Abstract
Objective
To evaluate the feasibility of real-time reverse transcriptase–polymerase chain reaction (RT-PCR) detection of free cancer cells in the peritoneal washes as a prognostic indicator for patients with gastric carcinoma.
Summary Background Data
Peritoneal lavage cytology (CY) is an excellent prognostic determinant but lacks sensitivity. This can be improved by using RT-PCR to quantitate carcinoembryonic antigen (CEA) mRNA in peritoneal washes.
Methods
Peritoneal washes were obtained from 189 patients with gastric carcinoma during laparotomy. CEA mRNA levels and CEA/GAPDH mRNA ratios were quantified using a real-time PCR system with fluorescent hybridization probes. Receiver-operating characteristic plots were used to determine which of these parameters should be used as a marker for the intraperitoneal cancer cells. The prognostic significance of its positivity was then evaluated by Kaplan-Meier curves, and its value as an independent prognostic factor was evaluated by multivariate analysis.
Results
The sensitivity and specificity of real-time RT-PCR with an optimal cutoff value were 80% and 94%; those for conventional cytology were 56% and 91%. The survival of 16 patients who were CY-PCR+ was poor and approached that of 35 CY+ patients. Recurrence as peritoneal carcinomatosis was frequent among PCR+ patients but rare for their PCR- counterparts. PCR+ was a significant independent prognostic factor, along with the presence of node metastasis and serosal invasion, but CY+ was not.
Conclusions
Quantitative RT-PCR of peritoneal washes can replace cytologic examination as a tool for the sensitive evaluation of the risk of intraperitoneal recurrence in patients with gastric carcinoma.
Gastric carcinoma remains one of the leading causes of cancer death in Japan. Peritoneal carcinomatosis represents the most common route of tumor dissemination in patients with this disease, 1 and recurrence is most likely caused by the presence in the abdominal cavity of metastatic free cancer cells exfoliated from the serosal surfaces of primary cancers. 2 Cytologic examination of peritoneal washes has therefore been performed at laparotomy to detect such cells and evaluate the risk of recurrent disease, 3–7 and is now recognized as an important prognostic determinant in the Western Hemisphere 4,6 as well as in the East. Conventional examination with Papanicolaou staining, however, is reported to lack sensitivity, 8 and improvement in this aspect has been reported by investigators using immunohistochemistry with panels of antibodies. 1,9
We have recently applied the reverse transcriptase–polymerase chain reaction (RT-PCR) for sensitive detection of micrometastases in the peritoneal cavity, using carcinoembryonic antigen (CEA) as a target gene. 10 A decrease in the incidence of false-negative results with this technique has been shown, as evidenced by the fact that peritoneal carcinomatosis was seldom observed after several years of follow-up among patients negative for the examination. 11 The problem with this system is that gene amplification and analysis of PCR products are so time-consuming that results could not be obtained during the operation. In addition, some false-positive results, which may be attributable to CEA-expressing noncancerous cells, have been encountered. CEA mRNA in these cases may be either illegitimately expressed by the noncancerous cells or expressed after induction by various cytokines. 12 Because CEA mRNA levels are expected to be higher in cancer cells, a quantitative technique may be useful to distinguish between the presence of cancer cells and contamination with other CEA-producing cells.
With recent innovations in PCR technology, a new generation of thermal cycler (LightCycler; Roche Diagnostics, Mannheim, Germany) that combines continuous fluorescence monitoring of PCR and rapid-cycle PCR within glass capillaries has become available. 13,14 This real-time fluorescence PCR system allows accurate quantification of initial template copy number, based on the fact that the cycle number at which the sample fluorescence exceeds the background level is correlated with the starting copy number. 15,16 To circumvent the weaknesses of the conventional RT-PCR system, we have established a new protocol for rapid, quantitative detection of free cancer cells in peritoneal washes using the LightCycler system with a hybridization probe format. 17 In the present study, we reviewed the association between intraabdominal CEA mRNA levels quantified by the LightCycler system and survival. The significance of positive CEA mRNA as an independent prognostic factor was also evaluated by multivariate analysis.
METHODS
Peritoneal Washes
At the beginning of each operation, 100 mL saline was introduced into the Douglas cavity and aspirated after gentle stirring. These washes were centrifuged at 1,800 rpm for 5 minutes to collect intact cells, rinsed with phosphate-buffered saline, dissolved in ISOGEN RNA extraction buffer (Nippon Gene, Tokyo, Japan), and stored at −80°C until use. A part of each peritoneal wash was examined cytopathologically after conventional Papanicolaou and Giemsa staining.
cDNA Synthesis
Frozen samples in ISOGEN were thawed and total RNA was extracted using a guanidinium-isothiocyanate-phenol-chloroform-based method. Because the number of cells in the wash fluids is usually small, glycogen (40 μg/mL) for molecular biology (Roche Diagnostics, Mannheim, Germany) was added as a carrier to improve the recovery of RNA before isopropanol precipitation. Extracted total RNA (up to 5 μg) was preincubated with 50 ng random hexanucleotide primer (Pharmacia Biotech, Uppsala, Sweden) in 9 μL solution for 10 minutes at 70°C. After chilling on ice, 4 μL fivefold synthesis buffer (250 mmol/L Tris-HCl, pH 8.3, 375 mmol/L KCl, 15 mmol/L MgCl2), 2 μL of 100 mmol/L dithiothreitol, 4 μL of 2.5 mmol/L each dNTP and 1 μL SuperScript II RNase H− reverse transcriptase (200 units/μL, GIBCO BRL, Gaithersburg, MD) were added. The reaction mixture was then incubated for 40 minutes at 42°C, and the resultant first-strand cDNA was immediately used for PCR amplification with the LightCycler. Remaining cDNA solution was heated at 70°C for 15 minutes and stored at −80°C until further analysis.
