Abstract
Objective
The purpose of this study was to investigate the clinical usefulness of microvessel density (MVD) and an in vivo angiogenesis parameter, color Doppler vascularity index (CDVI), in patients with gastric cancer.
Summary Background Data
Many studies have reported a significant association between the degree of MVD-evaluated angiogenesis with the clinicopathologic factors and prognosis of patients with various solid tumors. All these studies were accomplished on tissue sections retrospectively obtained from surgical specimens. However, an in vivo method to assess tumor angiogenesis for human malignancies is highly desirable for diagnostic purpose, treatment planning, and follow-up. The CDVI is a new ultrasound parameter for evaluating in vivo angiogenesis, has a good correlation with status of lymph node metastasis in cervical carcinoma, and can predict distant metastasis and survival in colon cancer patients. Therefore, the CDVI may also be useful to assess in vivo angiogenesis in human gastric cancer.
Methods
A total of 79 patients with gastric cancer were enrolled in this study, and microvessel density was evaluated by using immunohistochemical staining of surgical specimens with anti-CD-34 antibody. Tumors were sonographically visible in 31 patients. The CDVI of each tumor was determined using transabdominal color Doppler ultrasound. The CDVI was defined as the ratio of the number of the colored pixels within a tumor section to the number of total pixels in that specific tumor section, and was calculated by using Encomate software (Electronic Business Machine Co. Ltd., Taipei, Taiwan). Correlation between MVD, CDVI and clinicopathologic factors and patient survival was studied.
Results
The MVD was significantly correlated with vascular invasion by multiple linear regression analysis. Although the survival of patients with high MVD (> 32) was significantly worse than those with low MVD (< 32) by univariate analysis, vascular invasion was an independent prognostic factor by Cox proportional hazard model. There was a linear correlation between CDVI and MVD (r = .495, P = .005). Moreover, in patients with a high CDVI (> 11%), the survival rate was significantly lower than that in those with low CDVI (≤ 11%, P = .005). None of the patients with high CDVI (> 11%) survived 2 years after curative resection. In addition to vascular invasion, the CDVI was another independent prognostic factor in the patients with stage III gastric cancer.
Conclusions
Vascular invasion was an important prognostic indicator in gastric cancer. The high CDVI was a good preoperative indicator of early death in stage III gastric cancer patients. Thus, the CDVI may be helpful in selecting patients with gastric cancer for neoadjuvant chemotherapy and/or anti-angiogenic therapy.
Angiogenesis is a very complex phenomenon and essential for the growth of solid tumors measuring more than a few millimeters. 1 It permits rapid tumor growth and potential presence of tumor metastasis. 2,3 It is not easy to develop a single method capable of detecting such a complex biological phenomenon. At present the most widely used method to assess angiogenesis in human malignancies is the quantification of microvessel density (MVD) of tumors using specific markers for endothelial cells including factor VIII-related antigen, CD31, and CD34. 4–11 These studies have reported an association between the degree of angiogenesis and the clinicopathologic factors and prognosis of patients with various solid tumors, such as breast, 4–6 lung, 7 prostate, 8 head and neck, 9 and gastrointestinal cancer. 10,11 All these studies were accomplished on tissue sections retrospectively obtained from surgical specimens. However, an in vivo method to assess tumor angiogenesis for human malignancies is highly desirable for diagnostic purpose, treatment planning, and follow-up.
With the current technique of color Doppler sonography, tumor vascularity can be assessed in vivo. 12 The correlation of the color Doppler vascular signals with the angiographic and histologic findings has also been shown in tumors of various human organs. 13,14 Incremental angiogenesis could be demonstrated in the tumorigenesis of ovarian and endometrial malignancies with color Doppler ultrasound. 15,16 Vascularity index is a new ultrasound parameter for evaluating in vivo angiogenesis. Several reports have revealed that vascularity index could be used to differentiate the nature of neck lymph node 17 and have a good correlation with status of lymph node metastasis in cervical carcinoma. 18 Conventional transabdominal sonography has become increasingly important not only in evaluating diseases of solid organ but also in diagnosis of the gastrointestinal diseases. 19-21 Recently, we also found that color Doppler vascularity index (CDVI) can predict distant metastasis and survival in colon cancer patients. 22 Therefore, the CDVI may also be used to assess in vivo angiogenesis in human gastric cancer.
To investigate the clinical usefulness of the CDVI and MVD in gastric cancer, this study was conducted to elucidate the correlation between CDVI and MVD, and evaluate association between these two angiogenesis parameters, clinicopathological factors, and survival in patients with sonographically visible gastric cancer.
