Abstract
Objective
To show the feasibility of performing surgery across transoceanic distances by using dedicated asynchronous transfer mode (ATM) telecommunication technology.
Summary Background Data
Technical limitations and the issue of time delay for transmission of digitized information across existing telecommunication lines had been a source of concern about the feasibility of performing a complete surgical procedure from remote distances.
Methods
To verify the feasibility and safety in humans, the authors attempted remote robot-assisted laparoscopic cholecystectomy on a 68-year-old woman with a history of abdominal pain and cholelithiasis. Surgeons were in New York and the patient in Strasbourg. Connections between the sites were done with a high-speed terrestrial network (ATM service).
Results
The operation was carried out successfully in 54 minutes without difficulty or complications. Despite a round-trip distance of more than 14,000 km, the mean time lag for transmission during the procedure was 155 ms. The surgeons perceived the procedure as safe and the overall system as perfectly reliable. The postoperative course was uneventful and the patient returned to normal activities within 2 weeks after surgery.
Conclusions
Remote robot-assisted surgery appears feasible and safe. Teletransmission of active surgical manipulations has the potential to ensure availability of surgical expertise in remote locations for difficult or rare operations, and to improve surgical training worldwide.
Remote surgical operations require both rapid and accurate transmission of information. Factors that influence significantly the rapidity and accuracy of this information are the time required to convert video images and gestures into electronic signals, and the bandwidth and time lag of existing telecommunication lines. 1,2 Using current technology, we recently showed the feasibility of performing remote surgical operations in an experimental animal model. 3 Results of our experimental tests allowed us to perform, for the first time, remote robot-assisted surgery on a human. Here we present the case and postoperative course and discuss the current limitations and the potential clinical and social impact of remote telesurgery.
METHODS
Patient
A 68-year-old woman with a history of recurrent abdominal pain in the right hypochondrium and epigastrium underwent abdominal ultrasound documenting the presence of cholelithiasis. There was no dilatation of the common bile duct, and laboratory findings were all in the normal range. The patient was scheduled for laparoscopic cholecystectomy. After approval was obtained from the Ethical Committee (Comité Consultatif de Protection des Personnes dans la Recherche Biomedicale d’Alsace; 19 June 2001, 01/42) and from the U.S. Food & Drug Administration, the patient gave her informed consent to the operation.
Robot Setup
The ZEUS system (Computer Motion, Galeta, CA) consists of two physically separated subsystems named “surgeon-side” (Fig. 1) and “patient-side” (Fig. 2). The surgeon’s subsystem (based in New York) has a console that takes the surgeon’s input; the patient’s subsystem (based in Strasbourg) includes two robotic arms that translate the input into actual instrument manipulation, and an additional robotic arm to control the endoscopic camera. A variety of surgical instruments can be connected to the robotic arms, so that the surgeon can activate graspers and so forth by simply manipulating the handles at the remote console. Two computers connected by the high-speed communication channels linked the two subsystems. Camera movements were directed from the computer in New York according to the operating surgeon’s instructions.
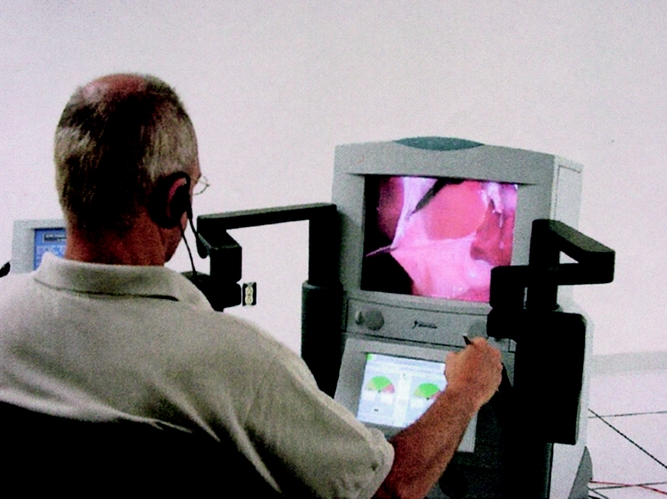
Figure 1. Operator and surgeon’s robotic console in New York.
