Abstract
Objective
To investigate whether monocyte paralysis resistant to interferon-gamma (IFN-γ) costimulation may exist before surgery and postoperative infection and may correlate with the outcome of postoperative sepsis.
Summary Background Data
Several studies have correlated monocyte paralysis during the course of sepsis with lethal outcome. Although the authors’ previous work indicated that preoperative defects in monocyte interleukin (IL)-12 production are associated with the development of severe postoperative sepsis, the functional state of monocytes before surgery and infection and its significance for sepsis requires further analysis.
Methods
In a prospective study, monocyte functions of 1,113 consecutive patients were examined before major visceral surgery. Monocytes were isolated from peripheral blood and were stimulated in vitro with IFN-γ and lipopolysaccharide. The secretion of IL-12 p70, IL-12 p40, IL-10, and tumor necrosis factor was measured.
Results
Preoperative monocyte secretion of IL-12 p70 and IL-12 p40 was significantly reduced in patients who developed lethal postoperative sepsis compared with sepsis survivors and patients with uneventful postoperative recovery. Moreover, preoperative monocyte IL-12 production was an independent predictive factor for the lethal outcome of postoperative sepsis by multivariate analysis. Preoperative monocyte IL-10 production was impaired in the sepsis group but did not correlate with death from sepsis. Preoperative monocyte tumor necrosis factor secretion was comparable between patients with uneventful recovery, sepsis survivors, and nonsurvivors. Thus, impaired preoperative monocyte IL-12 secretion in patients developing lethal postoperative sepsis did not result from an overproduction of IL-10 or from a generalized monocyte paralysis. The association between impaired preoperative monocyte IL-12 production and death from sepsis was also not explained by gender differences, underlying malignant disease, tumor type, neoadjuvant therapy, or age.
Conclusions
These results identify a selective preoperative defect in monocyte IL-12 production as a predictive factor for the lethal outcome of postoperative sepsis. These data suggest that a partial preoperative monocyte paralysis severely impairs the host defense against postoperative infection, resulting in an increased risk of lethal sepsis.
Numerous immune functions are known to be impaired as a consequence of major surgery or trauma. These include T-lymphocyte proliferation and cytokine secretion, delayed-type hypersensitivity skin test response, monocyte cytokine secretion and MHC class II expression, and neutrophil functions such as chemotaxis, phagocytosis, and oxygen radical production. 1–4 It further appears that the extent of surgical trauma directly correlates with the extent of postoperative immunosuppression. 5–7 Notably, unresponsiveness to hypersensitivity skin testing and loss of monocyte HLA-DR expression have been associated with the incidence and/or outcome of postoperative sepsis. 8–10 These studies suggest that suppression of immune defense mechanisms after major surgery or trauma may increase the susceptibility to postoperative infection and sepsis.
Recent work from our group has extended this concept by showing that immunosuppression and predisposition to sepsis may not only be induced by surgery or trauma but may even exist before major surgery. We have found that preoperative production of interleukin (IL)-12 p70 by lipopolysaccharide (LPS)-stimulated monocytes was markedly impaired in patients who developed postoperative sepsis, and that the extent of this functional defect directly correlated with sepsis severity. 11 Consistent with a critical role of IL-12 for the host defense in sepsis, neutralization of IL-12 or genetic ablation of the interferon-gamma (IFN-γ) receptor was shown to impair bacterial clearance and to markedly enhance the death rate in murine septic peritonitis. 12–14 Moreover, administration of IL-12 to mice after burn injury improves resistance against an intraabdominal septic challenge. 15,16 The importance of IL-12 for immune protection against bacterial pathogens is further emphasized by recent reports on recurrent infections in patients with genetic IL-12 deficiency. 17,18 Collectively, these studies are consistent with the concept that the IL-12-driven proinflammatory response is of critical importance for the immune defense against postoperative infection.
Biologically active IL-12 p70 is a heterodimeric cytokine comprising covalently linked p35 and p40 subunits and is produced by antigen-presenting cells such as macrophages and dendritic cells on exposure to microbial products or ligation of cell surface CD40. 19,20 The p40 subunit of IL-12 may also combine with a novel p19 protein to generate the cytokine IL-23, which shares several biologic activities with IL-12 p70. 21 IL-12 is required for the production of IFN-γ by natural killer (NK) cells and T lymphocytes and supports the development of the Th1 phenotype of CD4+ T cells. IFN-γ enhances IL-12 release, thereby inducing positive feedback interactions that are crucial for activation of the phagocyte system and T-cell differentiation. Thus, IL-12 regulates immediate defense mechanisms of innate immunity and directs the subsequent adaptive immune response.