PATIENTS AND METHODS
Patients
A total of 79 patients with gastric cancer, who had undergone radical gastrectomy at our institution from July 1995 to March 1999, were included in this study. They were all proved to have adenocarcinomas by panendoscopic biopsies. They were staged according to TNM system. Criteria for consideration as curative resection were the complete removal of a primary gastric tumor, D2 dissection of regional lymph nodes, and no macroscopic tumor being left behind. They had no detectable metastasis in liver, peritoneum, and distant organ at the time of surgery. No other previous or concomitant primary cancer was present. Abdominal ultrasound was performed before operation to evaluate liver and other intraperitoneal metastases routinely without hydrogastric preparation after overnight fasting. The tumor mass of gastric cancer could be clearly delineated by trans-abdominal ultrasound in 31 patients (39%) who constituted a subpopulation of this study. These 31 patients ranged in age from 43 to 85 years (average age, 63.7 years); 21 were men and 10 were women. Clinicopathologic characteristics were similar between the original group and the CDVI subgroup. All patients in the CDVI subgroup had stage III disease except one whose disease was stage II (Table 1). No patient had received chemotherapy and radiotherapy before surgery. Clinicopathologic factors including age, sex, gross types of tumors (Borrmann classification), histologic types of tumors (Lauren classification), depth of tumor invasion, status of lymph node metastasis, vascular invasion, tumor size (tumor length × width measured in resected stomach, cm2), and Helicobacter pylori (H. pylori) infection documented with histologic findings were reviewed and stored in patients’ data base. Vascular invasion was considered to be definite only when tumor cells and red blood cells were noted together in an endothelium-lined vascular space or when tumor cells were found in an endothelium-lined vascular space with a definite smooth muscle layer. The tissue was considered positive for H. pylori if faintly blue staining curved bacilli were seen in the mucus of crypts just adjacent to tumor using hematoxylin and eosin stain. The patients were followed up from 3 to 46 months after sugery. The follow-up intervals were calculated as survival intervals after surgery.
Table 1. CHARACTERISTICS OF PATIENTS STUDIED
Sonographic Technique and Quantification of Vascular Density in Color Doppler Images
The scanner we used was a color Doppler ultrasound unit (HDI 3000 or 5000, Advanced Technology Laboratories, Bothell, WA) with a 2–5 MHz curved array or with 5–10 MHz linear array transducers. Settings of the color Doppler ultrasound were standardized for the highest sensitivity in the absence of apparent noise by using a medium wall filter, pulsed repetition frequency of 1000 Hz, color gain of 78–79%, moderate-to-long persistence, and a slow sweep technique. Under these conditions, the lowest possible measurable velocity was claimed below 5 cm/second. Focusing depth was set between 1.5 and 5 cm. Each tumor was scanned thoroughly and tangential scanning was made to avoid the intraluminal air interference as much as possible. The color window was set to cover the whole tumor on the screen. The tumor of the gastric cancer was then scanned carefully in all directions, and the tumor section with subjectively maximal color signals was captured and stored for later analysis. Each tumor was scanned three times; thus, three tumor sections with maximal color signals were available for quantatitive analysis. After the examination, the previously stored images were retrieved and displayed on the monitor. The tumor margin was contoured using a cursor. Quantification of the vascular color signals within the demarcated tumor area was then automatically performed using the special software (Encomate, Electronic Business Machine Co., Ltd., Taiwan). The results were expressed as the “color Doppler vascularity index (CDVI)” (the number of colored pixels within the tumor section/the number of total pixels in that particular tumor section) (Fig. 1). For each tumor, the mean of the CDVI of three representative tumor sections was used for statistical analysis. 17,18,22
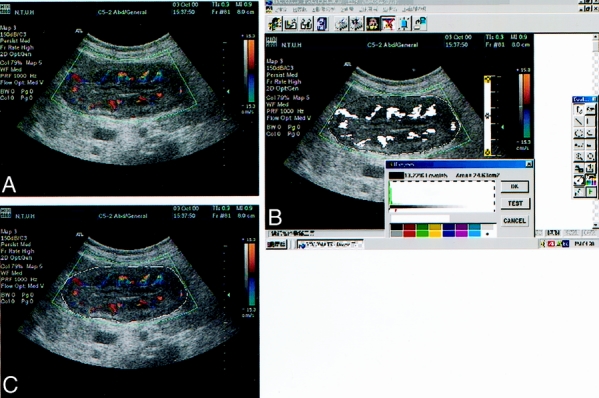
Figure 1. Color Doppler vascularity index assessment within the gastric cancer. The color window was set to cover the whole tumor on the screen and stored for later quantitative analysis (A). The tumor margin was contoured using a cursor (B). Quantification of the vascular color signals within the demarcated tumor was then automatically executed by special software called Encomate (Electronic Business Machine Co., Ltd., Taiwan). The results were expressed as the ‘color Doppler vascularity index‘ (the number of colored pixels within the tumor section/the number of total pixels in that particular tumor section) (C).
