Abstract
Objective
To determine whether high-volume hospitals (HVHs) have lower in-hospital death rates after abdominal aortic aneurysm (AAA) repair compared with low-volume hospitals (LVHs).
Summary Background Data
Select statewide studies have shown that HVHs have superior outcomes compared with LVHs for AAA repair, but they may not be representative of the true volume–outcome relationship for the entire United States.
Methods
Patients undergoing repair of intact or ruptured AAAs in the Nationwide Inpatient Sample (NIS) for 1996 and 1997 were included (n = 13,887) for study. The NIS represents a 20% stratified random sample representative of all U.S. hospitals. Unadjusted and case mix-adjusted analyses were performed.
Results
The overall death rate was 3.8% for intact AAA repair and 47% for ruptured AAA repair. For repair of intact AAAs, HVHs had a lower death rate than LVHs. The death rate after repair of ruptured AAA was also slightly lower at HVHs. In a multivariate analysis adjusting for case mix, having surgery at an LVH was associated with a 56% increased risk of in-hospital death. Other independent risk factors for in-hospital death included female gender, age older than 65 years, aneurysm rupture, urgent or emergent admission, and comorbid disease.
Conclusions
This study from a representative national database documents that HVHs have a significantly lower death rate than LVHs for repair of both intact and ruptured AAA. These data support the regionalization of patients to HVHs for AAA repair.
Each year in the United States, many patients are in need of elective or emergent repair of abdominal aortic aneurysms (AAAs). Health policy initiatives to reduce surgical death rates are therefore an important public health issue. 1,2 Recently, there has been increased interest in regionalization of high-risk surgical procedures, such as aortic aneurysmectomy, to high-volume hospitals (HVHs) in an effort to reduce the number of perioperative deaths.
Hospital volume has clearly been shown to be associated with improved outcomes for several complex vascular surgical procedures, including AAA repair. 3–5 Several population-based studies using state discharge data have consistently shown lower surgical death rates at HVHs. 6–12 However, the validity of studies conducted at the state level is questionable because there are often only a few HVHs in each state, and the effect of volume on outcome may be overestimated if a single HVH “overperforms” relative to other HVHs. 13 Conversely, the effect may be underestimated if certain low-volume hospitals (LVHs) “overperform” compared with their LVH colleagues.
Obtaining a precise estimate for the magnitude of the “volume–outcome effect” of each high-risk surgical procedure is extremely important from a health policy perspective. The stronger the association between volume and the death rate, the more incentive exists for regionalization of patients to HVHs. The current study was performed using a database representative of the entire United States to gain a precise and generalizable estimate of the effect of hospital volume on the in-hospital death rate after repair of intact and ruptured AAAs.
METHODS
Data Source
The Nationwide Inpatient Sample (NIS) is a 20% stratified random sample of all hospital discharges in the United States. It is maintained by the Agency for Health Care Policy and Research as part of the Healthcare Cost and Utilization Project. 14 This study’s data were derived from 1996 and 1997 versions of the NIS. During this period, 507 hospitals from 19 states in 1996 and 536 hospitals from 22 states in 1997 performed AAA repair.
All patients who were discharged from these hospitals in 1996 and 1997 with an International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) primary procedure code for resection of abdominal aorta with replacement (ICD-9-CM code 3844) were included in the study. 15 In addition, a primary diagnostic code for either AAA (ICD-9-CM code 4414) or ruptured AAA (ICD-9-CM code 4413) was necessary to select patients who underwent an operation specifically for AAA. Patients with these two diagnostic codes represented approximately 86% of patients with a primary procedure ICD-9-CM code of 3844 in the NIS database in 1996 and 1997. Secondary ICD-9-CM diagnostic codes were abstracted to ascertain the presence of several comorbid diseases. 16,17 For all included patients, data regarding age, gender, race, nature of admission, vital status at discharge, length of stay (LOS), and hospital charges were abstracted directly from the database.
The majority of the excluded patients had primary diagnostic codes for thoracoabdominal aneurysm without rupture (8%) (ICD-9-CM code 4417), dissection of abdominal aorta (2%) (ICD-9-CM code 44102), and rupture of thoracoabdominal aneurysm (1%) (ICD-9-CM code 4416). In an effort to further eliminate patients who underwent surgery for traumatic aortic injuries, patients younger than 40 years of age and those who had a diagnostic code for injury to a blood vessel (ICD-9-CM code 902) were also excluded from the study.
