore CH, Owen DA, et al. Radiofrequency ablation of porcine liver in vivo: effects of blood flow and treatment time on lesion size. Ann Surg 1998; 227: 559–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Goldberg SN, Gazelle GS. Radiofrequency tissue ablation: physical principles and techniques for increasing coagulation necrosis. Hepato-Gastroenterology 2001; 48: 359–367. [PubMed] [Google Scholar]
- 159.Livraghi T, Goldberg SN, Lazzaroni S, et al. Hepatocellular carcinoma: radio-frequency ablation of medium and large lesions. Radiology 2000; 214: 761–768. [DOI] [PubMed] [Google Scholar]
- 160.Rossi S, Di Stasi M, Buscarini E. Percutaneous RF interstitial thermal ablation in the treatment of hepatic cancer. AJR Am J Roentgenol 1996; 167: 759–768. [DOI] [PubMed] [Google Scholar]
- 161.Rossi S, Buscarini E, Garbagnati F, et al. Percutaneous treatment of small hepatic tumors by an expandable RF needle electrode. AJR Am J Roentgenol 1998; 170: 1015–1028. [DOI] [PubMed] [Google Scholar]
- 162.Allgaier HP, Deibert P, Zuber I, et al. Percutaneous radiofrequency interstitial thermal ablation of small hepatocellular carcinoma. Lancet 1999; 353: 1676–1677. [DOI] [PubMed] [Google Scholar]
- 163.Francica G, Marone G. Ultrasound-guided percutaneous treatment of hepatocellular carcinoma by radiofrequency hyperthermia with a “cooled-tip needle.” A preliminary clinical experience. Eur J Ultrasound 1999; 9: 145–153. [DOI] [PubMed] [Google Scholar]
- 164.Poggi G, Gatti C, Cupella F, et al. Percutaneous US-guided radiofrequency ablation of hepatocellular carcinomas: results in 15 patients. Anticancer Res 2001; 21: 739–742. [PubMed] [Google Scholar]
- 165.Cuschieri A, Bracken J, Boni L. Initial experience with laparoscopic ultrasound-guided radiofrequency thermal ablation of hepatic tumours. Endoscopy 1999; 31: 318–321. [DOI] [PubMed] [Google Scholar]
- 166.Goletti O, Lencioni R, Armillotta N, et al. Laparoscopic radiofrequency thermal ablation of hepatocarcinoma: preliminary experience. Surg Laparosc Endosc Percutan Tech 2000; 10: 284–290. [PubMed] [Google Scholar]
- 167.Montorsi M, Santambrogio R, Bianchi P, et al. Radiofrequency interstitial thermal ablation of hepatocellular carcinoma in liver cirrhosis. Role of the laparoscopic approach. Surg Endosc 2001; 15: 141–145. [DOI] [PubMed] [Google Scholar]
- 168.Curley SA, Izzo F, Delrio P, et al. Radiofrequency ablation of unresectable primary and metastatic hepatic malignancies: results in 123 patients. Ann Surg 1999; 230: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Nicoli N, Casaril A, Marchiori L, et al. Intra-operative and percutaneous radiofrequency thermal ablation in the treatment of hepatocellular carcinoma. Chir Ital 2000; 52: 29–40. [PubMed] [Google Scholar]
- 170.Curley S, Izzo F, Ellis LM, et al. Radiofrequency ablation of hepatocellular carcinoma in 110 patients with cirrhosis. Ann Surg 2000; 232: 381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wood TF, Rose DM, Chung M, et al. Radiofrequency ablation of 231 unresectable hepatic tumours: indications, limitations and complications. Ann Surg Oncol 2000; 7: 593–600. [DOI] [PubMed] [Google Scholar]
- 172.Sironi S, Livraghi T, Meloni F, et al. Small hepatocellular carcinoma treated with percutaneous RF ablation: MR imaging follow-up. AJR Am J Roentgenol 1999; 173: 1225–1229. [DOI] [PubMed] [Google Scholar]
- 173.Catalano O, Esposito M, Nunziata A, et al. Multiphase helical CT findings after percutaneous ablation procedures for hepatocellular carcinoma. Abdom Imaging 2000; 25: 607–614. [DOI] [PubMed] [Google Scholar]