Microvessel Staining and Evaluation
The paraffinized tumor blocks of 79 patients with gastric cancers were stained for endothelial cell CD34 antigen using the labelled streptavidin-biotin after antigen retrieval (Fig. 2). Deparaffinized sections were briefly heated in a pressure cooker. After endogenous peroxidase was blocked with 3% hydrogen peroxide in the section, each section was incubated with nonimmuned horse serum. The sections were incubated in an anti-CD34 monoclonal antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) at a dilution of 1:20, or the control nonimmune serum at 4° C overnight. The sections were incubated with link antibodies followed by peroxidase conjugated streptavidin complex (LSAB kit, DAKO Corporation, Carpinteria, CA). The peroxidase activity was visualized with diaminobenzidine tetrahydroxychioride solution (DAB, DAKO corporation, Carpinteria, CA) as the substrate. The sections were lightly counterstained with hematoxylin. After screening the areas with intense neovascularized spots at low power field (100X), microvessels in the area with the highest number of discrete microvessels were counted in a 400X field. Three separate intense neovascularized areas were assessed, and the mean was calculated as microvessel density of each tumor evaluated.
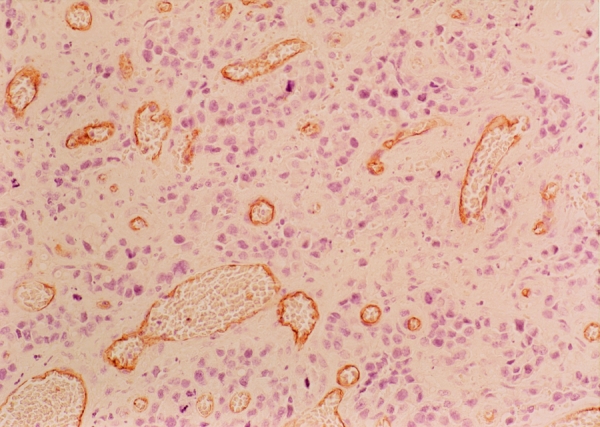
Figure 2. Immunohistochemical staining for CD-34 in gastric cancer tissues (original magnification × 400). Microvessels are represented by brown clusters, which stand out sharply from other tissues.
Statistics
The relationship between MVD, CDVI and the various clinicopathologic factors was examined using a chi-square test. One-way analysis of variance (ANOVA) was used to test the correlation among different TNM stages. Survival curves were calculated using the Kaplan-Meier method and analyzed by the log-rank test. The CDVI and clinicopathologic variables influencing survival was assessed by the Cox proportional hazards model. Statistical significance was defined as P < .05.
RESULTS
Microvessel density of the 79 patients ranged from 5 to 68 with a mean value of 32.4. A significantly higher MVD was found in positive vascular invasion (P = .000), depth of tumor invasion (P = .021), diffuse type cancer (P = .023) and positive Helicobacter pylori infection (P = .013). However, multiple linear regression analysis showed only vascular invasion was significantly correlated with MVD (P = .016) (Table 2). Microvessel density of stage I gastric cancer was significantly lower than that of stage II and III (P = .0007), and there was no significant difference among stage II, IIIa, and IIIb. The outcome of the 79 patients was then analyzed. Because the mean MVD of these patients was 32.4, we classified them into two subgroups: group of MVD > 32 and group of MVD ≤ 32. The survival rates were calculated using the Kaplan-Meier method. The survival rate of the group with high MVD was significantly lower than that with low MVD (P = .0002). The effects of variables presumably associated with patient survival were studied by multivariate analysis using Cox proportional hazards model. As a result, vascular invasion was the only independent prognostic factor in these 79 patients (Table 3).
Table 2. RELATIONSHIP BETWEEN MVD AND THE CLINICOPATHOLOGIC FACTORS BY MULTIVARIATE ANALYSIS IN 79 PATIENTS WITH GASTRIC CANCER
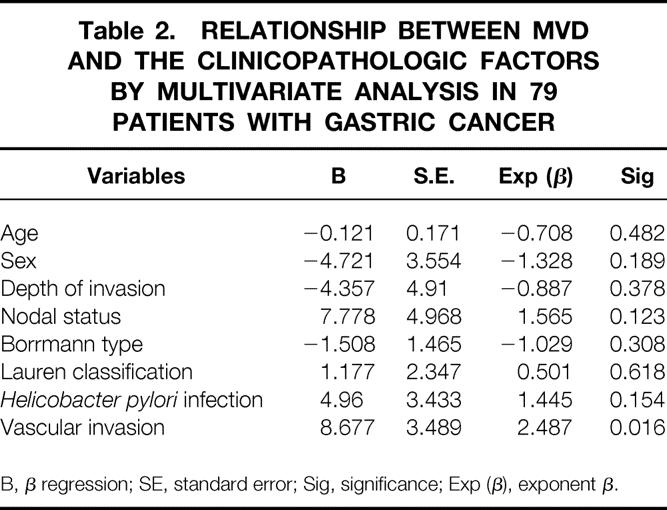
B, β regression; SE, standard error; Sig, significance; Exp (β), exponent β.