Outcome Variables
The primary outcome variable was vital status at discharge (in-hospital death rate). LOS was a secondary endpoint and was used to compare the relative use of resources between HVHs and LVHs. Analyses were conducted for patients with both intact and ruptured AAAs. Rupture was determined by querying the primary and secondary diagnostic codes for the ICD-9-CM code 4413 (diagnostic code for rupture of aneurysm).
Unadjusted and risk-adjusted analyses were conducted. Risk adjustment included demographics (age, gender, race), 10 comorbid diseases, nature of admission (elective, urgent, or emergent), and ruptured versus intact AAAs. The Romano modification of the Charlson comorbidity score was used with ICD-9-CM codes from an index hospitalization to account for comorbid disease in patients’ risk adjustments. 16,17 Each comorbid disease was coded as a dichotomous variable and entered individually into the multivariate model. Not all hospitals reported the nature of admission. Data were available for 12,306 (89%) of the patients in the database. Therefore, a “dummy” variable was created to represent the missing values in the multivariate analysis.
Hospital Volume
The number of procedures performed at each hospital during 1996 and 1997 was calculated using an anonymous hospital identification number available in the NIS database. The definition of HVHs and LVHs was derived from a recent report that used previously specified criteria to choose the highest-quality study assessing the effect of hospital volume on outcomes for several surgical procedures. 4 For repair of intact AAAs, high volume was found to be greater than 30 procedures per hospital each year. This threshold was consistent with previous studies conducted using state administrative databases. 5–12 Hospitals were assigned either high- or low-volume status for each year, and volume was encoded as a dichotomous variable.
Hospital volume rather than surgeon volume was used as a marker of improved outcomes for several reasons. First, hospital volume and surgeon volume tend to be colinear and cannot be simultaneously compared in a multivariate analysis. Second, hospital volume is a complex variable that more accurately captures the capability of the healthcare system to manage a high-risk patient, including preoperative optimization and selection, intraoperative surgeon-related factors, and postoperative care. 13,18 Further, recent data have shown that low-volume surgeons practicing in a HVH have similar outcomes to high-volume surgeons working in the same hospital for some surgical procedures. 19,20
Statistical Analysis
Univariate comparisons of hospital volume, patient characteristics, and outcome variables were performed using the chi-square test, Wilcoxon rank-sum test, Student t test, simple logistic regression, and simple linear regression where appropriate. Multiple logistic regression of the in-hospital death rate was used to test its association with hospital volume after adjusting for potentially confounding patient case mix variables. The multivariate model of death was tested for goodness of fit according to the Hosmer-Lemeshow method, and the area under the receiver operator characteristic (ROC) curve was calculated. Any patient characteristic that had P < .1 in the univariate analysis was included in the multivariate analysis. LOS was not normally distributed and was skewed to the left, so multiple linear regression of log-transformed LOS was used for the multivariate analysis. The Shapiro-Wilk test was used to ensure normality of the log-transformed data. 21P < .05 was considered statistically significant in all final analyses. STATA Version 6.0 (College Station, TX) was used for all statistical analyses.
RESULTS
Hospital and Patient Characteristics
During 1996 and 1997, 13,387 patients were discharged from hospitals in the NIS after undergoing AAA repair. For 1996, there were 507 hospitals that performed AAA repair; 76 (15%) of these hospitals were classified as HVHs and 431 (85%) were classified as LVHs. In 1997, 536 hospitals performed AAA repair; 91 (17%) of these were HVHs and 445 (83%) were LVHs.
Baseline characteristics of the patients undergoing surgery at HVHs or LVHs revealed several differences (Table 1). Most patients were men (79%). Patients at HVHs were more likely than those at LVHs to have a history of myocardial infarction (12.2% vs. 7.2%). Patients at LVHs were more likely to have a history of chronic obstructive pulmonary disease (25% vs. 21.7%) and more likely to have repair of a ruptured aneurysm (16% vs. 10%). Otherwise, patients who had surgery at HVHs and LVHs had similar demographics and comorbid diseases.