Real-Time RT-PCR With the LightCycler
Real-time RT-PCR was performed with a single-step method using hybridization probes as fluorophores as reported previously. 17 In brief, a CEA-specific oligonucleotide primer pair consisted of sense primer: 5′-AACTTCTCCTGGTCTCTCAGCT, and an antisense primer: 5′-GCAAATGCTTTAAGGAAGAAG, which were designed to recognize sequences in the M/3′ exon and the 3′ exon of the CEA gene and to span large intron sequences. The donor probe (5′-TGAAATGAAGAAACTACACCAGG-FL) was labeled at the 3′ end with fluorescence, whereas the acceptor probe (5′-LC-CTGCTATATCAGAGCAACCCCAA-P) was labeled at the 5′ end with LightCycler-Red640 and modified at the 3′ end by phosphorylation to block extension. To quantify and prove the integrity of the isolated RNA, real-time RT-PCR analysis for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was carried out using specific primers (sense: 5′-TGAACGGGAAGCTCACTGG, antisense: 5′-TCCACCACCCTGTTGCTGTA). Nucleotide sequences of the hybridization probes used were as follows: donor, 5′-TCAACAGCACACCCACTCCT- FL; acceptor, 5′-LC-CACCTTTGACTCTGGGGCT-P. All primers and probes were synthesized and purified by reverse-phase HPLC by Nihon Gene Research Laboratories (Sendai, Japan).
PCR amplification of CEA mRNA using a LightCycler was carried out in 10 μL reaction mixture consisting of a master mixture containing Taq DNA polymerase, dNTP mixture and buffer (LightCycler DNA Master hybridization probes, Roche Diagnostics), 4.0 mmol/L MgCl2, 0.25 μmol/L sense and antisense primer, 0.4 μmol/L of each probe, and 1 μL template cDNA in a LightCycler capillary. Before amplification, 0.16 μL anti-Taq DNA polymerase antibody (TaqStart antibody, Clontech Lab, Palo Alto, CA) was added to the reaction mixture with incubation at room temperature for 5 minutes to block primer elongation. For CEA amplification, 95°C (90 seconds) for antibody inactivation was followed by 50 rounds of amplification at 95°C (0 seconds) for denaturation, 50°C (10 seconds) for annealing, and 72°C (10 seconds) for extension, with a temperature slope of 20°C per second performed in the LightCycler instrument. Real-time PCR monitoring was achieved by measuring the fluorescent signal at the end of the annealing phase for each cycle. For GAPDH amplification, the same temperature profile was used except for the extension step, 72°C for 20 seconds.
A moderately differentiated, CEA-producing adenocarcinoma cell line derived from a liver metastasis in a patients with colon cancer (COLM-2) was used for preparing external standards for CEA mRNA, with 10-fold serial dilutions of cDNA (1–105 COLM-2 cells). The slope of this standard curve was less than 3.50, indicating a PCR amplification efficiency of at least 90% based on the equation (1 + 0.9)3.59 = 10, which means sufficient amplification efficiency for reliable quantitative estimation. Thus, the current assay system consisting of single-step RT-PCR could detect reproducibly from 100 (=1) to 105 COLM-2 colon carcinoma cells mixed with 1 × 107 peripheral blood leukocytes, indicating a comparable sensitivity to conventional nested-primer RT-PCR, although the low copy range (100–10 cells) is relatively less reproducible than the high copy range (102–105 cells). External standards for GAPDH mRNA were also prepared by 10-fold serial dilutions (102–107 leukocytes) of cDNA equivalent to 1 × 107 peripheral blood leukocytes. Each run consisted of six external standards, a negative control without a template, and patient samples with unknown mRNA concentrations. Quantitation of mRNA in each sample was then performed automatically with reference to the standard curve constructed each time by the LightCycler software.
Patients
Between 1995 and 1998, there were 193 patients who had histologically proven gastric adenocarcinomas, underwent laparotomy at Aichi Cancer Center Hospital, underwent RT-PCR analysis of the peritoneal washes, and underwent regular postoperative surveillance for at least 2 years or until death. Two patients who died within 30 days of surgery as a result of perioperative complications were excluded. GAPDH mRNA was undetectable from samples of another two patients, possibly because of a small number of cells. Data from the remaining 189 patients were analyzed in the current study. The depth of cancer invasion (pT categories) was evaluated histologically according to the TNM classification, 18 except for the seven patients who were considered to have unresectable disease. T categories for these patients were decided on surgical findings. Of the 182 patients with resectable cancer, 150 underwent potentially curative R0 resection 18 and the remaining 32 were treated with palliative resection. There were 68 patients with T1 cancer (cancer confined to the mucosa or invading as far as the submucosa), 52 patients with T2 tumors (invasion beyond the submucosa but not as far as the serosa), 55 with T3 tumors (serosal invasion), and 14 with T4 tumors (invasion to adjacent tissues). The population included 26 patients with synchronous peritoneal metastasis.
Surgical Procedure
After laparotomy, the abdominal cavity was thoroughly examined for tumor metastasis, peritoneal deposits in particular. Samples of the latter were taken whenever they were observed, and the diagnosis of cancer metastasis was histologically confirmed with frozen sections before closing the abdomen. When potentially curative R0 resection 18 was considered possible, gastrectomy with D2 lymphadenectomy was the treatment of choice. Palliative resection was performed and chemotherapy given at the discretion of the surgeons for the patients who were not treated with R0 resection. Gastrectomy was avoided for those with extensive invasion to the retroperitoneum and for those with extensive peritoneal dissemination.