A prospective study was performed to address the question of whether impaired monocyte functions before surgery may be related to the development or the course of postoperative sepsis. Results from the preoperative analysis of monocyte cytokine production of 1,113 surgical patients revealed that in addition to IL-12 p70, the reduced secretion of IL-12 p40 correlates with the development of lethal postoperative sepsis. These monocyte defects were observed despite cell stimulation with both IFN-γ and LPS.
METHODS
Patient Population and Study Design
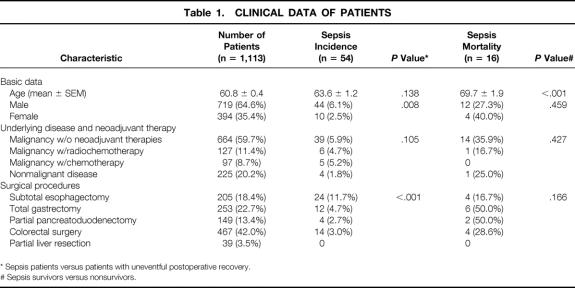
Patients undergoing major elective surgery of the upper and lower gastrointestinal tract (subtotal esophagectomy, total gastrectomy, partial pancreatoduodenectomy, colorectal surgery, partial liver resection) were included in a prospective study from January 1998 to July 2000. Theclinical profiles of all patients analyzed are detailed in Table 1. Patients with acquired or inherited immunodeficiency and patients receiving immunosuppressive medication were excluded from the study. The study group consisted of 1,113 patients; 1,059 had an uneventful postoperative recovery and 54 (4.8%) developed postoperative sepsis. Among the patients studied, 127 were treated with neoadjuvant radiochemotherapy and 97 were treated with polychemotherapy before surgery. Radiochemotherapy was applied with 2 Gy per day over a 3-week period (30 Gy) and continuous infusion of 5-fluorouracil (250 mg/body surface area over 7 days for 3 weeks). For polychemotherapy, patients received one to three cycles of PLF (cisplatin, leucovorin, and 5-fluorouracil over a period of 6 weeks per cycle (5-fluorouracil 2,000 mg/BSA continuously over 24 hours for 6 weeks, leucovorin 500 mg for 2 hours every 7 days, cisplatin 50 mg/BSA for 1 hour every 14 days). Three weeks after radiochemotherapy and 4 weeks after chemotherapy, a clinical restaging was performed.
Table 1. CLINICAL DATA OF PATIENTS
* Sepsis patients versus patients with uneventful postoperative recovery.
# Sepsis survivors versus nonsurvivors.
From each patient, a venous blood sample was collected at least 1 day before surgery. For inclusion of patients in the group with sepsis, established criteria were used. 22 Because of the low stringency of these criteria, however, patients were included in the sepsis group only if these sepsis criteria were met for 3 or more consecutive days. The study received local hospital ethical committee approval. Informed consent was obtained from all patients.
Monocyte Cytokine Secretion
Peripheral blood mononuclear cells were isolated from 25 mL heparinized blood using Ficoll-metrizoate density gradient centrifugation, and monocytes were purified by plastic adherence. Duplicate cultures of 5 × 104 monocytes/well were incubated with 1 μg/mL IFN-γ (Biosource Intl., Nivelles, Belgium) for 16 hours in 1 mL RPMI 1640 medium supplemented with 10% fetal calf serum. Thereafter, 1 μg of endotoxin from Escherichia coli serotype 0127:B8 (Sigma Chemical Co., St. Louis, MO) was added at a final concentration of 1 μg/mL, and cells were incubated for an additional 24 hours. Supernatants were centrifuged to remove residual cells and stored at −20°C. The levels of IL-12 p70, IL-12 p40 (Amersham, Braunschweig, Ger-many), tumor necrosis factor-alpha (TNF-α), and IL-10 (Biosource) in supernatants of stimulated monocytes were determined by the enzyme-linked immunosorbent assay (ELISA) technique according to the manufacturer’s instructions. All cytokine assays were standardized by including a titration of the appropriate purified recombinant cytokine of known concentration. Sensitivity levels of the ELISA assays were 3 pg/mL (IL-12 p70), 5 pg/mL (IL-12 p40), 3 pg/mL (TNF), and 1 pg/mL (IL-10). The absorbance of the samples was determined on a MRX Microplate Reader (Dynatech, Denkendorf, Germany) using 450 nm as the primary and 630 nm as the reference wave length.