Table 1. DEMOGRAPHIC CHARACTERISTICS AND COMORBID DISEASES
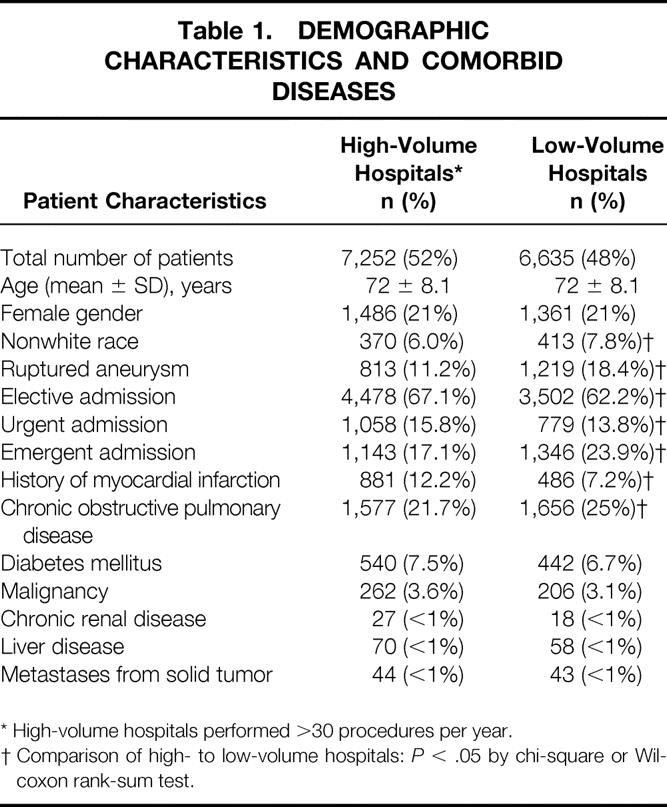
* High-volume hospitals performed >30 procedures per year.
† Comparison of high- to low-volume hospitals:P < .05 by chi-square or Wilcoxon rank-sum test.
Patients were divided according to several age groups and whether they had surgery for intact versus ruptured AAA at HVHs or LVHs (Table 2). There were no significant differences in age distribution between HVHs and LVHs. However, patients who had surgery for ruptured AAA tended to be older than those who had repair of intact AAA.
Table 2. DISTRIBUTION OF PATIENTS BY AGE GROUP
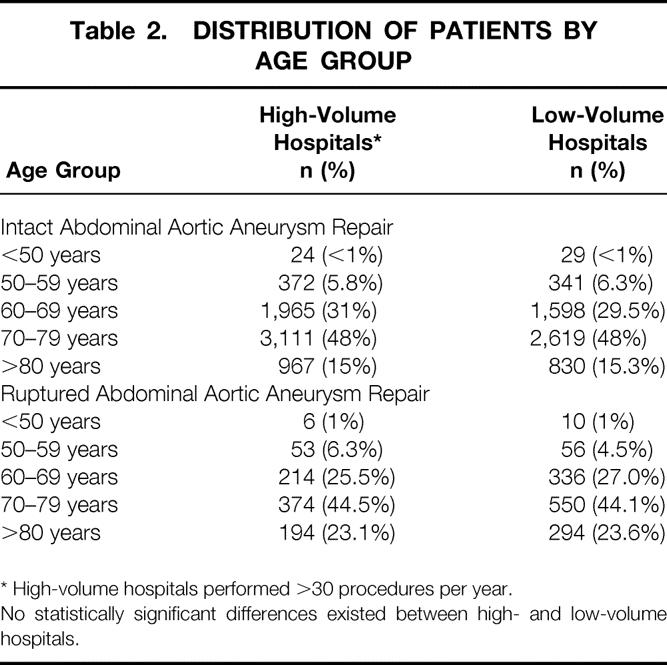
* High-volume hospitals performed >30 procedures per year.
No statistically significant differences existed between high- and low-volume hospitals.
