Abstract
Objective
To examine the expression of adhesion molecules by serosal and dermal fibroblasts in patients with inflammatory bowel disease.
Summary Background Data
The pathophysiologic process that leads to stricture formation in Crohn’s disease (CD) is unknown. Serosal fibroblasts in these patients have an enhanced ability to contract collagen. This property may be reflected in fibroblast adhesion molecule expression, which in turn may be constitutive or secondary to the inflammatory process in patients with CD.
Methods
Fibroblasts were isolated from inflamed and macroscopically normal serosa of patients with CD or ulcerative colitis (UC) and from normal serosa of patients with colon cancer. Dermal fibroblasts were also isolated from the wound edge. Cell surface and whole cell expression of ICAM-1 were evaluated by flow cytometry and Western blot analysis, respectively. NFκB was measured by mobility shift assay in parallel experiments. Interleukin 1β was added to the culture medium.
Results
Expression of ICAM-1 and NFκB, increased in patients with both CD and UC, was unaltered by interleukin 1β. The whole cell concentration of ICAM-1 was greater in patients with CD than in patients with UC. Dermal fibroblasts did not display these features.
Conclusions
Patients with inflammatory bowel disease display enhanced ICAM-1 expression in serosal fibroblasts but not dermal fibroblasts, indicating a secondary response to inflammation.
Most patients with Crohn’s disease (CD) ultimately require surgical resection of their disease. 1 Stricture formation and intestinal obstruction is the precipitating factor in many of these patients. Despite the significant morbidity associated with this complication, the etiology of stricture formation remains unclear. Efforts to explain the stricturing process characteristic of CD have focused on identification of the cell types and the connective tissue matrix present within CD strictures. Smooth muscle cell accumulation and excessive deposition of collagen may contribute toward the stricturing process. 2,3 In addition, there are alterations in the predominant collagen subtypes found in the connective tissue matrix of gastrointestinal strictures in patients with CD. 2,3
The fibroblast plays a key role in the collagen production, rearrangement, and fibrosis that is essential in wound healing. 4 Fibroblasts are influenced by multiple cytokines 5,6 secreted both by acute inflammatory cells and neighboring fibroblasts. During wound healing, fibroblasts express cellular adhesion molecules (e.g., ICAM-1 and CD11b) that are essential for intercellular interaction 7 and part of normal fibroblast function.
Extensive research in the field of rheumatology has produced new insights into the molecular mechanisms involved in fibroblast activity. 8,9 Altered fibroblast adhesion molecule expression has been described among patients with fibrotic conditions such as scleroderma. 10 It is unclear whether these findings are a primary phenomenon or secondary to the disease process, but these observations may have important implications regarding the pathophysiology of inflammatory bowel disease (IBD).
Expression of cellular adhesion molecules, including ICAM-1, is controlled by gene transcription. Cytokines affect adhesion molecule expression through their effect on intracellular enzyme cascades, which culminate in the release of cellular transcription factors (e.g., NFκB). Control of gene expression by transcription factors is central to regulated cellular function. NFκB regulates the expression of genes responsible for promoting inflammation and regulating apoptosis. NFκB is a pleiotropic transcription factor with key functions in the intestinal immune system. The NFκB family controls transcriptional activity of various promoters of proinflammatory cytokines, cell surface receptors, transcription factors, and cellular adhesion molecules that are involved in intestinal inflammation. 11 NFκB can be found in the cytoplasm of most cells as an inactive complex with unprocessed precursor proteins or IκB proteins. 12 Activation of cells with various stimuli initiates a signaling cascade that induces site-specific phosphorylation of IκBα and leads to dissociation of the complex. 13 NFκB then translocates into the nucleus and binds to specific DNA gene promoter regions, thus activating or augmenting transcription.
Increased production of proinflammatory cytokines in the intestinal mucosa may be important in the pathophysiology of intestinal inflammation in IBD. Many of the genes that code for these cytokines are transcriptionally regulated by NFκB binding site proteins. 14,15 The presence of nuclear transcription factors in fibroblasts and other inflammatory cells would therefore appear to be a common denominator among all cell types, including fibroblasts, in patients with IBD. This intracellular final common pathway may provide an attractive target for therapeutic strategies in patients with CD.