Postoperative Surveillance
The follow-up program consisted of interim history, physical examination, hematology, and blood chemistry panels including tests for CEA and CA19-9, performed every 3 months for the first postoperative year and every 6 months thereafter. Either abdominal ultrasonography or computed tomography was carried out every 6 months. Peritoneal recurrence, evident on the basis of clinical symptoms, digital examination, and physical and radiologic findings of bowel obstruction and ascites, was confirmed by paracentesis, laparotomy, and autopsy performed at the discretion of the surgeon.
Statistical Analysis
Survival analyses were made by Kaplan-Meier curves with death and clinical diagnosis of the peritoneal carcinomatosis as the endpoints. For the analysis of survival with diagnosis of peritoneal metastasis as the endpoint, cancer deaths resulting from other types of metastasis in the absence of clinical signs of peritoneal carcinomatosis were treated as censored cases.
Receiver-operating characteristic (ROC) curves 19 were used to compare the accuracies of two parameters, CEA mRNA levels and CEA/GAPDH mRNA ratios, for distinguishing between the patients positive and negative for the intraperitoneal metastatic cancer cells. Being positive for the intraperitoneal cancer cells in this analysis was arbitrarily defined either as having macroscopic dissemination at surgery or being diagnosed clinically as having peritoneal carcinomatosis during the 2 years of follow-up. ROC curves were constructed by plotting all possible sensitivity/specificity pairs for the two parameters, resulting from continuously varying the cutoff values over the entire range of results obtained. Sensitivity in this context was defined as the positive rate for CEA mRNA or the CEA/GAPDH mRNA ratio with peritoneal washes of patients who had synchronous peritoneal seeding or relapse in the form of peritoneal carcinomatosis within 2 years of postoperative surveillance. Specificity was the negative rate for CEA mRNA or the CEA/GAPDH ratio among the patients free from peritoneal metastasis at surgery and without signs of peritoneal carcinomatosis thereafter. For both CEA mRNA and CEA/GAPDH ratio, sensitivity on the y axis was plotted against the false-positive fraction (1 - specificity) on the x axis with various cutoff values ranging from 0 to 50,000. The sensitivity increases at the expense of specificity as the cutoff value is lowered, and a plot lying above and to the left of another plot indicates greater observed accuracy.
Independent prognostic factors were identified through analysis of the 189 patients by multivariate analysis using the Cox regression hazards model. Node metastasis, peritoneal metastasis, serosal invasion, and positive finding by the conventional cytology examination were selected as covariates, along with the result of RT-PCR. Multivariate analysis was performed also with 150 patients who underwent curative R0 resection, because the knowledge of the peritoneal fluid CEA mRNA level may be irrelevant for predicting the outcome of other 39 patients.
RESULTS
CEA mRNA Levels, GAPDH mRNA Levels, and CEA/GAPDH Ratios According to Depth of Tumor Invasion
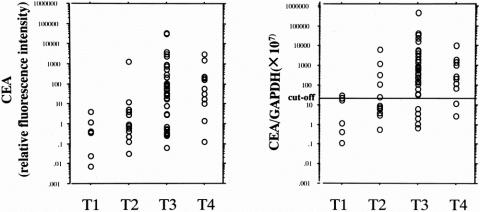
The integrity and amount of extracted RNA were examined by RT-PCR analysis of GAPDH mRNA. The values for GAPDH mRNA were then used as internal controls to calculate the CEA/GAPDH ratio, which represents the amount of CEA mRNA per given number of viable cells. The results of quantitative analysis by the LightCycler for CEA mRNA and CEA/GAPDH mRNA ratios are summarized in Figure 1. Median CEA mRNA values for peritoneal washes from the Douglas cavity varied according to the T category and were 0 for T1, 0 for T2, 3.1 for T3, and 24 for pT4. Median GAPDH mRNA values for each of the T categories were 3.5 × 105, 4.0 × 105, 1.3 × 106, and 1.9 × 106, and there was a trend toward a greater number of viable cells in the abdominal cavity as the cancers invaded the serosa. Accordingly, the median CEA/GAPDH mRNA ratios (CEA mRNA/GAPDH mRNA × 107) for each of the T categories were 0, 0, 64, and 163, respectively. Six samples of peritoneal washes obtained during surgery for benign disease had been tested during the preliminary phase of this study, and CEA mRNA was not detected in any of these samples.
Figure 1. Relative values for CEA mRNA and CEA:GAPDH mRNA ratios in peritoneal washes of gastric carcinoma patients stratified by T categories of the TNM classification. Quantification was performed by real-time PT-PCR using a LightCycler.
Accuracies of CEA mRNA Levels and CEA/GAPDH Ratios for Detecting Intraperitoneal Cancer Cells
ROC curve analyses were performed for CEA mRNA levels and CEA/GAPDH ratios using data from all 189 patients evaluated (Fig. 2). Sensitivities and specificities were calculated based on the diagnosis of peritoneal metastases made at surgery or during the postoperative surveillance period of 2 years. There was no difference in area under the curve between CEA mRNA and CEA/GAPDH ratio, indicating that the correction of CEA mRNA values with respect to GAPDH may not be strictly necessary. However, as described above, there were 2 samples out of 193 in which GAPDH mRNA was not detected. Because a small number of cells are being dealt with in the current assay system, quality control of the RNA samples through measuring the CEA/GAPDH ratio was considered mandatory. It was therefore decided that the subsequent survival analyses should be made based on the CEA/GAPDH ratio. A plot for conventional cytology examination with a sensitivity of 56% and a specificity of 91% was observed far below the curves for CEA mRNA and CEA/GAPDH ratio.