In-Hospital Death Rate
The overall in-hospital death rate was 3.8% for intact AAA repair. There was a marked variation in the incidence of surgical death after intact AAA repair. For example, men younger than 65 years old who had surgery at HVHs had a surgical death rate of 0.8%, compared with 7.1% for women older than 65 years who had surgery at LVHs; this is nearly a ninefold variation (Table 3). HVHs had a lower death rate than LVHs (3.1% vs. 4.7%;P < .001). This represents an unadjusted relative risk (RR) of 1.54 (95% confidence interval [CI], 1.28–1.85) for having surgery at an LVH.
Table 3. UNADJUSTED IN-HOSPITAL DEATH RATES
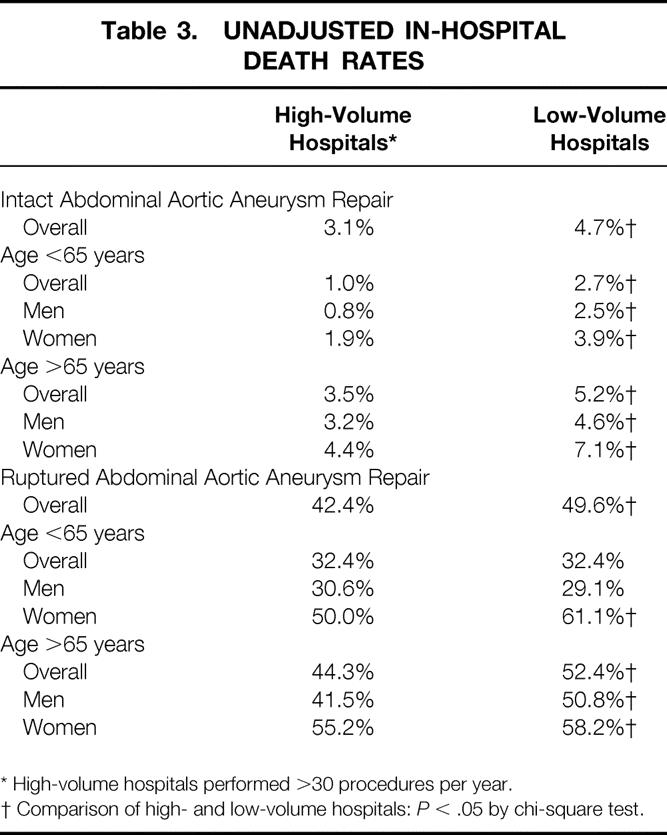
* High-volume hospitals performed >30 procedures per year.
† Comparison of high- and low-volume hospitals:P < .05 by chi-square test.
In a simple logistic regression analysis, increasing age was associated with a higher in-hospital death rate (P < .001). There was a stepwise increase in the death rate associated with increasing age, with age changed to a categorical variable (Fig. 1). Using a dichotomous age variable with a cutoff of 65 years, patients older than 65 years had a 4.2% in-hospital death rate, significantly (P < .001) greater than the 1.8% for patients younger than 65 years. When examining both hospital volume and the dichotomous age variable in a univariate analysis, it was clear that the effect of hospital volume persists for both patients older than and younger than 65 years old (Fig. 2). Specifically, for patients younger than 65 years, the in-hospital death rate was 1.0% at HVHs and 2.7% at LVHs (P = .004). For patients older than 65 years, the in-hospital death rate was 3.5% at HVHs and 5.2% at LVHs (P < .001).
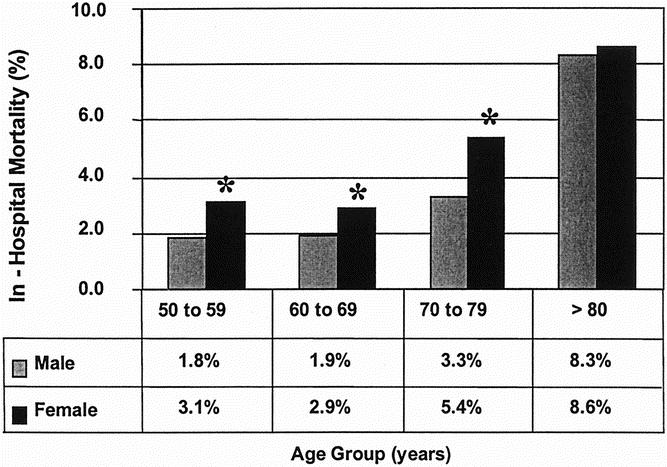
Figure 1. Comparison of in-hospital death rates by age group and gender for patients undergoing repair of intact abdominal aortic aneurysm in the United States, 1996 to 1997. There is a significant difference in the death rate between men and women until they reach an age greater than 80 years old. *P < .05.