Previous work from our laboratory has shown that fibroblasts isolated from the serosa of patients with CD exhibit an increased ability to contract a collagen matrix in vitro. 16 Whether this phenomenon represents a constitutive abnormality in patients with CD or is a secondary response to transmural inflammation is unknown and is the subject of this study.
METHODS
Eighteen patients were recruited into the study. Eight patients with CD and five with UC who were undergoing surgical resection for their disease formed the experimental group. Five patients undergoing bowel resection for colon cancer acted as a control group. Patients with evidence of acute sepsis were excluded.
At laparotomy, a 1-cm2 serosal biopsy sample was harvested from macroscopically normal and macroscopically diseased bowel of patients with CD and UC. Similar biopsy samples were taken from patients with colon cancer. All serosal biopsy samples were harvested before devascularization of the bowel segment. In addition, small samples of dermis were taken from the wound edge. Biopsy specimens were transported immediately to the laboratory in sterile RPMI culture medium and incubated for a maximum of 4 hours before processing.
Reagents and Antibodies
RPMI medium, penicillin and streptomycin solution, l-glutamine, and fetal calf serum (FCS) were purchased from GIBCO Life Technologies Ltd., Paisley, UK. Murine antihuman ICAM-1 was purchased from Becton Dickinson, Cambridge, UK. All remaining chemicals were purchased from Sigma-Aldrich Company Ltd., Dorset, UK, if not otherwise stated.
Tissue Culture
Serosal biopsy samples were placed into petri dishes within a laminar flow hood. Each biopsy specimen was dissected into 0.5-mm pieces of tissue. Four tissue culture dishes were coated with 10% FCS, and the 0.5-mm pieces of tissue were placed onto the dishes, approximately 20 per dish. Culture medium comprised 500 mL RPMI, l-glutamine 5 mL, 1% penicillin, 1% streptomycin, 1% Fungizone, and 10% FCS. Ten milliliters of culture medium was added to each dish gradually over a 10-minute period. All dishes were then incubated for 3 weeks until confluent fibroblasts were evident. Cells were fed on day 4 and weekly thereafter. When confluence was achieved, cells were trypsinized and transferred into tissue culture flasks so that a pure fibroblast culture could be isolated.
Trypsin EDTA was used to trypsinize the cells. Culture medium was removed and the base of the flask was coated in trypsin, which was then removed immediately. Five milliliters of trypsin was then added to the flask, and it was incubated at 37°C for 3 minutes. The trypsin was then neutralized by the addition of 10 mL culture medium. The suspension was then removed from the flask and spun at 1,100 rpm for 5 minutes. The resultant pellet was then resuspended and the cells split between two tissue culture flasks. Cells were studied between the third and fifth passages.
Cell Surface ICAM-1 Expression
Once confluent, fibroblasts were isolated by trypsinization and expression of ICAM-1 was assessed by flow cytometry. Ten thousand fibroblasts in 100 μL medium were incubated with 10 μL murine antihuman ICAM-1 antibody at 4°C for 30 minutes and washed with RPMI and analyzed.