Table 3. CLINICOPATHOLOGIC FACTORS AFFECTING SURVIVAL RATE BY MULTIPLE LINEAR REGRESSION ANALYSIS IN 79 PATIENTS WITH GASTRIC CANCER WHO UNDERWENT RESECTION
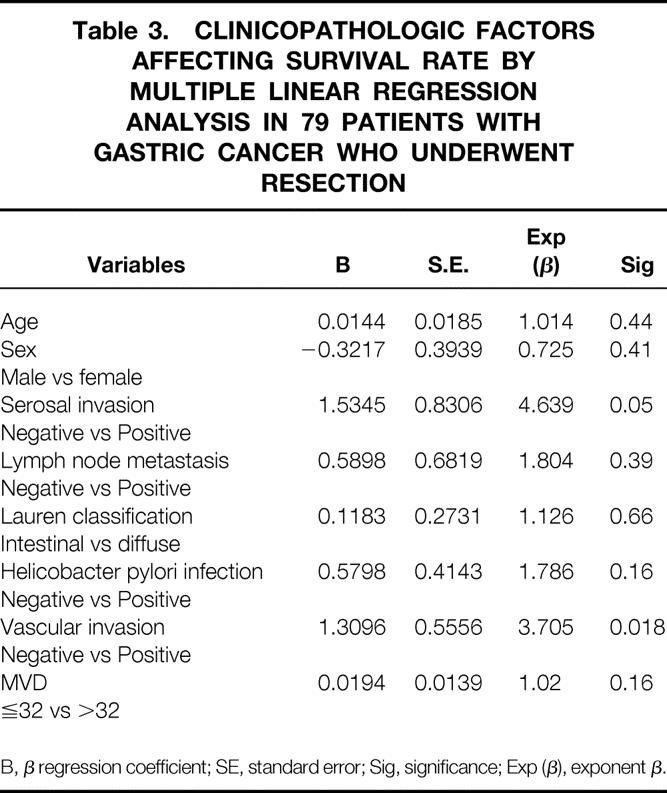
B, β regression coefficient; SE, standard error; Sig, significance; Exp (β), exponent β.
In the CDVI subgroup, the CDVI of the sonographically visible gastric cancers ranged from 1.0% to 30% with a mean value of 11.4%. There was a linear correlation between CDVI and MVD (r = .495, P = .005). The CDVI in the patients with vascular invasion was significantly higher than in those without vascular invasion (P = .026). The CDVI of diffuse type gastric cancer was significantly higher than that of intestinal type (P = .034). There was no significant correlation between CDVI and other clinicopathologic factors such as age, sex, Borrmann types, Helicobacter pylori infection, T stage, tumor location, and tumor size.
The prognosis of the 31 patients who had sonographically visible gastric cancer was then analyzed. Because the mean MVD of these patients was 33.5, we classified them into two subgroups: group of MVD > 34 and group of MVD ≤ 34. The mean CDVI of these patients was 11.4%. Accordingly, the patients were divided into two subgroups: a group of high CDVI (> 11%) and a group of low CDVI (≤ 11%). The survival rates were calculated using the Kaplan-Meier method. The survival rate of the group with high CDVI was significantly lower (P = .005) than that with low CDVI (Fig. 3) and MVD was also the case (P = .0493). There was an intriguing finding that none of the stage III patients with the CDVI > 11% survived beyond 2 years after curative resection.
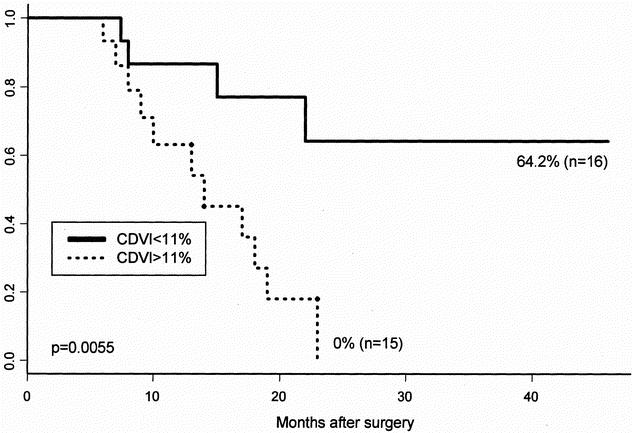
Figure 3. Survival curves of patients with high color Doppler vascularity index (> 11%) and that with low color Doppler vascularity index (≤ 11%).
Simultaneous consideration of the effects of variables, including vascular invasion, CDVI, and MVD on patients’ survival, was studied by multivariate analysis using Cox proportional hazards model. As a result, vascular invasion and CDVI were independent of prognostic factors (Table 4).