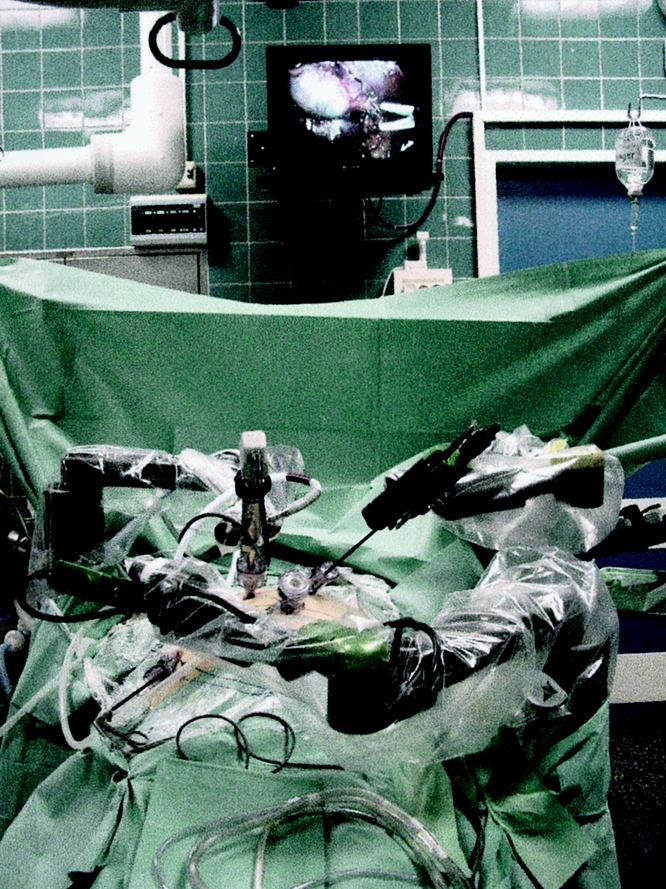
Figure 2. Robotic arms at the remote site in Strasbourg.
Network Connections and Monitoring
Connections between New York and Strasbourg were established through asynchronous transfer mode (ATM) technology (France Telecom/Equant’s, Paris, France). ATM network nodes are interconnected through a high-speed terrestrial fiberoptic network that transports data through virtual connections dedicated per customer. The ATM network provides a high quality of service for data transport. For instance, the probability of having no network outage is 99.99% (so-called network availability rate); the ATM network provides a low transport delay and low packet loss ratio.
A virtual pathway using ATM technology was set up between Equant’s point of presence in New York and the operating room within IRCAD-EITS (European Institute of Telesurgery) in Strasbourg. A bandwidth of 10 Mb/s has been reserved through network interconnecting applications at both sites using a network termination unit (NTU), which provides a multiservice path to different applications.
To monitor and measure its own level of quality, the NTU (the sender) inserted operating and maintenance (OAM) packets within user data flow, which were extracted and analyzed by the remote NTU (the receiver). Analyzing these packets and comparing the number of user packets initially sent to those actually received, we measured the number of lost packets.
An identical backup line was available in case of main line congestion. Data flow assigned to each application is merged into the 10-Mb/s virtual path according to a specific quality of service.
Robot motion data had a high priority and a rate guarantee of 512 Kb/s within the 10-Mb/s virtual path. Video packets were sent with a minimum guaranteed rate of 7 Mb/s, with the possibility to use more bandwidth if available within the 10-Mb/s virtual path. We have plugged a video conferencing system and intraperitoneal phone to NTU. Data coming from these two applications were merged, and they received a guaranteed minimum rate of around 3 Mb/s.
The two sites were also connected through a videoconference system and a large television screen at both sites.
Surgical Procedure
The operator site was set up in a nonmedical building in Manhattan (point of presence of France Telecom). Surgeons in New York performed the dissection of the cystic duct and artery and the cholecystectomy, while a team in Strasbourg induced the pneumoperitoneum and performed robot arm setup, trocar placement, exposure of structures, and clip application. In Strasbourg, the surgical team monitored the procedure on a screen and was in constant connection through a phone line with the colleagues in New York to coordinate electrocoagulation. After completion of the dissection and introduction of a plastic bag for extraction, the gallbladder was then removed by the surgeon bedside. The abdomen was then exsufflated and the incisions were closed. The operative steps were carefully monitored and technical difficulties, complications, appropriateness of dissection, and operative times were recorded. After completion of the procedure, the three surgeons in the team in New York gave a subjective evaluation of the quality of the image and the overall safety of the procedure. Evaluation was on a 0-to-10 scale (0 = worst possible; 10 = best possible). Each surgeon was unaware of other colleagues’ evaluations.