Whole Cell ICAM-1 Expression
Total protein expression was determined from 2 × 106 fibroblasts using NP-40 isolation solution (0.5% NP-40, Tris 10 nmol/L pH 8.0, 60 nmol/L KCl, 1 mmol/L EDTA pH 8.0, 1 mmol/L DTT, 10 mmol/L PMSF, and 1 μmol/L leupeptin and aprotinin). Isolated protein was measured by the Bradford assay Protein Detection Kit (Bio-Rad, Hercules, CA) and loaded at 50 μg/well. Samples were then run on 12% SDS polyacrylamide gradient gel (140 V for 60 minutes) then electrophoretically transferred to Immobilon-P (Millipore, Bedford, MA) (100 V, 45 minutes). The remaining gel was stained after transfer with ponceau S solution (2%) to confirm equal loading. Blots were incubated with murine anti-ICAM-1 primary antibody (1:1,000) in 1% BSA TBS and 0.1% Tween 20 for 1 hour at room temperature and then incubated with horseradish peroxidase-conjugated antimouse IgG at 1:5,000 dilution for 1 hour. Blots were developed using an enhanced chemiluminescence system. 17
NFκB
Nuclear extracts were prepared from 20 × 106 isolated neutrophils. A total of 5 μg protein from the extracts was preincubated with the nonspecific DNA competitor poly(dl-dC) (5mg) for 10 minutes at room temperature. Probes were labeled with [32P] that contained two NFκB sites and derived from the HIV-1 enhancer were added for an additional 20 minutes at room temperature (Amersham, Unitec, Dublin, Ireland). DNA/protein complexes were resolved on a 5% nondenaturing polyacrylamide (60:1 cross-link)/Tris glycine gel and autoradiographs were prepared by exposure at −70°C using Kodak X-OMAT film. To demonstrate the specificity of the protein/DNA complex, either a 125 mol/L excess of the unlabeled probe or a mutated HIV enhancer probe was added to the nuclear extract before the addition of the radiolabeled probe. The sequence of the plus strand of the oligonucleotide used was HIV-1 enhancer 5′-AGG GAC TTT CCG CTG GGA CTT TCC-3′.
RESULTS
Cell surface ICAM-1 expression was increased in serosal fibroblasts isolated from patients with IBD (Fig. 1). Fibroblasts from patients with both CD and UC showed an increased expression of ICAM-1 compared with fibroblasts isolated from cancer controls (analysis of variance, P < .05).
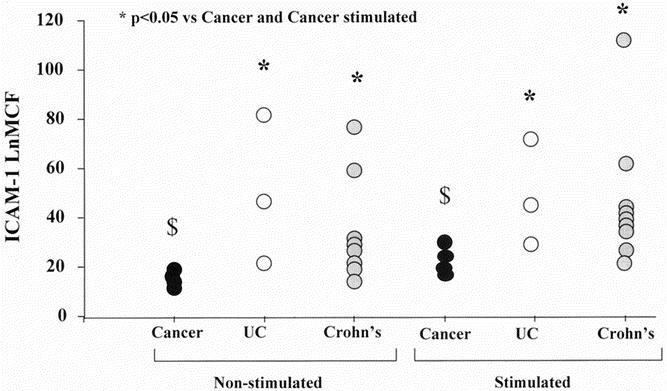
Figure 1. Serosal fibroblast cell surface ICAM-1 expression in inflammatory bowel disease. Serosal fibroblasts were cultured from patients with colon cancer, ulcerative colitis (UC), and Crohn’s disease. Fibroblasts were then stimulated with interleukin (IL) 1β at a concentration of 200 IU/mL. The expression of ICAM-1 was measured using flow cytometry and expressed graphically. The expression of ICAM-1 was significantly higher in patients with Crohn’s disease and UC compared with cancer both before and after stimulation with IL-1β. *P < .05 vs. cancer and cancer stimulated: IL-1β 200 IU/mL (analysis of variance). $ P < .05 versus cancer stimulated (analysis of variance). LnMCF, log of mean channel fluorescence.
Incubation of serosal fibroblasts in interleukin (IL) 1β at a concentration of 200 IU/mL significantly increased ICAM-1 expression in control fibroblasts (Mann-Whitney. P < .01)(Table 1) Dermal fibroblasts isolated from patients with CD did express increased ICAM-1 after stimulation with IL-1β, but this result did not achieve statistical significance. IL-1β at this concentration did not enhance ICAM-1 expression in serosal fibroblasts in patients with IBD.