Statistical Analysis
Statistical analysis of the data was performed using the Mann-Whitney test, Student t test, chi-square test, Fisher exact test, Spearman rank order correlation, or logistic regression analysis where appropriate. The level of significance was set at P < .05.
RESULTS
Patients
The study group included 1,113 patients undergoing major visceral surgery. One thousand fifty-nine had an uneventful postoperative recovery and sepsis occurred in 54 patients (38 survivors, 16 nonsurvivors). Postoperative sepsis was significantly more frequent in patients with subtotal esophagectomy compared with patients undergoing other surgical procedures. The sepsis incidence was also greater in male than in female patients. When patients were divided according to surgical procedures, however, gender differences in sepsis incidence were not significant (P = .059). Unlike sepsis incidence, the sepsis death rate was not affected by the type of surgical procedure applied or by gender. In contrast, sepsis survivors were significantly younger than sepsis nonsurvivors.
Preoperative Monocyte Cytokine Secretion
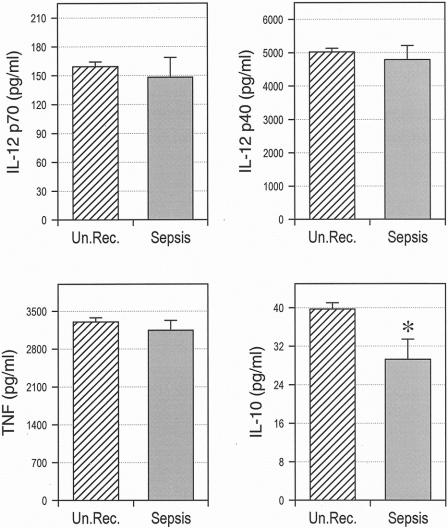
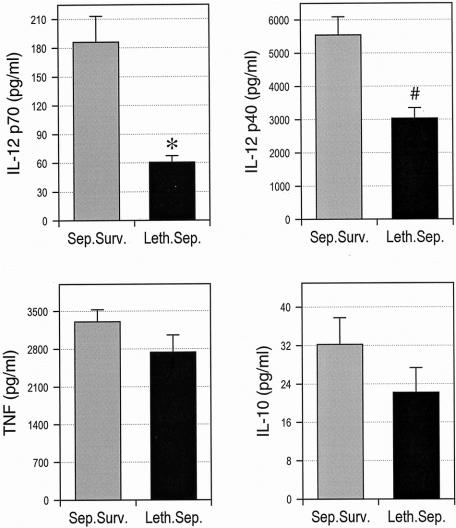
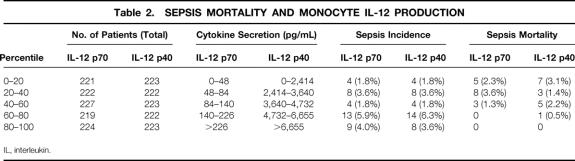
Peripheral blood monocytes were isolated from all patients before surgery, and cytokine production was stimulated by consecutive incubation with IFN-γ and LPS. The results in Figure 1 show that monocytes of sepsis patients exhibited an impaired preoperative production of IL-10, but not of other cytokines, compared with monocytes of patients with uneventful recovery. When the group of sepsis patients was divided into survivors and nonsurvivors, however, we found that monocytes from nonsurvivors produced substantially less IL-12 p70 and IL-12 p40 than cells from sepsis survivors (Fig. 2). Splitting the total patient population into quintiles according to preoperative monocyte IL-12 p70 and IL-12 p40 production further revealed that the death rate from sepsis was significantly higher in patients with low IL-12 p70 and IL-12 p40 secretion (Table 2). Lethal postoperative sepsis was not observed in patients with IL-12 p70 production greater than 140 pg/mL, although 22 of the 54 sepsis patients were in this group.