- 174.Choi D, Lim HK, Kim SH, et al. Hepatocellular carcinoma treated with percutaneous radio-frequency ablation: usefulness of power Doppler US with a microbubble contrast agent in evaluating therapeutic response: preliminary results. Radiology 2000; 217: 558–563. [DOI] [PubMed] [Google Scholar]
- 175.Livraghi T, Golberg SN, Lazzaroni S, et al. Small hepatocellular carcinoma: treatment with radiofrequency ablation versus percutaneous ethanol injection. Radiology 1999; 210: 655–661. [DOI] [PubMed] [Google Scholar]
- 176.Llovet JM, Vilana R, Bru C, et al. Increased risk of tumor seeding after percutaneous radiofrequency ablation for single hepatocellular carcinoma. Hepatology 2001; 33: 1124–1129. [DOI] [PubMed] [Google Scholar]
- 177.Buscarini L, Buscarini E, Di Stasi M, et al. Percutaneous radiofrequency ablation of small hepatocellular carcinoma: long-term results. Eur Radiol 2001; 11: 914–921. [DOI] [PubMed] [Google Scholar]
- 178.Lencioni R, Cioni D, Paolicehi A, et al. Percutaneous treatment of small hepatocellular carcinoma: radiofrequency ablation versus percutaneous ethanol injection: a prospective randomised trial (second report). Radiology 1998; 209: 174. [Google Scholar]
- 179.Livraghi T, Goldberg SN, Meloni F, et al. Percutaneous ablation of small hepatocellular carcinoma (HCC): a prospective comparison between percutaneous ethanol instillation (PEI) and radiofrequency (RF) ablation therapy. Radiology 1998; 209: 175.9769829 [Google Scholar]
- 180.Bilchik AJ, Wood TF, Allegra D, et al. Cryosurgical ablation and radiofrequency ablation for unresectable hepatic malignant neoplasms: a proposed algorithm. Arch Surg 2000; 135: 657–662. [DOI] [PubMed] [Google Scholar]
- 181.Shibata T, Niinobu T, Ogata N. Comparison of the effects of in-vivo thermal ablation of pig liver by microwave and radiofrequency coagulation. J Hepatobiliary Pancreat Surg 2000; 7: 592–598. [DOI] [PubMed] [Google Scholar]
- 182.Tanaka K, Nakamura S, Numata K, et al. Hepatocellular carcinoma: treatment with percutaneous ethanol injection and transcatheter arterial embolization. Radiology 1992; 185: 457–460. [DOI] [PubMed] [Google Scholar]
- 183.Koda M, Okamoto K, Miyoshi Y, et al. Combination therapy with transcatheter arterial embolization and percutaneous ethanol injection for advanced hepatocellular carcinoma. Hepato-Gastroenterology 1994; 41: 25–29. [PubMed] [Google Scholar]
- 184.Allgaier HP, Deibert P, Olschewski M, et al. Survival benefit of patients with inoperable hepatocellular carcinoma treated by a combination of transarterial chemoembolization and percutaneous ethanol injection—a single-center analysis including 132 patients. Int J Cancer 1998; 79: 601–605. [DOI] [PubMed] [Google Scholar]
- 185.Bartolozzi C, Lencioni R, Caramella D, et al. Treatment of large HCC: transcatheter arterial chemoembolization combined with percutaneous ethanol injection versus repeated transcatheter arterial chemoembolization. Radiology 1995; 197: 812–818. [DOI] [PubMed] [Google Scholar]
- 186.Lencioni R, Paolicchi A, Moretti M, et al. Combined transcatheter arterial chemoembolization and percutaneous ethanol injection for the treatment of large hepatocellular carcinoma: local therapeutic effect and long-term survival rate. Eur Radiol 1998; 8: 439–444. [DOI] [PubMed] [Google Scholar]
- 187.Ishii H, Okada S, Sato T, et al. Effect of percutaneous ethanol injection for postoperative recurrence of hepatocellular carcinoma in combination with transcatheter arterial embolization. Hepato-Gastroenterology 1996; 43: 644–650. [PubMed] [Google Scholar]
- 188.Sato M, Watanabe Y, Iseki N, et al. Chemoembolization and percutaneous ethanol injection for intrahepatic recurrence of hepatocellular carcinoma after hepatic resection. Hepato-Gastroenterology 1996; 43: 1421–1426. [PubMed] [Google Scholar]