Table 1. ICAM-1 EXPRESSION IN PATIENTS WITH CROHN’S DISEASE
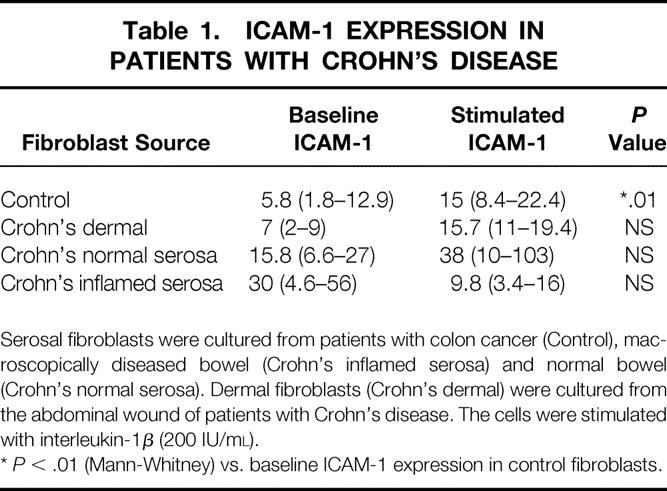
Serosal fibroblasts were cultured from patients with colon cancer (Control), macroscopically diseased bowel (Crohn’s inflamed serosa) and normal bowel (Crohn’s normal serosa). Dermal fibroblasts (Crohn’s dermal) were cultured from the abdominal wound of patients with Crohn’s disease. The cells were stimulated with interleukin-1β (200 IU/ml).
*P < .01 (Mann-Whitney) vs. baseline ICAM-1 expression in control fibroblasts.
There was no significant difference in cell surface ICAM-1 expression between patients with CD and UC (see Fig. 1). When whole cell ICAM-1 expression was measured, patients with CD and UC again expressed increased cellular ICAM-1 compared with cancer controls (Fig. 2). Patients with CD showed a significantly greater concentration of whole cell ICAM-1 compared with patients with UC (analysis of variance, P < .05). Stimulation with IL-1β did not affect whole cell ICAM-1 expression in patients with IBD.
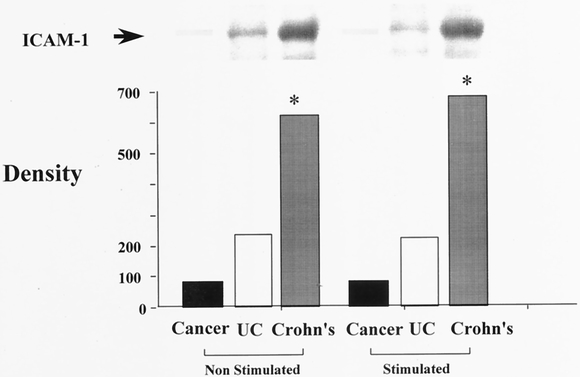
Figure 2. Serosal fibroblast whole cell ICAM-1 expression in inflammatory bowel disease. Serosal fibroblasts cultured from patients with Crohn’s disease, ulcerative colitis (UC), and colon cancer were stimulated with interleukin-1β at a concentration of 200 IU/mL. Fibroblast protein was isolated and whole cell ICAM-1 concentration measured using Western blot analysis. Band densitometry is represented graphically beneath the Western blot. *P < .05 vs. cancer and UC (analysis of variance). LnMCF, log of mean channel fluorescence.
Among patients with CD, expression of ICAM-1 was increased in both macroscopically normal and inflamed tissue compared with cancer controls and fibroblasts isolated from the dermis of patients with CD (Fig. 3).