Figure 1. Reduced preoperative monocyte IL-10 production in patients developing postoperative sepsis. Monocytes were purified from peripheral blood and stimulated by consecutive exposure to interferon-gamma and lipopolysaccharide. The concentrations of interleukin (IL)-12 p70, IL-12 p40, tumor necrosis factor, and IL-10 in supernatants were determined by specific enzyme-linked immunosorbent assay. Un.Rec., patients with uneventful postoperative recovery. *P = .012.
Figure 2. Preoperative defects of monocyte IL-12 production are associated with the development of lethal postoperative sepsis. Monocytes were purified from peripheral blood and stimulated by consecutive exposure to interferon-gamma and lipopolysaccharide. The concentrations of interleukin (IL)-12 p70, IL-12 p40, tumor necrosis factor, and IL-10 in supernatants were determined by specific enzyme-linked immunosorbent assay. Sep.Surv., sepsis survivors; Leth.Sep., lethal sepsis. *P < .001; #P = .002.
Table 2. SEPSIS MORTALITY AND MONOCYTE IL-12 PRODUCTION
IL, interleukin.
Logistic regression analysis was used to determine whether impaired monocyte IL-12 production would represent an independent predictive factor for sepsis outcome. The results of multivariate analysis revealed that in the total population of patients, both age (P = .006) and monocyte production of either IL-12 p70 (P = .011) or IL-12 p40 (P = .030) were independent predictive factors for the development of lethal postoperative sepsis. Production of IL-12 p70 and IL-12 p40 by monocytes was highly correlated (r = 0.693;P < .001). When only patients developing postoperative sepsis were considered, age (P = .004) and monocyte production of either IL-12 p70 (P = .025) or IL-12 p40 (P = .014) were also identified as independent predictive factors for lethal outcome. For the total population of patients as well as for the patient subgroup developing postoperative sepsis, monocyte IL-10 production, gender, underlying disease, neoadjuvant tumor therapy, and surgical procedures were not predictive for the development of lethal postoperative sepsis.
In contrast to IL-12, preoperative monocyte IL-10 secretion did not significantly differ between sepsis survivors and nonsurvivors, suggesting that defective monocyte IL-12 p70 and IL-12 p40 release in patients with lethal postoperative sepsis is not caused by an overproduction of IL-10 (see Fig. 2). Monocyte TNF production was also not different between survivors and nonsurvivors, thereby arguing against a general monocyte paralysis in these patients. Considered together, these findings are consistent with our previous observations 11 and show that patients developing lethal postoperative sepsis exhibit a significantly reduced preoperative monocyte production of IL-12 p70 and IL-12 p40.
Correlation of Monocyte IL-12 Production With Clinical Parameters
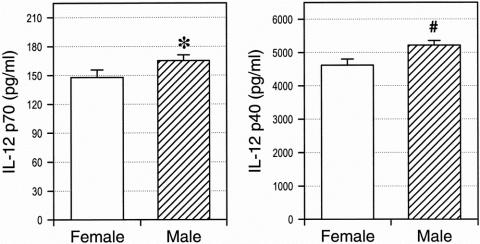
To further examine the association of impaired preoperative monocyte IL-12 production with the development of lethal postoperative sepsis, monocyte cytokine release was correlated with various clinical parameters. A multivariate analysis was performed to control for possible influences by gender, age, underlying disease, and neoadjuvant tumor therapy. The results indicate that production of IL-12 p70 (P = .063) and IL-12 p40 (P = .010) correlated with gender. Figure 3 depicts a univariate comparison of monocyte IL-12 production in male and female patients and demonstrates that both IL-12 p70 release and IL-12 p40 release were significantly elevated in male versus female patients. In contrast, the other parameters tested did not significantly correlate with monocyte IL-12 production.
Figure 3. Gender differences of monocyte interleukin (IL)-12 p40 production. Monocytes were purified from peripheral blood and stimulated by consecutive exposure to interferon-gamma and lipopolysaccharide. The concentrations of IL-12 p70 and IL-12 p40 in supernatants were determined by specific enzyme-linked immunosorbent assay. *P = .001; #P < .001.