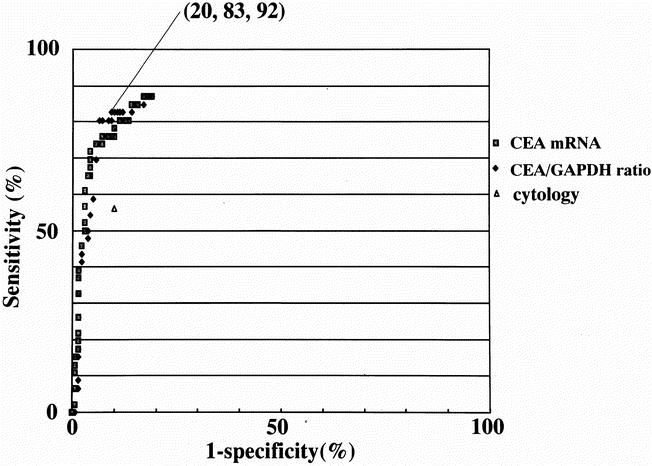
Figure 2. Receiver operating characteristics (ROC) curves for CEA mRNA levels and CEA:GAPDH mRNA ratios in peritoneal washes of patients with gastric carcinomas. These curves are constructed by plotting all possible sensitivity/specificity pairs for CEA mRNA levels or CEA/GAPDH mRNA ratios, resulting from continuously varying the cut-off values over the entire range of results obtained (CEA mRNA [•], CEA/GAPDH mRNA ratio [⋄], and cytology examination [▴]). Sensitivity is defined as the positive rate for CEA mRNA of the CEA/GAPDH mRNA ratio with peritoneal washes of patients with synchronous peritoneal seeding or relapse in the form of peritoneal carcinomatosis within 2 years of postoperative surveillance. Specificity is the negative rate for CEA mRNA of the CEA/GAPDH ratio among the patients free from peritoneal metastasis at surgery and without signs of peritoneal carcinomatosis thereafter. For both CEA mRNA levels and CEA/GAPDH mRNA ratios, sensitivity on the y-axis was plotted against the false-positive fraction (1-specificity) on the x-axis for various cut-off values ranging from 0 to 50,000. A plot lying above and to the left of another plot indicates greater observed accuracy. One of the ROC plots with the CEA/GAPDH ratio of 30 was selected as a cut-off value for the survival analysis in this study. This plot is highlighted with the figures in parenthesis indicating sensitivity and specificity.
Any of the ROC plots can be selected as a cutoff value for this assay system, depending on the sensitivity and specificity required. At a cutoff value for the CEA/GAPDH ratio of 0.6, for instance, the maximum sensitivity obtained by the LightCycler was 87%, superior to the sensitivity of 56% achieved by conventional cytology. At this cutoff value, however, specificity was 83%, and 5 of 68 patients with T1 tumors (with invasion confined to the mucosa or the submucosa) were diagnosed as positive. Of these, all five patients are disease-free to date, so these results will have to be considered as false-positives. If no case of these T1 tumors were to be ranked as positive, the cutoff value had to be raised to 30, where the specificity of 94% was obtained but the sensitivity was reduced to 80%. In this study, a cutoff value of 30 was used for the subsequent analyses. The correlations between the results of RT-PCR at this cutoff value and T categories, presence or absence of node metastasis, and presence or absence of peritoneal deposits at the time of surgery were statistically significant (Table 1). The positivities for CEA/GAPDH ratio for each of the T categories were 0%, 9%, 58%, and 64%; those of conventional cytology were 1.5%, 5.8%, 45%, and 43%, respectively.
Table 1. POSITIVITIES OF THE CEA/GAPDH RATIO OF THE PERITONEAL WASHES AND CYTOLOGIC EXAMINATION FOR VARIOUS PROGNOSTIC VARIABLES
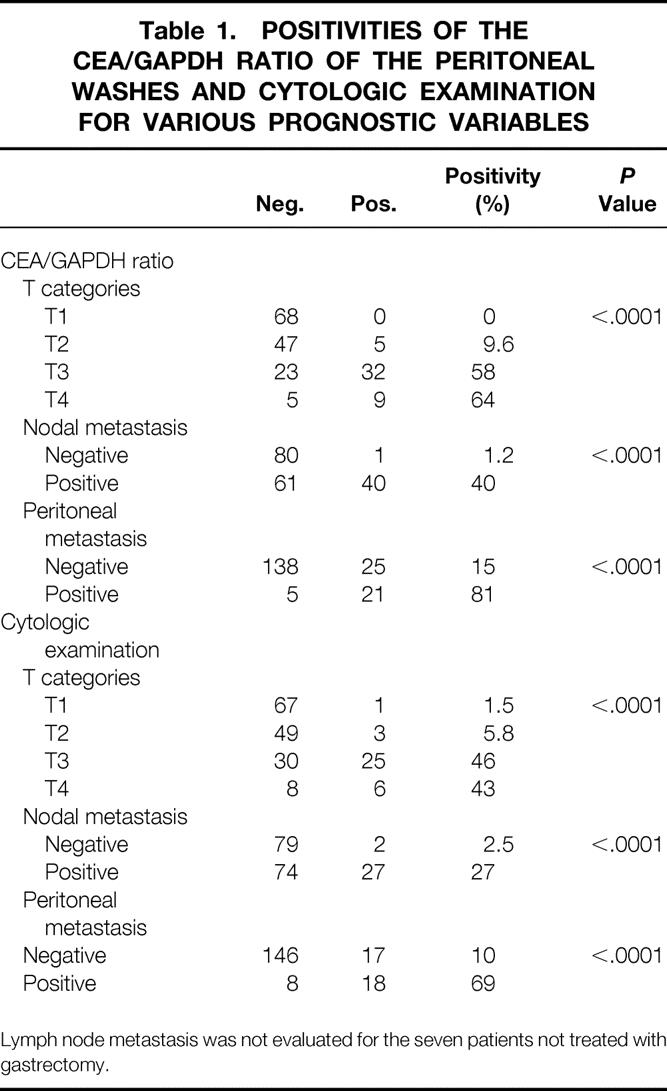
Lymph node metastasis was not evaluated for the seven patients not treated with gastrectomy.