RESULTS
Robot arm setup and trocar placement required 16 minutes. The laparoscopic cholecystectomy was performed in 54 minutes; this includes the time lost for switching instruments at the robot arms for the different steps of the procedure (a total of 10.30 minutes). There were no complications. Coordination of electrocautery, as ordered by the surgeon in New York, was excellent, and there was no damage related to the use of coagulation. No bleeding occurred. During the surgical procedures, reproduction of image details on the video monitor at the operative site in New York was highly accurate, resulting in perfect visualization of structures. For the duration of the operation, there were no interruptions in the transmission of surgical movements or degradation of video signals. We measured a constant time delay of 155 ms through the procedure. OAM tools found no ATM packet lost during surgery. The subjective evaluation of the quality of image by surgeons had an average score of 9.5. The overall safety of the procedure was intended as the combination of high-quality video images for appropriate visualization of structural and anatomic details, ability to control surgical movements, and perfect coordination in the use of cautery for coagulation of vessels. All three surgeons rated as 10 the score of “perception of the safety of the operation”; this reflects the confidence of the surgeons and the reliability of the total system. In no instances throughout the operation was there any risk to the patient related to the teletransmission or to the use of the robot system.
The patient recovered well from anesthesia and her postoperative course was uneventful. She was discharged from the hospital 48 hours after the operation; during the following week, she was monitored by daily telephone calls to rule out postoperative complications. Two and 4 weeks after surgery, the patient was seen in the office by the surgeons. Her wounds were well healed, with no sign of infection. At the time of her first visit she was already back to her routine daily activities. Pathologic examination of the specimen documented the presence of chronic cholecystitis and a 4-mm benign polyp in the gallbladder mucosa.
DISCUSSION
Laparoscopic surgery is performed under the guidance of images displayed on a video monitor using specific instruments through the abdominal wall. With laparoscopy, for the first time the surgeon was separated from direct contact with tissues and organs, allowing robotic and computer technologies to be introduced into surgery.
Robotic and computer technologies have the potential to enhance precision and dexterity 4–6 and to allow performance of surgical procedures from a remote distance. 3
Robotic Enhancement of Dexterity
Enhancement of dexterity is accomplished by improving accuracy, precision, and endurance. When working under image magnification, as in laparoscopy or microsurgery, the surgeon’s normal tremor is also magnified, increasing the incidence of purposeless movements; to compensate, the surgeon must slow the procedure, increasing operative time. This and the fixed posture of the surgeon lead to fatigue that, in turn, further increases tremor and unwanted movements. 7,8 Robotic systems have computer programs that filter out hand tremors, and the chair’s arm at the surgeon’s console adds stability and comfort during the procedure, improving endurance. These features and the possibility of modulating the amplitude of surgical motions by downscaling and stabilization translate into smooth and precise surgical maneuvers. Enhanced dexterity is suitable in multiple instances. Devices that cancel physiologic tremor have been used for vitroretinal microsurgery. 9 Robotic systems have been used for retinal vein cannulation, with a needle for administration of local therapy for retinal vein thrombosis; this involves cannulation of a 100-μm structure. 10 Others have reported efficient performance of sutured coronary artery bypass anastomoses in a plastic model using robotic enhancement technology. 11
Clinical Applications
Although clinical trials verifying the potential advantages of robotic over conventional surgery are not yet available, a number of series show that using robotic devices for human surgery is feasible and safe. Our group performed laparoscopic robotic cholecystectomy in 25 patients with no robot-related complications and with operative time and patient recovery similar to those of conventional laparoscopy. 12 Cadiere et al 13 recently reported a series of 146 patients undergoing robot-assisted laparoscopic surgeries, including antireflux procedures, gastroplasties, cholecystectomies, inguinal hernia repair, hysterectomies, and prostatectomies. There were no complications related to the system, and robotic assistance was found to be most beneficial when microsuturing within the abdomen or in very confined spaces. Falcone et al 14 reported successful robotic assistance for reversal of tubal ligation using 8-0 sutures and suggested that robotic technology may make laparoscopic microsuturing easier. Robotic assistance has been used for laparoscopic nephrectomy 15 and laparoscopic radical prostatectomy. 16,17
Robotic systems have also facilitated the performance of endoscopic cardiac surgery, including coronary artery bypass and mitral valve repair. 18–20 Current efforts aim to provide the capability to perform surgery on the beating heart through motion compensation, which would allow the surgeon to operate on any moving structure with the same precision as if it was perfectly still. 21
Remote Robot-Assisted Telesurgery
In addition to enhancing human performance, robotic systems provides the unique ability to perform surgery in remote locations. There are several challenges involved, but the most important limitations have been the reliability (or quality of service) of the telecommunication lines and the issue of latency (the delay time from when the hand motion is initiated by the surgeon until the remote manipulator actually moves and the image is shown on the surgeon’s monitor). Due to the latency factor, it was believed that the feasible distance for remote surgery was no more than a few hundred miles over terrestrial telecommunications;21 geosynchronous satellite systems, which have a latency of nearly 1.5 seconds, are considered unsuitable for performing long-distance surgery. 22 Our research estimated that about 300 ms was the maximum time delay compatible with safe performance of surgical manipulations, and we measured a mean time delay of 155 ms over transoceanic distances 3 when using dedicated ATM fibers.