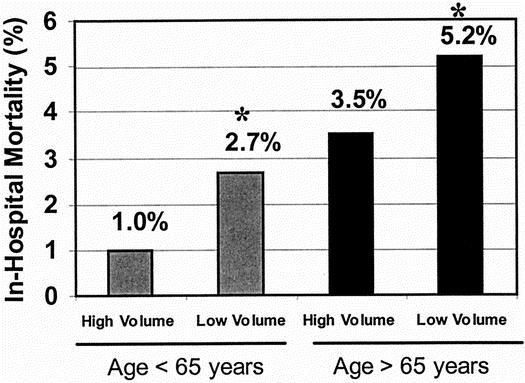
Figure 2. Comparison of in-hospital death rates by age group for patients undergoing repair of intact abdominal aortic aneurysm at high- and low-volume hospitals in the United States, 1996 to 1997. Patients older than and younger than 65 years old have significantly lower death rates at high-volume hospitals versus low-volume hospitals. *P < .05.
Women had an increased in-hospital death rate of 5.3% for intact AAA repair compared with 3.2% for men (P < .001). When the influence of gender was compared over each age category (see Fig. 2), women were at a higher risk of in-hospital death compared with men for all age groups except for patients older than 80 years. Women younger than 80 years accounted for 78% (2,229) of the NIS patients who underwent repair of intact AAAs in 1996 and 1997.
There was also an association between the nature of admission and in-hospital death, with death rates of 3.4% for elective admission, 9.4% for urgent admission, and 30.8% for emergent admission. Most ruptured aneurysms (80%) were categorized as an emergent admission, explaining the high death rate in this group. Other univariate risk factors for an increased in-hospital death rate include history of myocardial infarction (P < .001), history of malignancy (P < .001), and history of liver disease (P < .001).
The death rate after repair of ruptured AAA was slightly lower at HVHs versus LVHs (43% vs. 49%;P = .001) (see Table 3). Age was a significant risk factor for an increased death rate, with patients older than 65 years having a death rate of 49% compared with 32% for patients younger than 65 years (P < .001). Women who had repair of ruptured AAA were also at a higher risk than men of in-hospital death (57% vs. 44%;P < .001). When comparing across all age categories, there were not enough patients younger than 60 years and older than 80 years with ruptured aneurysm repair to give a statistically precise estimate of the death rate. However, for patients 60 to 69 years old, women had a death rate of 46.1% compared with 34.2% for men in that age group (P < .001). Similarly, women aged 70 to 79 had a death rate of 56.7% compared with 46.0% for men in that age group (P < .001). Other univariate risk factors for death after ruptured AAA repair include nonwhite race (P = .005), chronic obstructive pulmonary disease (P = .05), concurrent malignancy (P = .03), and a history of myocardial infarction (P = .02).
In a multivariate analysis adjusting for case mix, AAA surgery at an LVH was associated with a 56% increase in the surgical death rate (odds ratio [OR] 1.56; 95% CI, 1.33–1.82. Other independent risk factors for in-hospital death include female gender (OR 1.50; 95% CI, 1.24–1.80), age older than 65 years (OR, 2.05; 95% CI, 1.59–2.64), a ruptured aneurysm (OR 10.8; 95% CI, 8.8–13.3), an urgent admission (OR 1.69; 95% CI, 1.32–2.16), an emergent admission (OR 2.78; 95% CI, 2.21–3.51), history of myocardial infarction (OR 1.70; 95% CI, 1.22–2.37), and mild liver disease (OR 5.20; 95% CI, 2.73–9.79). The multivariate model was not rejected after goodness of fit testing, and the area under the ROC was calculated as 0.831, showing the good predictive capacity of the model.