Results of Cytology and Real-Time RT-PCR and Their Relation to Survival
The 189 patients with gastric carcinoma were stratified into the following three groups according to the results of cytology and RT-PCR: a group of 35 patients who were positive for cytology (CY+), a group of 16 patients positive for PCR but negative for cytology (CY-PCR+), and a group of 138 patients negative for both cytology and PCR (CY-PCR-). The median CEA/GAPDH ratio for CY+, CY-PCR+, and CY-PCR- were 338, 320, and 0, respectively. Survival curves for these three groups showed the prognosis of the CY-PCR+ group to approach that of the CY+ group, indicating that the knowledge of the peritoneal fluid CEA mRNA level has a place as a prognostic indicator even after the result of cytologic examination has been obtained (Fig. 3).
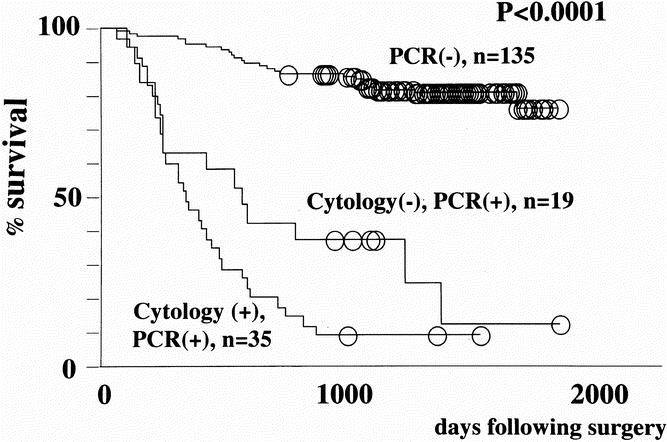
Figure 3. Survival curves of all 189 patients with gastric carcinomas stratified according to the results of cytology and real-time RT-PCR. A cut-off value of 30 for CEA/GAPDH ratio was employed.
The predictive power of the current system is more strikingly illustrated with survival analysis made with diagnosis of peritoneal carcinomatosis as the endpoint (Fig. 4). The 26 patients with macroscopic peritoneal deposits at surgery were excluded from this analysis. Whereas 9 of 11 patients in the CY-PCR+ group had peritoneal carcinomatosis, this pattern of failure was a rarity (5/135) among the CY-PCR- group.
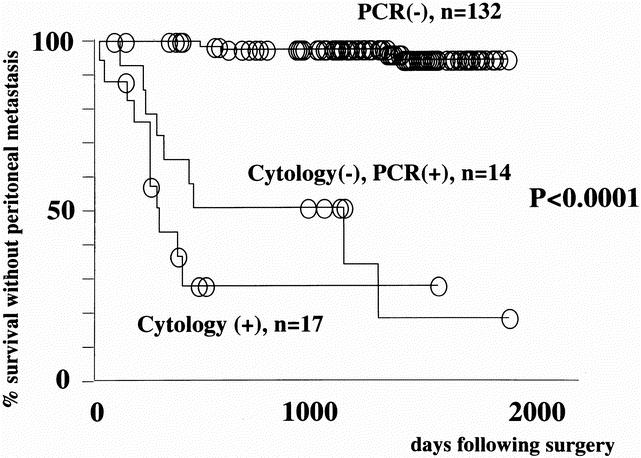
Figure 4. Peritoneal recurrence-free survival of gastric carcinoma patients stratified according to the results of cytology and real-time RT-PCR. The patients with peritoneal dissemination at surgery (n = 26) were excluded from the analysis. Deaths due to other types of metastasis were treated as censored cases.
Value of Cytology and RT-PCR as Independent Prognostic Factors
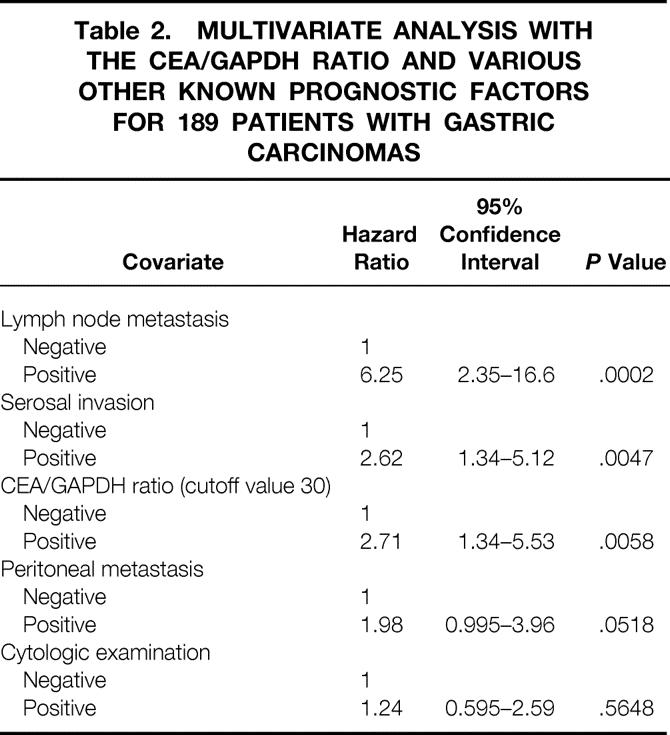
The results of cytologic examination and RT-PCR were both significant by the univariate analysis as prognostic factors. They were evaluated as covariates in the multivariate analysis involving all 189 patients along with other known significant factors: presence of serosal invasion, nodal metastasis, and peritoneal deposits at surgery (Table 2). The CEA/GAPDH mRNA ratio above the cutoff value of 30 was confirmed as a significant independent prognostic factor, along with the histologic finding of nodal metastasis and serosal invasion, whereas detection of free cancer cells with cytology examination was not.