The case reported here, the world’s first human long-distance operation, shows the feasibility and safety of performing a complete surgical operation from a remote location. There were no specific difficulties or complications resulting from the use of the teletransmission of the surgical procedure. The surgeons perceived the procedure as safe and the robotic movements as fluid and appropriately responsive to their manipulations. These results support the use of existing high-bandwidth, dedicated telecommunication lines for performing intercontinental surgery on humans with adequate efficacy and safety.
Technical feasibility and clinical safety, however, are not the only issues to solve to permit implementation of telesurgical procedures into routine clinical practice. The use of remote telesurgery will depend on a balance between real benefits and limitations.
Current Limitations for Remote Telesurgery
There are several limitations. First, although a “backbone” of high-speed terrestrial ATM fibers is present in more than 200 countries worldwide, currently most hospitals are not equipped with ATM technology. This was why we performed the surgery from an office of our partner company of telecommunications rather than from a major U.S. hospital, as we had planned before.
The cost of remote operations may be a reasonable concern. The robotic machines cost approximately $1 million. For our research development other significant costs involved the use of telecommunication lines and human resources, including several professionals, such as surgeons, computer scientists, and engineers. Of course, the costs of remote surgery when performed on a routine basis would have to cover solely the cost of the robotic machine and the teletransmission. It is difficult at present to give an exact estimate of the cost of the telecommunication component, because this varies depending on the distance and location of the sites connected (i.e., transoceanic connections would reasonably be more expensive than connections within the same continent or country). Roughly, the cost for a 1-year availability of ATM lines point-to-point ranges between $100,000 and $200,000. If remote telesurgery is evaluated solely as the expansion of existing surgical practice, it is not certainly cost-effective. Some have suggested, however, that if telesurgery achieves the goals of increasing access to healthcare and improving training and efficiency with enhanced outcomes, it may prove less costly to healthcare systems. 23 The cost of technologies is also expected to decrease with time.
The lack of face-to-face contact between the patient and the surgeon is another important aspect of telesurgery that might be an issue in malpractice actions. Because telesurgery may involve more than one state or country, conflicts of jurisdictions may also arise. Other legal issues also need to be addressed, such as whether the surgeon should or not be liable for errors related to delays in transmission or equipment failure or whether a special consent should be obtained, and who is the person responsible for it. In our opinion, the telemedicine community should set up an ad hoc international committee to address these and other legal issues to provide clear and internationally valid rules to regulate the practice of telesurgery.
The technical issue of performing surgeries on a ship in the ocean or in space stations has not yet been solved because they are currently reachable only through satellite transmission, which at present has an estimated latency incompatible with safe surgical operations. 22 Low earth orbit satellites might overcome this technical limitation.
Applications of Remote Surgery
Benefits of remote robot-assisted surgery are multiple. Geographic constraints will no longer determine the type of treatment the patient receives because of lack of surgical expertise. Ideally, any patient can receive the form of treatment more appropriate for his or her condition or more advantageous, such as new minimally invasive techniques. This may have an even more profound impact on developing countries, where healthcare is often provided by volunteers who do not necessarily have expertise in all fields of medicine and surgery.