DISCUSSION
The present results establish a direct correlation between impaired preoperative monocyte IL-12 production and the development of lethal sepsis after major visceral surgery. The results confirm and substantially extend our previous study 11 by showing that monocyte production of both IL-12 p70 and IL-12 p40 is markedly reduced in these patients. Moreover, impaired monocyte IL-12 production was identified as an independent predictive factor for the development of lethal postoperative sepsis. In the present study, monocytes were primed with IFN-γ before LPS stimulation, because IFN-γ is known to augment production of inflammatory cytokines, including IL-12, and because IFN-γ treatment may restore some aspects of monocyte paralysis or deactivation. 23–25 However, we observed defective IL-12 production despite costimulation of monocytes with IFN-γ and LPS. Based on these studies, we therefore propose that in surgical patients, IL-12 may represent a crucial component of host resistance against postoperative infection.
Association of a single cytokine defect with lethal outcome of postoperative sepsis was observed, although a complex array of both pro- and antiinflammatory mediators is thought to regulate host defense against a septic challenge. It should be considered, however, that IL-12 is a key cytokine in immune regulation with an important role for instructing the immune response to infection and directing it toward a Th1 phenotype. 20,26 Consistent with this notion, numerous studies have shown that IL-12 deficiency, even when present as a single cytokine defect, severely impairs host defense against a large number of pathogens, including viruses, intracellular bacteria, and parasites. 19,20,26 In models of polymicrobial septic peritonitis or E. coli-induced peritoneal sepsis, host defense was also found to be dependent on IL-12 and the IL-12-regulated cytokine IFN-γ. 12–14 Further, it was observed that suppressed IL-12 production after burn injury was associated with an increased death rate from septic peritonitis and that IL-12 therapy after injury restored resistance to infection. 15,16 The available data are therefore consistent with the concept that a reduced preoperative monocyte IL-12 production may severely impair host defense against postoperative infection and therefore may contribute to the development of lethal sepsis. Nonetheless, it is conceivable that in addition to IL-12, other as yet unknown immune mediators will be discovered that are associated with sepsis outcome and may therefore allow for an improved risk prediction.
The heterodimeric cytokine IL-23 was recently reported to be composed of a novel p19 protein associated with the IL-12 p40 subunit. 21 IL-23 therefore shares the p40 protein with the biologically active IL-12 p70 heterodimer, which consists of p35 and p40 subunits. 19,20,26 Moreover, IL-23 binds to the IL-12 receptor β1 chain and exhibits important biologic activities that are similar to those of IL-12, including stimulation of IFN-γ production and T-cell activation. 21 It is therefore conceivable that some of the functions in host defense previously attributed to IL-12 might in fact be mediated or at least affected by IL-23. Because our data show that preoperative monocyte IL-12 p40 production is significantly diminished in patients developing lethal postoperative sepsis, it seems possible that IL-23 production is also impaired in these patients. If proven to be correct, the proposed IL-23 deficiency could substantially aggravate preoperative immunosuppression. Direct analysis of this assumption, however, has to await the availability of assays specific for IL-23.
Although not correlating with the death rate, preoperative monocyte IL-10 production was impaired in patients with postoperative sepsis. This observation supports the view that endogenous IL-10 plays an important role for the immune response against a septic challenge. 27,28 Due to the known function of IL-10 as a potent antiinflammatory cytokine, 29–31 impaired production of IL-10 during sepsis could lead to an unopposed inflammatory response similar to the cytokine release syndrome induced by bacterial toxins, which is mitigated by IL-10. 32–36 The antiinflammatory activity of IL-10 was also suggested to be necessary for maintaining the balance of inflammatory mediators during experimental septic peritonitis. 27,28 An alternative role for IL-10 in sepsis might be deduced, however, from data showing that IL-10 exhibits various proinflammatory activities. For example, NK cell proliferation, cytotoxicity, and production of cytokines such as IFN-γ are markedly augmented by IL-10 when it is combined with IL-2, IL-12, or IL-18. 37–39 Infection of SCID mice with a recombinant Vaccinia virus expressing IL-10 resulted in greater NK cell activity and lower virus replication than infection with control virus, 40 suggesting that the immunostimulatory activities of IL-10 may operate in vivo and promote host defense. Consistent with this view, IL-10 was recently shown to promote the differentiation of immature dendritic cells into macrophage-like cells and to inhibit the intracellular growth of Mycobacterium tuberculosis in these cells. 41 Moreover, administration of IL-10 to healthy human volunteers was found to increase the serum levels of cytokines such as IFN-γ and IL-12 and to activate cytotoxic T cells and NK cells during endotoxemia. 42 It is therefore tempting to speculate that in addition to its antiinflammatory activities, IL-10 might also exhibit critical proinflammatory functions in sepsis. According to this hypothesis, reduced IL-10 production in surgical patients might result in an increased susceptibility to postoperative sepsis. Under certain conditions during the course of infection, IL-10 might act in concert with other cytokines, such as IL-12 and IFN-γ, to promote efficient pathogen clearance and immune defense.