Table 2. MULTIVARIATE ANALYSIS WITH THE CEA/GAPDH RATIO AND VARIOUS OTHER KNOWN PROGNOSTIC FACTORS FOR 189 PATIENTS WITH GASTRIC CARCINOMAS
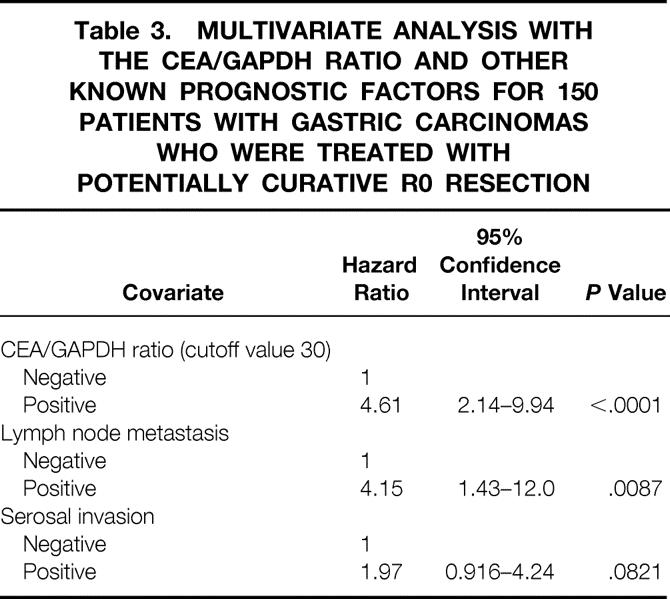
Multivariate analysis was performed also with 150 patients who underwent curative R0 resection (Table 3), because the knowledge of the peritoneal fluid CEA mRNA level may be irrelevant for predicting the outcome of the other 39 patients who have residual disease. In this group, the CEA/GAPDH mRNA ratio was the most prominent independent prognostic factor, followed by the presence of nodal metastasis.
Table 3. MULTIVARIATE ANALYSIS WITH THE CEA/GAPDH RATIO AND OTHER KNOWN PROGNOSTIC FACTORS FOR 150 PATIENTS WITH GASTRIC CARCINOMAS WHO WERE TREATED WITH POTENTIALLY CURATIVE R0 RESECTION
DISCUSSION
Unlike colorectal cancer, the recurrence of which frequently takes the form of hematogenous metastasis to the liver, locally advanced gastric carcinoma is most likely to develop into a state of peritoneal carcinomatosis. 1 This is the basis for application of detection of free cancer cells in peritoneal washes by cytologic examination for assessing the likelihood of early recurrence. No patient with a positive cytologic result accompanied by serosal invasion survived for more than 3 years in two series. 5,7 Intraperitoneal recurrence, on the other hand, was observed in several patients with negative cytologic results, 8 indicating a lack of sensitivity for conventional cytologic examination. With the current real-time RT-PCR technique, peritoneal carcinomatosis among the patients who had been negative for the test became a rarity. At the same time, it was shown clearly by the multivariate analysis that the CEA/GAPDH ratio is a powerful independent prognostic factor for the patients who underwent curative resection, and is capable of replacing conventional cytologic examination.
In addition to the quantitative detection of CEA mRNA, 17 steps for the quantitative detection of GAPDH mRNA were taken in this study to confirm the quality of the extracted RNA. This highlighted 2 samples without a detectable amount of RNA among the original 193 samples, and use of the CEA/GAPDH ratio is now recommended for clinical application of the LightCycler system from the standpoint of reliability. Whether the CEA/GAPDH ratio could accurately predict the peritoneal metastasis, however, had been a matter of concern because the total number of cancer cells in the samples represented by CEA mRNA levels and not the cancer cell/noncancer cell ratio was considered as the hallmark of risk for metastatic spread. The areas under the ROC curves revealed nevertheless that the CEA/GAPDH ratio and CEA mRNA levels have essentially the same accuracy in making the intended discrimination between those positive and negative for intraperitoneal metastatic cancer cells. When calculating the sensitivities and specificities for the ROC plots, the decision regarding positivity of the intraperitoneal cancer cells was arbitrarily made based on the results of 2 years of postoperative surveillance. Although more patients with recurrent disease may emerge after a longer period of observation, alterations in data resulting from these late recurrences can be estimated to be marginal, given that the mean length of time to peritoneal recurrence is reported to be 18.1 months after curative surgery. 20 It has been reported also that approximately 75% of tumor-related deaths occur within 2 years. 21
Some investigators have attempted to improve on the sensitivity of cytologic examination by using immunohistochemistry. 1,9 Among the patients who underwent potentially curative resection for gastric carcinoma with invasion to the serosa (T3), free cancer cells were detected in no more than 21%5 by conventional cytologic examination in our previous report. The detection rates reported by other groups were also in the range of 18% to 24%. 1,2,8 In contrast, free cancer cells were detected in 33% of patients with curatively resected T3 tumors 1 and in 38% of stage II-III gastric carcinomas 9 through immunostaining of cytospins. Despite this clear advantage over conventional Papanicolaou staining, sensitivities achieved by these attempts still fell short of the sensitivity obtained with RT-PCR. Further, use of at least three different antibodies is recommended for immunostaining to circumvent antigen heterogeneity of solid tumors, 9 adding complexity to the detection system. There are further advantages with the LightCycler system in that it is less time-consuming 17 and more easily automated and standardized than methods involving immunostaining. 22