Emergency operations in small rural hospitals are sometimes challenging for young surgeons on call. The availability of a network connecting the hospital to a major center would allow expert surgeons to assist or carry out the procedure themselves. The availability of expert surgeons might very well help in remote areas where military or scientific missions are being performed or in remote islands, especially in the case of emergency operations.
In addition to all these potential advantages for the patient, active intervention from remote locations opens new avenues for surgical education. The objective of performing remote procedures is not in fact to replace surgeons. It can improve surgery through teaching and mentoring to reduce the learning curve of surgeons for new procedures. Various degrees of interaction between the expert and the surgeon at the bedside are possible. Assistance of an expert may range from complete performance of the procedure to help just with exposure of anatomic structures to facilitate the work of the surgeons at the bedside. It has been estimated that 44,000 to 98,000 deaths annually occur due to errors in hospital care, and that as many as 54% of surgical errors could be prevented. 24 We believe that by enabling the direct intervention of an expert, remote telesurgery will likely reduce the errors that are caused by lack of experience or related to the early phase of the learning curve of new procedures. As a result of this potential impact on training and education, telesurgery might eventually improve the standard of surgical care throughout the world.
However, cautions and strict controls and standards must be put in place before telerobotic surgery becomes common. Teleperformance of a surgical operation requires the expert surgeon to be familiar with robotic devices. Simple expertise in a particular procedure or disease would not be sufficient to provide active support. At present, there are not enough surgeons specifically trained in robotic surgery and remote performance to contribute to the implementation of telesurgery. Admittedly, until such a workforce is available, telesurgery will not likely be capable of reducing errors and related costs on a large scale, not enough at least to become cost-effective.
There is no doubt that remote telesurgery depends on robotic assistance and information technologies and therefore will benefit from the future development of robotic technologies and implementation of virtual reality and simulation in surgical practice. Rehearsal of surgical procedures might be optimized as a stored data set of movements, with false moves eliminated and the perfect surgical procedure delivered by computer-driven and computer-enhanced robotic arms. 25
The enhancement of human dexterity resulting from the use of a robot and performance of high-precision tasks from a distance may have other advantages that extend beyond surgery. Indeed, this achievement symbolizes and realizes an important technological revolution. Whether up-to-date, telecommunication technologies have allowed the sharing of information, voice, and images, here we show for the first time that complex gestures can be performed with high precision and in real time over long distances. Applications of teleperformance of precise tasks might be multiple and might well apply to distant manipulations of hazardous materials such as nuclear or biologic devices.
CONCLUSIONS
Remote robot-assisted telesurgery is feasible and safe using a designated ATM telecommunication line. The possibility of performing complex manipulations from remote locations allows an expert surgeon to teach or proctor the performance of an advanced or new technique by real-time intervention and actually eliminates geographic constraints for obtaining high surgical expertise where this is required.
Acknowledgments
The authors thank Mrs. Heather Smith for her fundamental work in the realization of this project. We also acknowledge the support of France Telecom (Paris, France) and Computer Motion, Inc. (Goleta, CA). A special thanks to Cristophe Rabadan (FT) and Moji Ghodoussi (CM) for their expertise and assistance and to Herve Maisonneuve for his help in editing the manuscript.
Footnotes
Correspondence: Jacques Marescaux, MD, IRCAD-EITS, 1 Place de l’Hopital, 67000 Strasbourg, France.
E-mail: jacques.marescaux@ircad.u-strasbg.fr
Accepted for publication December 5, 2001.
References
- 1.Fabrizio MD, Lee BR, Chan DY, et al. Effect of time delay on surgical performance during telesurgical manipulation. J Endourol 2000; 14: 133–138. [DOI] [PubMed] [Google Scholar]
- 2.Satava RM, Jones SB. Telepresence surgery. In Satava RM, ed. Cybersurgery: Advanced Technologies for Surgical Practice. New York: Wiley-Liss Inc; 1998: 141–154.
- 3.Marescaux J, Leroy J, Gagner M, et al. Transatlantic robot-assisted telesurgery. Nature 2001; 413: 379–380. [DOI] [PubMed] [Google Scholar]
- 4.Satava RM. Emerging technologies for surgery in the 21st century. Arch Surg 1999; 134: 1197–1202. [DOI] [PubMed] [Google Scholar]
- 5.Haluck RS, Krummel TM. Computers and virtual reality for surgical education in the 21st century. Arch Surg 2000; 135: 786–792. [DOI] [PubMed] [Google Scholar]
- 6.Buckingham RA, Buckingham RO. Robots in operating theatres. Br Med J 1995; 311: 1479–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cuschieri A, Szabo Z. Philosophy and set up. In: Cuschieri A, Szabo Z, eds. Tissue Approximation in Endoscopic Surgery. Oxford: Isis Medical Media; 1995: 68–81.