Impaired monocyte cytokine production may result from a large number of different factors (e.g., immunosuppressive mediators, cytokine gene polymorphisms, disease processes, age, or gender differences). To elucidate potential mechanisms that may explain the association of a mitigated preoperative IL-12 production with lethal postoperative sepsis, the influence of various clinical conditions on monocyte cytokine secretion was examined. However, the results suggest that clinical conditions such as underlying disease, neoadjuvant tumor therapy, age, or gender-associated factors do not account for the defects observed. Although male patients were found to produce more IL-12 than female patients, they developed sepsis more frequently. The results of the present report further indicate that reduced preoperative IL-12 production in patients developing lethal postoperative sepsis does not reflect a general monocyte paralysis, because monocyte TNF release was not altered in these patients. Normal TNF production despite diminished secretion of IL-12 also argues against a general role for immunosuppressive mediators other than IL-10 (e.g., transforming growth factor-β). Thus, as an alternative explanation for our present and previous findings, partial defects in LPS signaling may have to be considered that affect only a subset of cytokines. An example of such an alteration may be provided by recent work showing that CD11b/CD18 deficiency of murine macrophages substantially reduces LPS-stimulated production of IL-12 but not TNF. 43 It remains to be determined, however, whether impaired IL-12 production in surgical patients developing lethal postoperative sepsis is caused by defects in CD11b/CD18 function or by other, as yet unknown, factors.
In summary, our results are consistent with the concept that immunosuppression before surgery predisposes patients to a severe course and lethal outcome of postoperative sepsis. Detection of impaired preoperative IL-12 production might thus be useful to identify high-risk patients. In conjunction with immunomodulatory strategies, the incidence and course of infection in these patients might be reduced by less aggressive surgical procedures that are associated with fewer complications, by split operations applying second-step reconstruction procedures after initial resection, or by alternate regimens of tumor therapy.
Acknowledgments
The authors thank Martina Rump, Felicitas Altmayr, Kerstin Weber, and Simone Kaiser-Moore for expert technical assistance.
Footnotes
Supported by grant Si 208/5-4 from the Deutsche Forschungsgemeinschaft.
Correspondence: Bernhard Holzmann, MD, Department of Surgery, Klinikum rechts der Isar, Technische Universität München, Ismaninger Str. 22, 81675 Munich, Germany.
E-mail: holzmann@nt1.chir.med.tu-muenchen.de
Accepted for publication October 10, 2001.
References
- 1.Guillou PJ. Biological variation in the development of sepsis after surgery or trauma. Surgery 1993; 342: 217–220. [DOI] [PubMed] [Google Scholar]
- 2.Faist E. The mechanisms of host defense dysfunction following shock and trauma. In Rietschel ET, Wagner H, eds. Pathology of Septic Shock. Heidelberg: Springer; 1996: 259–274. [DOI] [PubMed]
- 3.Windsor AC, Klava A, Somers SS, et al. Manipulation of local and systemic host defense in the prevention of perioperative sepsis. Br J Surg 1995; 82: 1460–1467. [DOI] [PubMed] [Google Scholar]
- 4.Hensler T, Hecker H, Heeg K, et al. Distinct mechanisms of immunosuppression as a consequence of major surgery. Int Immunol 1997; 65: 2283–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brune IB, Wilke W, Hensler T, et al. Down-regulation of Th1 immune response and altered pro- and anti-inflammatory T cell cytokine balance following conventional, but not laparoscopic surgery. Am J Surg 1999; 177: 55–60. [DOI] [PubMed] [Google Scholar]