The problem consistently encountered with methods that involve RT-PCR technique concerns specificity. After a number of reports describing the successful application of this technique for the detection of micrometastases, 23–26 several criticisms have emerged in the literature pointing to unexpectedly high false-positive rates. 27–29 These false-positive results may be attributable to the illegitimate expression of target mRNA in noncancerous cells, notably leukocytes in the peripheral blood. This becomes a considerable problem when the samples to be evaluated are of peripheral blood or bone marrow aspirates. For instance, CEA mRNA expression has been detected in stem cells of peripheral blood from cancer patients treated with granulocyte-colony stimulating factor (G-CSF) 12 and even in peripheral blood leukocytes from healthy volunteers, 27,28 rendering detection of circulating cancer cells unreliable when using a PCR system that amplifies CEA. A definite way of overcoming this problem is to find and make use of a novel marker gene that is expressed in all cancer cells while not being detectable in any noncancerous cells. Although this is an ultimate goal for all investigators, no success has so far been reported, particularly with gastrointestinal cancer. However, other approaches may also allow a reduction in the false-positive rate. In a previous report, we found that levels of CEA mRNA that had been detected in the peripheral blood samples from healthy volunteers were relatively low. 17 This implies that leukocytes and other blood cell components do not usually express CEA mRNA at levels equivalent to cancer cells. In addition, samples of peritoneal washes contain even smaller amounts of blood cell components than bone marrow aspirates or peripheral blood samples. It was therefore predicted that false-positive results due to weak expression of CEA mRNA from contamination by these noncancerous cells could be eliminated if the PCR were performed quantitatively and the CEA levels below a certain cutoff value were ignored as insignificant. By raising the cutoff value of CEA/GAPDH ratio to 30, none of the patients with T1 tumors became positive for the current assay. However, some other patients with T2-T3 tumors with CEA/GAPDH ratio of above 30 also survived disease-free for more than 2 years. At least some of these patients may thus represent additional cases of false-positive results. To summarize the issue of specificity, false-positive results could be reduced but not eliminated, even with the application of quantitative analysis.
Finally, quantitative measurement of CEA mRNA by the LightCycler with subsequent automatic data analysis can be completed well within 3 hours. 17 Besides its use simply as a prognostic determinant, the results of RT-PCR can now be used to augment macroscopic findings for decisions concerning the application of treatment options in addition to surgical resection. For example, the current detection system can be used for selecting patients who are eligible for intraperitoneal chemotherapy 30 or chemohyperthermia 31, which could be effective against microscopic dissemination while showing limited activity toward gross metastases. Future clinical trials of adjuvant chemotherapy versus surgery alone may be conducted for populations with detectable intraperitoneal CEA mRNA, in the absence of other evidence of residual disease.
Acknowledgments
The authors thank Miss N. Yamada for expert technical assistance and Dr. N. Hamajima of the Department of Epidemiology, Aichi Cancer Center Research Institute, for critically reviewing the statistical methods used in the study.
Footnotes
Supported in part by a grant from the Ministry of Education, Science and Culture, Japan, and a Grant-in-Aid from the Imanaga Memorial Foundation.
Correspondence: Yasuhiro Kodera, MD, Department of Gastroenterological Surgery, Aichi Cancer Center Hospital, 1-1 Kanokoden, Chikusa-ku, Nagoya 464, Japan.
E-mail: ykodera@mvi.biglobe.ne.jp
Accepted for publication October 5, 2001.
References
- 1.Benevolo M, Mottolese M, Cosimelli M, et al. Diagnostic and prognostic value of peritoneal immunocytology in gastric cancer. J Clin Oncol 1998; 16: 3406–3411. [DOI] [PubMed] [Google Scholar]
- 2.Koga S, Kaibara N, Iitsuka Y, et al. Prognostic significance of intraperitoneal free cancer cells in gastric cancer patients. J Cancer Res Clin Oncol 1984; 108: 236–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boku T, Nakane Y, Minoura T, et al. Prognostic significance of serosal invasion and free intraperitoneal cancer cells in gastric cancer. Br J Surg 1990; 77: 436–439. [DOI] [PubMed] [Google Scholar]
- 4.Bonenkamp JJ, Songun I, Hermans J, et al. Prognostic value of positive cytology findings from abdominal washings in patients with gastric cancer. Br J Surg 1996; 83: 672–674. [DOI] [PubMed] [Google Scholar]
- 5.Kodera Y, Yamamura Y, Shimizu Y, et al. Prognostic value of positive findings in patients with gastric carcinoma undergoing a potentially curative resection. J Surg Oncol 1999; 72: 60–65. [DOI] [PubMed] [Google Scholar]
- 6.Hayes N, Wayman J, Wadehra V, et al. Peritoneal cytology in the surgical evaluation of gastric carcinoma. Br J Cancer 1999; 79: 520–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bando E, Yonemura Y, Takeshita Y, et al. Intraoperative lavage for cytological examination in 1297 patients with gastric carcinoma. Am J Surg 1999; 178: 256–262. [DOI] [PubMed] [Google Scholar]
- 8.Abe S, Yoshimura H, Tabata H, et al. Curative resection of gastric cancer: Limitation of peritoneal lavage cytology in predicting the outcome. J Surg Oncol 1995; 59: 226–229. [DOI] [PubMed] [Google Scholar]
- 9.Juhl H, Stritzel M, Wroblewski A, et al. Immunocytological detection of micrometastatic cells: Comparative evaluation of findings in the peritoneal cavity and the bone marrow of gastric, colorectal, and pancreatic cancer patients. Int J Cancer 1994; 57: 330–335. [DOI] [PubMed] [Google Scholar]