In a second multivariate analysis, intact and ruptured AAA repairs were examined in separate models to determine differences in independent variables predictive of death. In this analysis, having surgery at an LVH was a significant predictor of increased in-hospital death for both intact (OR 1.71; 95% CI, 1.37–2.14) and ruptured (OR 1.43; 95% CI, 1.15–1.78) AAA repairs. The magnitude of this effect was greater for intact AAA repair (Table 4). Other independent risk factors for death were similar for intact and ruptured AAA repairs, except race (OR 1.60; 95% CI, 1.1–2.4) and malignancy (OR 2.76; 95% CI, 1.1–7.0), which were associated with an increased risk of death after ruptured AAA repair but not intact AAA repair.
Table 4. RESULTS OF THE MULTIVARIATE ANALYSIS: INDEPENDENT VARIABLES ASSOCIATED WITH AN INCREASED RISK OF IN-HOSPITAL DEATH
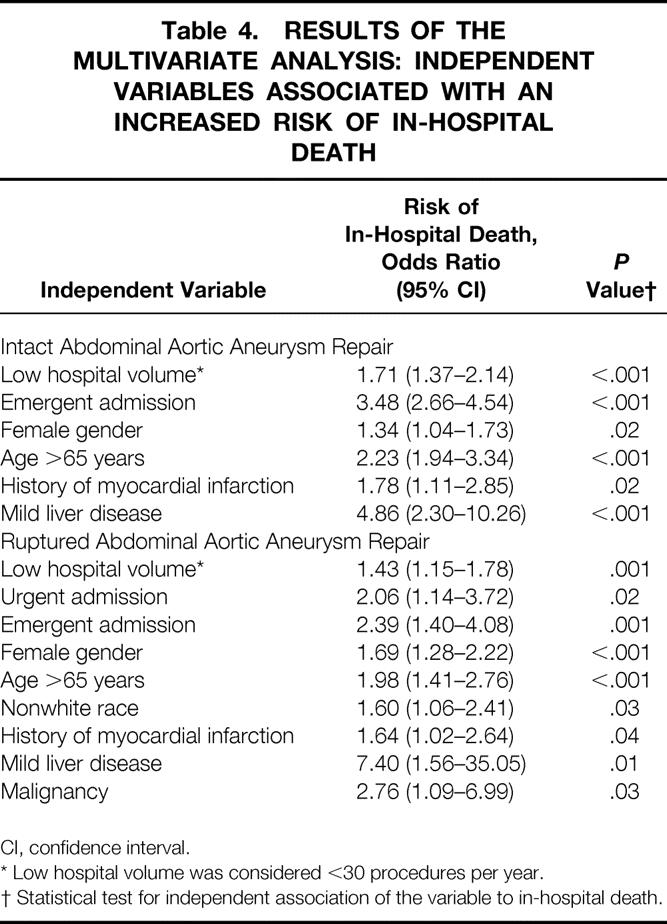
CI, confidence interval.
* Low hospital volume was considered <30 procedures per year.
† Statistical test for independent association of the variable to in-hospital death.
Length of Stay
The overall median LOS was 8 days (interquartile range [IQR], 6–10) for patients with intact AAA repair. HVHs had a median LOS 1 day shorter than LVHs after intact AAA repair (7 days [IQR, 6–10] vs. 8 days [IQR, 6–10]); this was statistically significant (P = .002) but represents only a modest clinical difference. Patients older than 65 years had a median LOS 1 day longer than those younger than 65 years (7 days [IQR, 6–9] vs. 8 days [IQR, 6–11]), which was statistically significant (P < .001). Other risk factors for increased LOS in the univariate analysis include nonwhite race (P < .001), chronic obstructive pulmonary disease (P < .001), diabetes mellitus (P = .03), a history of myocardial infarction (P < .001), and chronic renal disease (P = .001).
Median LOS for patients who survived repair of AAA after rupture was significantly longer (P < .001) than those who had elective repair, with a median of 12 days (IQR, 8–18). There were no statistically significant differences between LOS at HVHs and LVHs after ruptured AAA repair. Once again, there were statistically significant but modest clinical differences in median LOS between patients older than and younger than 65 years (12 days [IQR, 8–18] vs. 11 days [IQR, 7–17];P = .03). Another univariate predictor of increased LOS after repair of a ruptured AAA was a history of myocardial infarction (P = .06). In the multivariate analysis for LOS, there was no relationship between hospital volume and increased LOS. The only variable with a significant effect on LOS in the multivariate analysis was surviving the repair of a ruptured AAA. These patients had an estimated increase in LOS of 5 days (95% CI, 4.4–5.7 days;P < .001).