- 8.Soper NJ, Hunter JG. Suturing and knot tying in laparoscopy. Surg Clin North Am 1992; 72: 1139–1152. [DOI] [PubMed] [Google Scholar]
- 9.Gomez-Blanco M, Riviere CN, Khosla PK. Intraoperative tremor monitoring for vitreoretinal microsurgery. Stud Health Technol Inform 2000; 70: 99–101. [PubMed] [Google Scholar]
- 10.Riviere CN, Jensen PS. A study of instrument motion in retinal microsurgery. Abstract presented at 21st Annual Conference of IEEE Eng Med Biol Soc, June 26, 2000, Chicago.
- 11.Garcia-Ruiz A, Smedira NG, Loop FD, et al. Robotic surgical instruments for dexterity enhancement in thoracoscopic coronary artery bypass graft. J Laparoendosc Adv Surg Tech A 1997; 7: 277–283. [DOI] [PubMed] [Google Scholar]
- 12.Marescaux J, Smith MK, Folscher D, et al. Telerobotic laparoscopic cholecystectomy: initial clinical experience with 25 patients. Ann Surg 2001; 234: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cadiere GB, Himpens J, Germay O, et al. Feasibility of robotic laparoscopic surgery: 146 cases. World J Surg 2001; 25: 1467–1477. [DOI] [PubMed] [Google Scholar]
- 14.Falcone T, Goldberg JM, Margossian H, et al. Robotic-assisted laparoscopic microsurgical tubal anastomosis: a human pilot study. Fertil Steril 2000; 73: 1040–1042. [DOI] [PubMed] [Google Scholar]
- 15.Guillonneau B, Jayet C, Tewari A, et al. Robot-assisted laparoscopic nephrectomy. J Urol 2001; 166: 200–201. [PubMed] [Google Scholar]
- 16.Pasticier G, Rietbergen JB, Guillonneau B, et al. Robotically assisted laparoscopic radical prostatectomy: feasibility study in men. Eur Urol 2001; 40: 70–74. [DOI] [PubMed] [Google Scholar]
- 17.Abbou CC, Hoznek A, Salomon L, et al. Laparoscopic radical prostatectomy with a remote controlled robot. J Urol 2001; 165: 1964–1966. [DOI] [PubMed] [Google Scholar]
- 18.LaPietra A, Grossi EA, Derivaux CC, et al. Robotic-assisted instruments enhance minimally invasive mitral valve surgery. Ann Thorac Surg 2000; 70: 835–838. [DOI] [PubMed] [Google Scholar]
- 19.Carpentier A, Loulmet D, Aupecle B, et al. Computer-assisted cardiac surgery. Lancet 1999; 353: 379–380. [DOI] [PubMed] [Google Scholar]
- 20.Kappert U, Schneider J, Cichon R, et al. Development of robotic enhanced endoscopic surgery for the treatment of coronary artery disease. Circulation 2001; 104: 1102–1107. [DOI] [PubMed] [Google Scholar]
- 21.Mack MJ. Minimally invasive and robotic surgery. JAMA 2001; 285: 568–572. [DOI] [PubMed] [Google Scholar]
- 22.Satava RM. Emerging technologies for surgery in the 21st century. Arch Surg 1999; 134: 1197–1202. [DOI] [PubMed] [Google Scholar]
- 23.Forde KA. Ethical, legal and moral issues of advanced technologies. In Satava RM, ed. Cybersurgery: Advanced Technologies for Surgical Practice. New York: Wiley-Liss Inc; 1998: 179–187.
- 24.Kohn LT, Corrigan JM, Donaldson MS. To Err is Human: Building a Safer Health System. Washington, DC: National Academy Press; 1999. [PubMed]
- 25.Krummel TM. Surgical simulation and virtual reality: the coming revolution. Ann Surg 1998; 228: 635–637. [DOI] [PMC free article] [PubMed] [Google Scholar]