Table 4. CLINICOPATHOLOGIC FACTORS AFFECTING OVERALL SURVIVAL RATE DETERMINED BY COX PROPORTIONAL HAZARD MODEL IN STAGE III PATIENTS
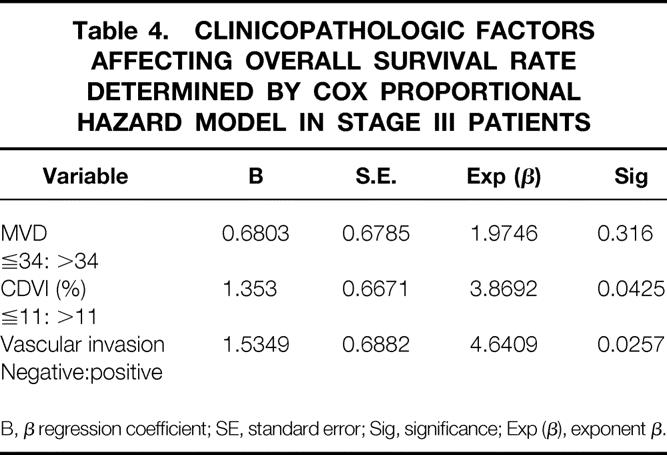
B, β regression coefficient; SE, standard error; Sig, significance; Exp (β), exponent β.
Discussion
In this study, MVD of gastric cancer was correlated with tumor differentiation, tumor invasion depth, lymph node metastasis, and vascular invasion. However, vascular invasion was found to be an independent prognostic factor in the original group and the CDVI subgroup. The CDVI was statistically significantly correlated with MVD, though the correlation between these two parameters was not strong. In addition to vascular invasion, the CDVI was an independent prognostic factor in the CDVI subgroup.
Because the stomach is a curved, horn-shaped and air-filled viscus and located near diaphragm, detection of gastric lesions by transabdominal ultrasound tends to be more difficult and complicated than that in the colon. The major technical limitations are detectability of tumor, color signal noise from cardiac pulse, and peristalsis wave. The measurement of the CDVI needs to delineate the tumor margin clearly and demonstrate reliable color Doppler signals without noise in order to obtain consistent data. In our experience, detection rate of gastric cancer by transabdominal ultrasound was related to tumor size and tumor location. Tumor size seems to be the most important factor. The larger the tumor size is, the higher the detection rate is. Tumor location is another important factor. Tumors located at anterior wall near lesser curvature of the antrum and low body can be visualized more frequently than those at posterior wall and fundus. Therefore, they are the reasons why the CDVI could be measured in only 39% of gastric cancers.
After the tumor was clearly visualized, the color window was set in size to just cover the gastric mass of interest and to improve the color sensitivity and frame rate. The plane with the maximum vascular signals within a gastric mass was examined by adjusting the scanning planes in all directions. With this procedure and constant setting of color Doppler ultrasound as described in the above method, noise produced by cardiac pulse and peristalsis wave was mostly omitted, and a good correlation of intraobserver and interobserver reproducibility was obtained. 17,18
Current ultrasound technology is not capable of detecting tumor neovascularization itself (approximately 15 μm or less in diameter), which was usually demonstrated immunohistochemically. 23 The color Doppler signals seen within the tumor represented the larger and functional vessels (approximately 100 μm or more in diameter), possibly intratumoral arterioles, venules, and arteriole-vanule shunting, 23,24 in which blood-flow ran. Although there was a loose linear correlation between CDVI and MVD in this study, we hypothesized that the more neovascularization existed, the more supplying intratumoral arterioles and draining venules was present. The distribution of angiogenesis in a tumor is usually uneven and heterogenous, and MVD, the microvessel count in a tiny portion of tumor, may be not sufficient to represent the global tumor angiogenesis. Recently, Maniotis et al. 25 reported that aggressive melanoma cells generate nonendothelial cell-lined channels delineated by extracellular matrix, and these “vasculogenic mimicry” channels that are undetectable in MVD assay link directly to larger normal vessels. Thus, CDVI, by quantitatively depicting the density of the larger supplying arterioles and draining venules, can better reflect global vascularization of a tumor.
Liotta et al. 26,27 developed a tumor perfusion study with C57BL/6J male mice, and found that the tumor vessel size and density are important determinants of the size of tumor cell clumps, as well as the concentration of effluent tumor cells released into the circulation and developed distant metastasis. 28 Therefore, increased density of larger vessels that can be evaluated with CDVI may facilitate distant metastases by allowing the intravasation and transportation of larger cancer cell clumps. In our previous study, colon cancer patients with high CDVI has a higher incidence of distant metastasis after curative resection and poorer prognosis than patients with low CDVI. 22 This in vivo observation in humans is in line with Liotta’s in mice. As a result, CDVI can better reflect the tumor invasiveness, metastasis, and prognosis. This may be the reason why high CDVI can predict early death in patients with stage III gastric cancer.
Currently, MVD is widely employed for assessing angiogenesis in human solid cancers. 10,11,29–32 In fact, many studies found a significant association between MVD and clinical outcome of the patients, and demonstrated that MVD is an independent prognostic factor in multivariate analysis. 33 In the present study, higher MVD was correlated with positive vascular invasion, depth of tumor invasion, positve lymph node metastasis, diffuse type cancer, and positive Helicobacter pylori infection. Vascular invasion was the only factor that significantly correlated with MVD in multivariate analysis, and was also an independent prognostic factor. However, MVD was not an independent prognostic factor. Vascular invasion is a well-known factor for hematogenous metastasis and is also an independently prognostic factor in gastric cancer. 34–39 Local shedding of cancer cells into the tumor vascular stream that can commence at the onset of angiogenesis is quantitatively related to the surface area of intratumoral vessels. 26,40 The CDVI as a measure of global vascularity of the tumor might better reflect the surface area of intratumoral vessels. In the present study, the patients with vascular invasion had significantly higher CDVI than those without. Poor prognosis of high CDVI patients may be related with hematogenous spreading for high frequency of vascular invasion.