- 10.Nakanishi H, Kodera Y, Torii A, et al. Detection of carcinoembryonic antigen-expressing free tumor cells in peritoneal washes from patients with gastric carcinoma by polymerase chain reaction. Jpn J Cancer Res 1997; 88: 687–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kodera Y, Nakanishi H, Yamamura Y, et al. Prognostic value and clinical implications of disseminated cancer cells in the peritoneal cavity detected by reverse transcriptase-polymerase chain reaction and cytology. Int J Cancer 1998; 79: 429–433. [DOI] [PubMed] [Google Scholar]
- 12.Goeminne JC, Guillaume T, Salmon M, et al. Unreliability of carcinoembryonic antigen (CEA) reverse transcriptase-polymerase chain reaction (RT-PCR) in detecting contaminating breast cancer cells in peripheral blood stem cells due to induction of CEA by growth factors. Bone Marrow Transplant 1999; 24: 769–775. [DOI] [PubMed] [Google Scholar]
- 13.Wittwer CT, Herrmann MG, Moss AA, et al. Continuous fluorescence monitoring of rapid cycle DNA amplifications. Biotechniques 1997; 22: 130–138. [DOI] [PubMed] [Google Scholar]
- 14.Wittwer CT, Ririe KM, Andrew RV, et al. The LightCycler: a microvolume multisample fluorimeter with rapid temperature control. Biotechniques 1997; 22: 176–181. [DOI] [PubMed] [Google Scholar]
- 15.Gerard CJ, Olsson K, Ramanathan R, et al. Improved quantitation of minimal residual disease in multiple myeloma using real-time polymerase chain reaction and plasmid-DNA complementarity determining region III standards. Cancer Res 1998; 58: 3957–3964. [PubMed] [Google Scholar]
- 16.Kreuzer KA, Lass U, Bohn A, et al. LightCycler technology for the quantitation of bcr/abl fusion transcripts. Cancer Res 1999; 59: 3171–3174. [PubMed] [Google Scholar]
- 17.Nakanishi H, Kodera Y, Yamamura Y, et al. Rapid quantitative detection of carcinoembryonic antigen-expressing free tumor cells in the peritoneal cavity of gastric-cancer patients with real-time RT-PCR on the LightCycler. Int J Cancer 2000; 89: 411–417. [DOI] [PubMed] [Google Scholar]
- 18.Sobin LH, Wittekind C. TNM Classification of Malignant Tumors, 5th ed. New York: John Wiley & Sons; 1997.
- 19.Zweigh MH, Campbell G. Receiver-operating characteristics (ROC) plots, a fundamental evaluation tool in clinical medicine. Clin Chem 1993; 39: 561–577. [PubMed] [Google Scholar]
- 20.Yoo CH, Noh SH, Shin DW, et al. Recurrence following curative resection for gastric carcinoma. Br J Surg 2000; 87: 236–242. [DOI] [PubMed] [Google Scholar]
- 21.Siraishi N, Inomata M, Osawa N, et al. Early and late recurrence after gastrectomy for gastric carcinoma. Univariate and multivariate analyses. Cancer 2000; 89: 255–261. [DOI] [PubMed] [Google Scholar]
- 22.Goeminne J-C, Guillaume T, Symann M. Pitfalls in the detection of disseminated non-hematological tumor cells. Ann Oncol 2000; 11: 785–792. [DOI] [PubMed] [Google Scholar]
- 23.Gerhard M, Juhl H, Kalthoff H, et al. Specific detection of carcinoembryonic antigen-expressing tumor cells in bone marrow aspirates by polymerase chain reaction. J Clin Oncol 1994; 12: 725–729. [DOI] [PubMed] [Google Scholar]
- 24.Soeth E, Vogel I, Roder C, et al. Comparative analysis of bone marrow and venous blood isolates from gastrointestinal cancer patients for the detection of disseminated tumor cells using reverse transcription PCR. Cancer Res 1997; 57: 3106–3110. [PubMed] [Google Scholar]
- 25.Mori M, Mimori K, Ueo H, et al. Clinical significance of molecular detection of carcinoma cells in lymph nodes and peripheral blood by reverse transcription-polymerase chain reaction in patients with gastrointestinal or breast carcinomas. J Clin Oncol 1998; 16: 128–132. [DOI] [PubMed] [Google Scholar]
- 26.Ghossein RA, Bhattacharya S, Rosai J. Molecular detection of micrometastases and circular tumor cells in solid tumors. Clin Cancer Res 1999; 5: 1950–1960. [PubMed] [Google Scholar]
- 27.Zippelius A, Kufer P, Honold G, et al. Limitations of reverse-transcriptase polymerase chain reaction analyses for detection of micrometastatic epithelial cancer cells in bone marrow. J Clin Oncol 1997; 15: 2701–2708. [DOI] [PubMed] [Google Scholar]
- 28.Ko Y, Klinz M, Totzke G, et al. Limitations of the reverse transcription-polymerase chain reaction method for the detection of carcinoembryonic antigen-positive tumor cells in peripheral blood. Clin Cancer Res 1998; 4: 2141–2146. [PubMed] [Google Scholar]
- 29.Ko Y, Grunewald E, Totzke G, et al. High percentage of false-positive results of cytokeratin 19 RT-PCR in blood: A model for the analysis of illegitimate gene expression. Oncology 2000; 59: 81–88. [DOI] [PubMed] [Google Scholar]
- 30.Yu W, Whang I, Suh I, et al. Prospective randomized trial of early postoperative intraperitoneal chemotherapy as an adjuvant to resectable gastric cancer. Ann Surg 1998; 228: 347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fujimoto S, Takahashi M, Mutou M, et al. Successful intraperitoneal hyperthermic chemoperfusion for the prevention of postoperative peritoneal recurrence in patients with advanced gastric carcinoma. Cancer 1999; 85: 529–534. [PubMed] [Google Scholar]