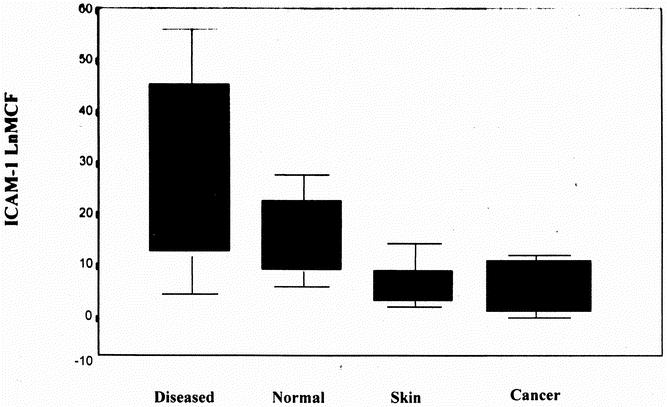
Figure 3. Differential ICAM-1 expression in patients with Crohn’s disease. Serosal fibroblasts were cultured from macroscopically inflamed (Diseased; n = 6) and normal (n = 4) areas of the bowel of patients with Crohn’s disease. Fibroblasts were also cultured from the dermis of the abdominal wound of patients with Crohn’s disease (Skin; n = 5) and from control fibroblasts isolated from patients with colon cancer (n = 5). Cellular surface ICAM-1 expression was measured using flow cytometry (LnMCF). The results are expressed as median, interquartile range, and range. *P < .05 vs. Cancer and Skin (analysis of variance).
The expression of NFκB was increased in serosal fibroblasts isolated from patients with UC and CD (Fig. 4).
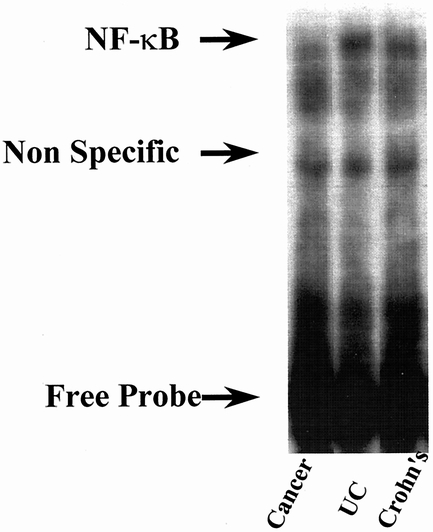
Figure 4. NFκB is increased in fibroblasts isolated from patients with inflammatory bowel disease. Nuclear extracts were prepared from serosal fibroblasts cultured from patients with Crohn’s disease, ulcerative colitis (UC), and cancer controls. A representative radioactive electrophoretic mobility shift assay using consensus oligonucleotides to detect NFκB in nuclear extracts of fibroblasts is shown. Arrows indicate the position of the κB-specific DNA binding activity and the position of nonspecific control activity. In both Crohn’s and UC, NFκB activity was increased compared with controls, but there was no difference in activity between patients with UC and Crohn’s disease.
DISCUSSION
Inflammatory bowel disease is characterized by persistence of the acute inflammatory response. This persistent inflammation can be attributed to failure to clear antigenic stimuli and cells that initiate the acute inflammatory response. CD is associated with stricture formation, but the association between acute inflammation and eventual stricture formation is undefined. Because fibroblasts are intimately involved in tissue repair and scar formation, it is likely that there is an association between fibroblasts, the acute inflammatory response, and eventual stricture formation.
Previous work has shown that serosal fibroblasts in CD have an enhanced ability to contract collagen. 16,18 In the current study these observations are extended to show that serosal fibroblasts in IBD express increased levels of the adhesion molecule ICAM-1. Patients with CD not only express increased levels of ICAM-1 on the cell surface of fibroblasts, seen also in UC, but also store increased levels of this adhesion molecule within the cell cytoplasm. This finding may indicate that serosal fibroblasts in CD are primed with ICAM-1 and therefore have the capacity to display enhanced intercellular interaction. This may be an important prerequisite for enhanced collagen contraction and possible stricture formation. It may also explain why IL-1β at a concentration of 200 IU/mL failed to stimulate a further enhancement of ICAM-1 expression in fibroblasts in CD but did enhance that of control fibroblasts that may be in a resting state.