The majority of the patients with early gastric cancer can be cured by surgery. Unfortunately, most gastric cancer patients had advanced disease in Taiwan. The prognosis of patients with advanced gastric cancer who have undergone curative gastrectomy is still poor. It may depend on the biological nature of the resected tumor itself, as no grossly detectable residual tumors have been left behind after surgery. Unfortunately, there is currently still no definite place for adjuvant chemotherapy in resected gastric cancer outside the setting of a clinical trial. 41 One of the major clinical problems is that we don’t know how to select high-risk patients who can really benefit from chemotherapy or other modality of treatment. Therefore, selecting out a subset of patients from this group who may have a worse prognosis may be clinically useful, and future studies using new compounds and regimens, focusing on high-risk groups of patients may offer the best chance of demonstrating a definite role for adjuvant chemotherapy in this disease.
There is little doubt that the current staging system of gastric cancer still lumps together molecularly distinct diseases with distinct clinical phenotypes. The majority of human solid tumors are heterogeneous diseases, made up of multiple cell clones with diverse biological aggressiveness. It seems clear that stage III gastric cancers at least are heterogeneously angiogenic diseases, and this angiogenic heterogeneity was associated with diverse angiogenic tumor clones, 42 activation of oncogenes, 43,44 downregulation of tumor suppressor genes, 45 immunogenecity, 46,47 and the ability to metastatize. Therefore, the CDVI could be considered to represent the summation effect of complex biological processes of tumor vascularization, progression, and metastasis in gastric cancer, and was an excellent prognostic indicator to select out patients who might suffer from early death in stage III gastric cancer after curative gastrectomy. The results of initial studies of preoperative chemotherapy in resectable advanced gastric cancer are encouraging. 48,49 Thus, the preoperative CDVI in advanced gastric cancer patients may help to identify patients for appropriate preoperative chemotherapy. Other than chemotherapy, different types of antiangiogenic compounds have been developed and shown to inhibit angiogenesis and the growth of some tumors in preclinical trials and a few compounds are further down the clinical road. 50–52 Such agents may be valuable in enhancing the efficacy of preoperative chemotherapy of advanced gastric cancer patients with high CDVI tumors.
Footnotes
This work was supported by grant (NSC89-2314-B002-421) from National Science Council, Taipei, Taiwan.
Correspondence: King-Jen Chang, MD, PhD, Department of Surgery, National Taiwan University Hospital, No. 7, Chung-Shan South Road, Taipei, Taiwan.
E-mail: kingjen@ha.mc.ntu.edu.tw
Accepted for publication June 7, 2001.
References
- 1.Folkman J, Long D, Becker F. Growth and metastasis of tumor in organ culture. Cancer 1963; 16: 453–457. [DOI] [PubMed] [Google Scholar]
- 2.Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst 1990; 82: 4–6. [DOI] [PubMed] [Google Scholar]
- 3.Bicknell R, Harris AL. Novel growth regulatory factors and tumor angiogenesis. Eur J Cancer 1991; 27: 781–784. [DOI] [PubMed] [Google Scholar]
- 4.Weidner N, Semple SF, Welch WR, et al. Tumor angiogenesis and metastasis-correlation in invasive breast carcinoma. N Engl J Med 1991; 324: 1–8. [DOI] [PubMed] [Google Scholar]
- 5.Weidner N, Folkman J, Pozza F, et al. Tumor angiogenesis: a new significant and independent prognostic indicator in early stage breast carcinoma. J Natl Cancer Inst, 1992; 84: 1875–1887. [DOI] [PubMed] [Google Scholar]
- 6.Toi M, Kashitani J, Tominaga T. Tumor angiogenesis is an independent prognostic indicator in primary breast carcinoma. Int J Cancer 1993; 55: 371–374. [DOI] [PubMed] [Google Scholar]
- 7.Macchiiarini P, Fontanini G, Hardin MJ, et al. Relation of neovasculatue to metastasis of non-small-cell lung cancer. Lancet 1992; 40: 145–146. [DOI] [PubMed] [Google Scholar]
- 8.Weidner N, Carrol PR, Flax J, et al. Tumor angiogenesis correlates with metastasis in invasive prostate carcinoma. Am J Pathol 1993; 143: 401–409. [PMC free article] [PubMed] [Google Scholar]
- 9.Gasparini G, Weidner N, Maluta S, et al. Intratumoral microvessel density and p53 protein: correlation with metastasis in head-and-neck squamous-cell carcinoma. Int J Cancer 1993; 55: 739–744. [DOI] [PubMed] [Google Scholar]