- 6.Carlei F, Schietroma M, Cianca G, et al. Effects of laparoscopic and conventional (open) cholecystectomy on human leukocyte antigen-DR expression peripheral blood monocytes: correlations with immunologic status. World J Surg 1999; 23: 18–22. [DOI] [PubMed] [Google Scholar]
- 7.Decker D, Schöndorf M, Bidlingmaier F, et al. Surgical stress induces a shift in the type-1/type-2 T-helper cell balance, suggesting down-regulation of cell-mediated and up- regulation of antibody-mediated immunity commensurate to the trauma. Surgery 1996; 119: 316–325. [DOI] [PubMed] [Google Scholar]
- 8.Hershman MJ, Cheadle WG, Wellhausen SR, et al. Monocyte HLA-DR antigen expression characterizes clinical outcome in the trauma patient. Br J Surg 1990; 77: 204–207. [DOI] [PubMed] [Google Scholar]
- 9.Cheadle WG, Hershman MJ, Wellhausen SR, Polk HC Jr. HLA-DR antigen expression on peripheral blood monocytes correlates with surgical infection. Am J Surg 1991; 161: 639–645. [DOI] [PubMed] [Google Scholar]
- 10.Wakefield CH, Carey PD, Foulds S, et al. Changes in major histocompatibility complex class II expression in monocytes and T cells of patients developing infection after surgery. Br J Surg 1993; 80: 205–209. [DOI] [PubMed] [Google Scholar]
- 11.Hensler T, Heidecke CD, Hecker H, et al. Increased susceptibility to postoperative sepsis in patients with impaired monocyte IL-12 production. J Immunol 1998; 161: 2655–2659. [PubMed] [Google Scholar]
- 12.Steinhauser ML, Hogaboam CM, Lukacs NW, et al. Multiple roles for IL-12 in a model of acute septic peritonitis. J Immunol 1999; 162: 5437–5443. [PubMed] [Google Scholar]
- 13.Zisman DA, Kunkel SL, Strieter RM, et al. Anti-interleukin-12 therapy protects mice in lethal endotoxemia but impairs clearance in murine Escherichia coli peritoneal sepsis. Shock 1997; 8: 349–356. [DOI] [PubMed] [Google Scholar]
- 14.Zantl N, Uebe A, Neumann B, et al. Essential role of γ-interferon in survival of colon ascendens stent peritonitis, a novel murine model of abdominal sepsis. Infect Immun 1998; 66: 2300–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goebel A, Kavanagh E, Lyons A, et al. Injury induces deficient interleukin-12 production, but interleukin-12 therapy after injury restores resistance to infection. Ann Surg 2000; 231: 253–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Sullivan ST, Lederer JA, Horgan AF, et al. Major injury leads to predominance of the T helper-2-lymphocyte phenotype and diminished interleukin-12 production associated with decreased resistance to infection. Ann Surg 1995; 222: 482–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haraguchi S, Day NK, Nelson RP Jr, et al. Interleukin-12 deficiency associated with recurrent infections. Proc Natl Acad Sci USA 1998; 95: 13125–13129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Altare F, Lammas D, Revy P, et al. Inherited interleukin 12 deficiency in a child with bacille Calmette-Guérin and Salmonella enteritidis disseminated infection. J Clin Invest 1998; 102: 2035–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol 1995; 13: 251–276. [DOI] [PubMed] [Google Scholar]
- 20.Lamont AG, Adorini L. IL-12: a key cytokine in immune regulation. Immunol Today 1996; 17: 214–217. [DOI] [PubMed] [Google Scholar]
- 21.Oppmann B, Lesley R, Blom B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 2000; 13: 715–725. [DOI] [PubMed] [Google Scholar]
- 22.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest 1992; 101: 1644–1655. [DOI] [PubMed] [Google Scholar]
- 23.Döcke WD, Randow F, Syrbe U, et al. Monocyte deactivation in septic patients: restoration by IFN-γ treatment. Nat Med 1997; 3: 678–681. [DOI] [PubMed] [Google Scholar]
- 24.Kox WJ, Bone RC, Krausch D, et al. Interferon γ-1b in the treatment of compensatory anti-inflammatory response syndrome. A new approach: proof of principle. Arch Intern Med 1997; 24: 389–393. [PubMed] [Google Scholar]