- 189.Buscarini L, Buscarini E, Di Stasi M, et al. Percutaneous radiofrequency thermal ablation combined with transcatheter arterial embolization in the treatment of large hepatocellular carcinoma. Ultraschall Med 1999; 20: 47–53. [DOI] [PubMed] [Google Scholar]
- 190.Rossi S, Garbagnati F, Lencioni R, et al. Percutaneous radiofrequency thermal ablation of nonresectable hepatocellular carcinoma after occlusion of tumor blood supply. Radiology 2000; 217: 119–126. [DOI] [PubMed] [Google Scholar]
- 191.Seki T, Tamai T, Nakagawa T, et al. Combination therapy with transcatheter arterial chemoembolization and percutaneous microwave coagulation therapy for hepatocellular carcinoma. Cancer 2000; 89: 1245–1251. [PubMed] [Google Scholar]
- 192.Pacella CM, Bizzarri G, Cecconi P, et al. Hepatocellular carcinoma: long-term results of combined treatment with laser thermal ablation and transcatheter arterial chemoembolization. Radiology 2001; 219: 669–678. [DOI] [PubMed] [Google Scholar]
- 193.Kainuma O, Asano T, Aoyama H, et al. Combined therapy with radiofrequency thermal ablation and intraarterial infusion chemotherapy for hepatic metastases from colorectal cancer. Hepato-Gastroenterology 1999; 46: 1071–1077. [PubMed] [Google Scholar]
- 194.Poon RTP, Fan ST, Yu WC, et al. A prospective longitudinal study of quality of life after resection of hepatocellular carcinoma. Arch Surg 2001; 136: 693–699. [DOI] [PubMed] [Google Scholar]
- 195.Lo CM, Lai ECS, Liu CL, et al. Laparoscopy and laparoscopic ultrasonography avoid exploratory laparotomy in patients with hepatocellular carcinoma. Ann Surg 1998; 227: 527–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Dominique E, El Otmany A, Goharin A, et al. Intraductal cooling of the main bile duct during intraoperative radiofrequency ablation. J Surg Oncol 2001; 76: 297–300. [DOI] [PubMed] [Google Scholar]
- 197.Germer CT, Albrecht D, Roggan A, et al. Experimental study of laparoscopic laser-induced thermotherapy for liver tumours. Br J Surg 1997; 84: 317–320. [PubMed] [Google Scholar]
- 198.Meloni MF, Goldberg SN, Livraghi T, et al. Hepatocellular carcinoma treated with radiofrequency ablation: comparison of pulse inversion contrast-enhanced harmonic sonography, contrast-enhanced power Doppler sonography, and helical CT. AJR Am J Roentgenol 2001; 17: 375–380. [DOI] [PubMed] [Google Scholar]
- 199.Kato T, Tamura S, Tekin A, et al. Use of microwave coagulation therapy in liver transplant candidates with hepatocellular carcinoma: a preliminary report. Transplant Proc 2001; 33: 1469. [DOI] [PubMed] [Google Scholar]
- 200.Pulvirenti A, Garbagnati F, Regalia E, et al. Experience with radiofrequency ablation of small hepatocellular carcinomas before liver transplantation. Transplant Proc 2001; 33: 1516–1517. [DOI] [PubMed] [Google Scholar]
- 201.Cheng SQ, Zhou XD, Tang ZY, et al. High-intensity focused ultrasound in the treatment of experimental liver tumour. J Cancer Res Clin Oncol 1997; 123: 219–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zhao J, Wang Z, Guo D, et al. CT appearance and its diagnosis value in liver cancer after transcatheter oily chemoembolization combining with high intensity focused ultrasound therapy. Zhonghua Gan Zang Bing Za Zhi 2001; 9 (suppl): 61–63. [PubMed] [Google Scholar]
- 203.Koniaris LG, Chan DY, Magee C, et al. Focal hepatic ablation using interstitial photon radiation energy. J Am Coll Surg 2000; 191: 164–174. [DOI] [PubMed] [Google Scholar]
- 204.Rose SC, Hassanein TI, Easter DW, et al. Value of three-dimensional US for optimizing guidance for ablating focal liver tumors. J Vasc Interv Radiol 2001; 12: 507–515. [DOI] [PubMed] [Google Scholar]