- 10.Saclarides TJ, Speziale NJ, Drab E, et al. Tumor angiogenesis and rectal carcinoma. Dis Colon Rectum 1994; 37: 921–926. [DOI] [PubMed] [Google Scholar]
- 11.Frank RE, Saclarides TJ, Leurgans S, et al. Tumor angiogenesis as a predictor of recurrence and survival in patients with node-negative colon cancer. Ann Surg 1995; 222: 695–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dock W, Grabenwoger F, Metz V, et al. Tumor vascularization: assessment with duplex sonography. Radiology 1991; 181: 241–244. [DOI] [PubMed] [Google Scholar]
- 13.Taylor KJW, Ramos I, Carter D, et al. Correlation of Doppler US tumor signals with neovascular morphologic features. Radiology 1988; 166: 57–62. [DOI] [PubMed] [Google Scholar]
- 14.Newman JS, Bree RL, Rubin JM. Prostate cancer: diagnosis with color Doppler sonography with histologic correlation of each biopsy site. Radiology 1995; 195: 86–90. [DOI] [PubMed] [Google Scholar]
- 15.Wu CC, Lee CN, Chen TM, et al. Incremental angiogenesis assessed by color Doppler ultrasound in the tumorigenesis of ovarian neoplasm. Cancer 1994; 73: 1251–1256. [DOI] [PubMed] [Google Scholar]
- 16.Cheng WF, Chen TM, Chen CA, et al. Clinical application of intratumoral blood flow study in patients with endometrial carcinoma. Cancer 1998; 82: 1881–1886. [PubMed] [Google Scholar]
- 17.Wu CH, Hsu MM, Chang YL, et al. Vascular pathology of malignant cervical lymphadenopathy. Cancer 1998; 83: 1189–1196. [PubMed] [Google Scholar]
- 18.Cheng WF, Lee CN, Chu JS, et al. Vascularity index as a novel parameter for the in vivo assessment of angiogenesis in patients with cervical carcinoma. Cancer 1991; 85: 651–657. [DOI] [PubMed] [Google Scholar]
- 19.Bluth EI, Merrit CRB, Sullivan MA. Ultrasonic evaluation of the stomach, small bowel, and colon. Radiology 1979; 19: 677–680. [DOI] [PubMed] [Google Scholar]
- 20.Fakhry Jr., Berk RN. The “target” pattern: characteristic sonographic feature of stomach and bowel abnormalities. AJR 1981; 137:969–972. [DOI] [PubMed]
- 21.Rapaccini GL, Aliotta A, Pompili M, et al. Gastric wall thickness in normal and neoplastic subjects: a prospective study performed by abdominal ultrasound. Gastrointestinal Radiol 1988; 13: 197–199. [DOI] [PubMed] [Google Scholar]
- 22.Chen CN, Cheng YM, Liang JT, et al. Color Doppler vascularity index can predict distant metastasis and survival in colon cancer patients. Cancer Res 2000; 60: 2892–2897. [PubMed] [Google Scholar]
- 23.Fleischer AC, Wojcicki WE, Donnelly EF, et al. Quantified color Doppler sonography of tumor vascularity in an animal model. J Ultrasound Med 1999; 18: 547–551. [DOI] [PubMed] [Google Scholar]
- 24.Lassau N, Paturel-Asselin C, Guinebretiere J-M, et al. New hemodynamic approach to angiogenesis: color and pulsed Doppler ultrasonography. Invest Radiol 1999; 34: 194–198. [DOI] [PubMed] [Google Scholar]
- 25.Folberg R, Hendrix MJC, Maniotis AJ. Vasculogenic mimicry and tumor angiogenesis. Am J Pathol 2000; 156: 361–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liotta L, Kleinerman J, Saldel G. Quantitative relationships of intravascular tumor cells, tumor vessel, and pulmonary metastases following tumor implantation. Cancer Res 1974; 34: 997–1004. [PubMed] [Google Scholar]
- 27.Liotta LA, Kleinerman J, Saidel GM. The significance of hematogenous tumor cell clumps in the metastatic process. Cancer Res 1976; 36: 889–894. [PubMed] [Google Scholar]
- 28.Fidler IJ. The relationship of embolic homogeneity, number, size and viability to the incidence of experimental metastasis. Euro J Cancer 1973; 9: 223. [DOI] [PubMed] [Google Scholar]
- 29.Meada K, Chung YS, Takatsuka S, et al. Tumor angiogenesis as a predictor of recurrence in gastric carcinoma. J Clin Oncol 1995; 13: 477–481. [DOI] [PubMed] [Google Scholar]
- 30.Tanigawa N, Amaya H, Matsumura M, et al. Extent of tumor vascularization correlates with prognosis and hematogenous metastasis in gastric carcinomas. Cancer Res 1996; 56: 2671–2676. [PubMed] [Google Scholar]
- 31.Takebayashi Y, Akiyama SI, Yamada K, et al. Angiogenesis as an unfavorable prognostic factor in human colorectal carcinoma. Cancer 1996; 78: 226–231. [DOI] [PubMed] [Google Scholar]
- 32.Bossi P, Viale G, Lee AKC, et al. Angiogenesis in colorectal tumors: microvessel quantification in adenomas and carcinomas with clinicopathologic correlations. Cancer Res 1995; 55: 5049–5053. [PubMed] [Google Scholar]
- 33.Gasparini G. Prognostic and predictive value of intra-tumoral microvessel density in human solid tumors. In: Bicknell R, Lewis CE, Ferrara N. Tumor angiogenesis. New York: Oxford University Press Inc; 1997, 29–44.