Serosal fibroblasts in patients with CD and UC showed enhanced expression of NFκB compared with control fibroblasts from patients with colon cancer. Intracellular levels of the nuclear transcription factor NFκB relate to cellular cytokine production. This may reflect enhanced genetic transcription and protein production (e.g., ICAM-1), resulting from cellular cytokine stimulation in patients with IBD. Schreiber et al 19 showed increased expression of activated NFκB in the lamina propria cells of patients with IBD compared with controls. This work also showed that corticosteroids strongly inhibited activation of NFκB by stabilizing the cytosolic inhibitor IκBα against activation-induced degradation. Interestingly, Wahl et al 20 also showed that sulfasalazine, an agent frequently used in maintenance of disease remission in IBD, is a potent inhibitor of NFκB. Nonsteroidal antiinflammatory drugs such as indomethacin and aspirin, in addition to inhibiting cyclooxygenase, are also inhibitors of NFκB activation through their effects on Iκβ. 21
Dermal fibroblasts did not show enhanced ICAM-1 production. This indicates that changes in adhesion molecule expression in serosal fibroblasts do not represent a constitutive abnormality but merely a response to the high concentration of cytokines and other growth factors within the milieu of the acute inflammatory response.
Identification of such factors is the subject of ongoing research. Cytokines that have gained clinical relevance include IL-10 and tumor necrosis factor-alpha (TNFα). Monoclonal anti-TNFα has shown considerable promise in treatment of fistulating CD. 22
An exciting new development is the identification of a NOD 2 mutation at the IBD 1 susceptibility locus on chromosome 16 affecting individuals in families with CD. 23,24 This development opens the way for identification of extracellular and intracellular pathways that lead to persistent inflammation and protracted tissue remodeling and repair. Already clinical trials of antisense ICAM-1 have shown promise and provide a realistic expectation of therapeutic manipulation to modify stricture formation in CD. 25,26
Footnotes
Supported in part by a grant from the Mater College.
Correspondance: P. Ronan O’Connell, MD, Suite 12, Mater Private Hospital, Eccles Street, Dublin 7, Ireland.
E-mail: rocon@indigo.ie
Accepted for publication October 1, 2001.
References
- 1.Farmer RG, Whelan G, Fazio VW. Long-term follow-up of patients with Crohn’s disease: relationship between clinical pattern and prognosis. Gastroenterology 1985; 88: 1818. [DOI] [PubMed] [Google Scholar]
- 2.Graham MF, Diegelmann R, Elson CO, et al. Collagen content and types in the intestinal strictures of Crohn’s disease. Gastroenterology 1988; 94: 257–265. [DOI] [PubMed] [Google Scholar]
- 3.Stallmach A, Schuppan D, Riese HH, et al. Increased collagen type III synthesis by fibroblasts isolated from strictures of patients with Crohn’s disease. Gastroenterolgoy 1992; 102: 1920–1929. [DOI] [PubMed] [Google Scholar]
- 4.Kirschner CW, Shetler MR. Collagen and mucopolysaccharides in the hypertrophic scar. Connect Tissue Res 1974; 3: 205–213. [DOI] [PubMed] [Google Scholar]
- 5.Bendall LJ, Kortlepel K, Gottlieb DJ. Bone marrow fibroblast exposure to the inflammatory cytokines tumour necrosis factor-α and interferon-γ increases adhesion of acute myeloid leukaemia cells and alters the adhesive mechanism. Exp Haematol 1997; 25: 132–139. [PubMed] [Google Scholar]
- 6.Denton CP, Xu S, Black CM, Pearson JD. Scleroderma fibroblasts show increased responsiveness to endothelial cell derived IL-1 and bFGF. J Invest Dermatol 1997; 108: 269–274. [DOI] [PubMed] [Google Scholar]
- 7.Hynes RO. Integrins. Versatility, modulation, and signalling in cell adhesion. Cell 1992; 69: 11–25. [DOI] [PubMed] [Google Scholar]