- 25.Randow F, Döcke WD, Bundschuh DS, et al. In vitro prevention and reversal of lipopolysaccharide desensitization by IFN-γ, IL-12, and granulocyte-macrophage colony-stimulating factor. J Immunol 1997; 158: 2911–2918. [PubMed] [Google Scholar]
- 26.Gately MK, Renzetti LM, Magram J, et al. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu Rev Immunol 1998; 16: 495–521. [DOI] [PubMed] [Google Scholar]
- 27.van der Poll T, Marchant A, Burrman WA, et al. Endogenous IL-10 protects mice from death during septic peritonitis. J Immunol 1995; 155: 5397–5401. [PubMed] [Google Scholar]
- 28.Walley KR, Lukacs NW, Standiford TJ, et al. Balance of inflammatory cytokines related to severity and mortality of murine sepsis. Infect Immun 1996; 64: 4733–4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mosmann TR. Properties and functions of interleukin-10. Adv Immunol 1994; 56: 1–26. [PubMed] [Google Scholar]
- 30.Pretolani M. Interleukin-10: an anti-inflammatory cytokine with therapeutic potential. Clin Exp Allergy 1999; 29: 1164–1171. [DOI] [PubMed] [Google Scholar]
- 31.De Vries JE. Immunosuppressive and anti-inflammatory properties of interleukin 10. Ann Med 1995; 27: 537–541. [DOI] [PubMed] [Google Scholar]
- 32.Howard M, Muchamuel T, Andrade S, Menon S. Interleukin 10 protects mice from lethal endotoxemia. J Exp Med 1993; 177: 1205–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berg DJ, Kühn R, Rajewsky K, et al. Interleukin-10 is a central regulator of the response to LPS in murine models of endotoxic shock and the Shwartzman reaction but not endotoxin tolerance. J Clin Invest 1996; 96: 2339–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gérard C, Bruyns C, Marchant A, et al. Interleukin 10 reduces the release of tumor necrosis factor and prevents lethality in experimental endotoxemia. J Exp Med 1993; 177: 547–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marchant A, Bruyns C, Vandenabeele P, et al. Interleukin-10 controls interferon-γ and tumor necrosis factor production during experimental endotoxemia. Eur J Immunol 1994; 24: 1167–1171. [DOI] [PubMed] [Google Scholar]
- 36.Standiford TJ, Strieter RM, Lukacs NW, Kunkel SL. Neutralization of IL-10 increases lethality in endotoxemia: cooperative effects of macrophage inflammatory protein-2 and tumor necrosis factor. J Immunol 1995; 155: 2222–2229. [PubMed] [Google Scholar]
- 37.Cai G, Kastelein RA, Hunter CA. IL-10 enhances NK cell proliferation, cytotoxicity and production of IFN-γ when combined with IL-18. Eur J Immunol 1999; 29: 2658–2665. [DOI] [PubMed] [Google Scholar]
- 38.Shibata Y, Foster LA, Kurimoto M, et al. Immunoregulatory roles of IL-10 in innate immunity: IL-10 inhibits macrophage production of IFN-γ-inducing factors but enhances NK cell production of IFN-γ. J Immunol 1998; 161: 4283–4288. [PubMed] [Google Scholar]
- 39.Carson WE, Lindemann MJ, Baiocchi R, et al. The functional characterization of interleukin-10 receptor expression on human natural killer cells. Blood 1995; 85: 3577–3585. [PubMed] [Google Scholar]
- 40.Kurilla MG, Swaminathan S, Welsh RM, et al. Effects of virally expressed interleukin-10 on vaccinia virus infection in mice. J Virol 1993; 67: 7623–7628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Förtsch D, Röllinghoff M, Stenger S. IL-10 converts human dendritic cells into macrophage-like cells with increased antibacterial activity against virulent Mycobacterium tuberculosis. J Immunol 2000; 165: 978–987. [DOI] [PubMed] [Google Scholar]
- 42.Lauw FN, Pajkrt D, Hack CE, et al. Proinflammatory effects of IL-10 during human endotoxemia. J Immunol 2000; 165: 2783–2789. [DOI] [PubMed] [Google Scholar]
- 43.Perera P-Y, Mayadas TN, Takeuchi O, et al. CD11b/CD18 acts in concert with CD14 and Toll-like receptor (TLR) 4 to elicit full lipopolysaccharide and taxol-inducible gene expression. J Immunol 2001; 166: 574–581. [DOI] [PubMed] [Google Scholar]