- 34.Okada M, Kojima S, Murakami M, et al. Human gastric carcinoma: prognosis in relation to macroscopic and microscopic features of the primary tumor. J Natl Cancer Inst 1983; 71: 275–279. [PubMed] [Google Scholar]
- 35.Ribeiro MM, Seoxas M, Sobrinho-Simoes M. Prognosis in gastric carcinoma. The preeminence of staging and futility of histological classification. Dig Dis Pathol 1988; 1: 51–68. [Google Scholar]
- 36.Korenaga D, Haraguchi M, Okamura T, et al. DNA ploidy and tumor invasion in human gastric cancer. Arch Surg 1989; 14: 314–318. [DOI] [PubMed] [Google Scholar]
- 37.Gabbert HE, Meier S, Gerharz CD, et al. Incidence and prognostic significance of vascular invasion in 529 gastric cancer patients. Int J Cancer 1991; 49: 203–207. [DOI] [PubMed] [Google Scholar]
- 38.Setala LP, Kosma VM, Marin S, et al. Alhava EM. Prognostic factors in gastric cancer: the value of vascular invasion, mitotic rate and lymphoplasmacytic infiltration. Br J Cancer 1996; 74: 766–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maehara Y, Tomoda M, Hasuda S, et al. Prognostic value of p53 protein expression for patients with gastric cancer – a multivariate analysis. Br J Cancer 1999; 79: 1255–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liotta LA, Steeg PS, Stetler-Stevenson WG. Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell 1991; 64: 327–336. [DOI] [PubMed] [Google Scholar]
- 41.Ellis P, Cunningham D. Adjuvant and neoadjuvant chemotherapy. In: Sugimura T, Sasako M. Gastric cancer. New York: Oxford University Press Inc; 1997, 283–290.
- 42.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1995; 1: 27–31. [DOI] [PubMed] [Google Scholar]
- 43.Mitsuhashi R, Bayko L, Shirasawa S, et al. Mutant ras upregulates VEGF/PVF expression: implications for induction and inhibition of tumor angiogenesis. Cancer Res 1995; 55: 4575–4580. [PubMed] [Google Scholar]
- 44.Mazure N, Chen E, Yeh P, et al. Oncogenic transformation and hypoxia synergistically act to modulate vascular endothelial growth factor expression. Cancer Res 1996; 56: 3436–3440. [PubMed] [Google Scholar]
- 45.Dameron KM, Volpert OV, Tainsky MA, et al. Control of angiogenesis in fibroblasts by p53 regulation of thrombospondin-1. Science 1994; 265: 1582–1584. [DOI] [PubMed] [Google Scholar]
- 46.Camusssi G, Montrucchio G, Lupia E, et al. Angiogenesis induced in vivo by hepatocyte growth factor is mediated by platelet-activating factor synthesis from macrophage. J Immunol 1997; 158: 1302–1309. [PubMed] [Google Scholar]
- 47.Carlos TM, Harlen JM. Leukocyte-endothelial adhesion molecules. Blood 1994; 84: 2068–2101. [PubMed] [Google Scholar]
- 48.Ajani JA, Majer RJ, Ota DM, et al. Preoperative and postoperative combination chemotherapy for potentially resectable gastric carcinoma. J Natl Cancer Inst 1993; 85: 1839–1844. [DOI] [PubMed] [Google Scholar]
- 49.Findlay M, Cunningham D, Norman A, et al. A phase II study in advanced gastric cancer using epirubicin and cisplatin in combination with continuous infusion 5-fluorouracil (ECF). Ann Oncol 1994; 5: 609–616. [DOI] [PubMed] [Google Scholar]
- 50.Ingber D, Fujita T, Kishimoto S, et al. Synthetic analogues offumagillin that inhibit angiogenesis and suppress tumor growth. Nature 1990; 384: 555–557. [DOI] [PubMed] [Google Scholar]
- 51.Griffioen AW, Molema G. Angiogenesis: potentials for pharmacologic intervention in treatment of cancer, cardiovascular diseases, and chronic inflammation. Pharmacol Review 2000; 52: 237–268. [PubMed] [Google Scholar]
- 52.Kerbel RS. Tumor angiogenesis: past, present and the near future. Carcinogenesis 2000; 21: 505–515. [DOI] [PubMed] [Google Scholar]