- 8.Yellin MJ, Winikoff S, Fortune SM, et al. Ligation of CD40 on fibroblasts induces CD54 (ICAM01) and D106 (VCAM-1) up regulation and IL-6 production and proliferation. J Leukoc Biol 1995; 58: 209–216. [DOI] [PubMed] [Google Scholar]
- 9.Xu SW, Denton CP, Dashwood MR, et al. Endothelin-1 regulation of intracellular adhesion molecule-1 expression in normal and scleroderma fibroblasts. J Cardiovasc Pharmacol 1998; 31: S545–547. [DOI] [PubMed] [Google Scholar]
- 10.Shi-Wen X, Panesar M, Vancheeswaran R, et al. Expression and shedding of intercellular adhesion molecule 1 and lymphocyte function-associated antigen 3 by normal and scleroderma fibroblasts, effects of interferon-γ, tumour necrosis factor-α, and estrogen. Arthritis Rheum 1994; 37: 1689–1697. [DOI] [PubMed] [Google Scholar]
- 11.Neurath MF, Becker C, Barbulescu K. Role of NF-κB in immune and inflammatory responses in the gut. Gut 1998; 43: 856–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baeuerle P, Henkel T. Function and activation of NFκB in the immune system. Annu Rev Immunol 1994; 12: 141–153. [DOI] [PubMed] [Google Scholar]
- 13.Brown K, Gerstberger S, Carlson L, et al. Control of IκBα proteolysis by site-specific signal induced phosphorylation. Science 1995; 267: 1485–1487. [DOI] [PubMed] [Google Scholar]
- 14.Shakov AN, Collart MA, Vassali P, et al. Kappa B-type enhancers are involved in lipopolysaccharide-mediated transcriptional activation of the tumour necrosis factor α gene in primary macrophages. J Exp Med 1990; 171: 35–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kunsch C, Rosen CA. NF-kappa B subunit specific regulation in the interleukin-8 promoter. Mol Cell Biol 1993; 13: 6137–6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Regan MC, Flavin BM, Fitzpatrick JM, O’Connell PR. Stricture formation in Crohn’s disease: the role of intestinal fibroblasts. Ann Surg 2000; 231: 46–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harvey RF, Bradshaw JM. A simple index of Crohn’s disease activity. Lancet 1980; I: 514. [DOI] [PubMed] [Google Scholar]
- 18.Watson RWG, O’Neill A, Brannigan AE, et al. Regulation of Fas antibody induced neutrophil apoptosis is both caspase and mitochondrial dependent. FEBS Lett 1999; 453: 67–71. [DOI] [PubMed] [Google Scholar]
- 19.Schreiber S, Nikolaus S, Hampe J. Activation of nuclear factor kappa B in inflammatory bowel disease. Gut 1998; 42: 477–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wahl C, Liptay S, Adler G, Schmid RM. Sulfasalazine: a potent and specific inhibitor of nuclear factor kappa B. Immunology 1998; 156: 2119–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yin Min-Jean, Yamamoto Y, Gaynor R. The anti-inflammatory agents aspirin and salicylate inhibit the activity of I κB kinase. Nature 1998; 396: 77–80. [DOI] [PubMed] [Google Scholar]
- 22.Present DH, Rutgeerts P, Targan S, et al. Infliximab for the treatment of fistulas in patients with Crohn’s disease. N Engl J Med 1999; 340: 1398–1405. [DOI] [PubMed] [Google Scholar]
- 23.Hugot JP, Chamallard M, Zowall H, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature 2001; 411: 599–603. [DOI] [PubMed] [Google Scholar]
- 24.Ogura Y, Bonen D, Inohara N, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature 2001; 411: 603–606. [DOI] [PubMed] [Google Scholar]
- 25.Schreiber S, Nikolaus S, Malchow H, et al. Absence of efficacy of subcutaneous antisense ICAM-1 treatment of chronic active Crohn’s disease. Gastroenterology 2001; 120: 1339–1346. [DOI] [PubMed] [Google Scholar]
- 26.Yacyshyn BR, Bowen-Yacyshyn MB, Jewell L, et al. A placebo controlled trial of ICAM-1 antisense in the treatment of Crohn’s disease. Gastroenterology 1998; 114: 1133–1142. [DOI] [PubMed] [Google Scholar]