DISCUSSION
Several previous studies using statewide databases have shown the association of high hospital volume and improved outcomes after repair of intact and ruptured AAAs. The present study provides the first population-based estimate of the volume–outcome effect representative of the entire United States. In a risk-adjusted analysis, there was a 56% increase in the death rate at LVHs versus HVHs. This study also showed an incremental increase in the in-hospital death rate with increasing age and confirmed a significantly higher death rate for women undergoing AAA repair. In fact, there was a nearly ninefold variation in the surgical death rate for intact AAA repair (0.8% to 7.1%) that could be attributed to hospital volume, gender, and age alone. These factors are important when considering whether patients should be targeted for regionalization to HVHs. This study also showed that LOS was not increased at HVHs. Using LOS as a surrogate for resource use, the higher quality of care at HVHs appears to come at a similar cost to healthcare payers.
In 1979, Luft et al 3 published a landmark article on the effect of volume on outcomes after high-risk surgical procedures. In this study, AAA repair was among the procedures for which HVHs were associated with a decreased in-hospital death rate. Since that initial report, several others have documented this volume–outcome effect using state administrative databases. Hannan et al 6 found that both hospital volume and surgeon volume were significant predictors of in-hospital death after AAA repair in New York. For higher-volume surgeons, HVHs had an adjusted death rate of 11% versus 19% in LVHs. However, this report did not distinguish between ruptured and unruptured aneurysm repair, a major confounding variable. Further, the overall death rate for AAA repair has declined significantly since 1986, the year from which this report’s data were derived.
In Michigan, hospitals that performed more than 21 AAA repairs a year had a surgical death rate of 6.2% compared with 8.9% at lower-volume hospitals; this was a statistically significant reduction. 11 In a recent study of elective AAA repair in Maryland, Dardik et al 9 showed a twofold increased risk of case mix-adjusted in-hospital death for patients who had AAA repair at LVHs. In addition to these statewide studies, Kazmers et al 22 reported the results of patients undergoing AAA repair at Veterans Affairs medical centers across the United States. They found an in-hospital death rate of 4.2% at HVHs (>31 procedures/yr) compared with 6.7% at LVHs, a relationship that persisted after rigorous case mix adjustments.
Most studies on the volume–outcome effect for AAA repair from state databases are likely to overestimate the magnitude of the effect compared with the present study, which used a stratified sample of U.S. hospitals. This overestimation is because each state has only a few HVHs that may have superior outcomes compared with HVHs in other states, and those former hospitals may be national referral centers with greater expertise in AAA repairs. Comparisons between HVHs and LVHs in states with these national referral centers may not yield a valid estimate of the “true” volume–outcome effect for all hospitals in the United States. When calculating the potential reduction in deaths that might be obtained with regionalization, the impact of procedural volume on outcomes must be estimated from nationally representative studies.
Two recent reports have focused on estimating the number of lives that could be saved by referring patients to HVHs for several high-risk elective surgical procedures. In the first study, Dudley et al 4 applied the estimate of the volume–outcome effect from their review of the highest-quality study to the California population. In their analysis, they concluded that greater than 600 deaths in California and 4,000 deaths in the United States could be avoided each year by selective referral to HVHs. In a second study of the Medicare population, Birkmeyer et al 23 calculated the number of lives saved by regionalization for 10 high-risk surgical procedures. They estimated that 800 (5% death rate reduction) to 4,300 deaths (25% death rate reduction) could be avoided for elective surgery by implementing regionalization. These authors concluded that regionalization of care for common intermediate-risk procedures, such as cardiovascular procedures, would save more lives than regionalization of uncommon, high-risk procedures, such as pancreaticoduodenectomy. 23
There are several limitations to the current study. The NIS was created by merging administrative datasets from several states. Some argue that the administrative data should not be used to assess the quality of care in that they do not provide enough physiologic variables for robust case mix adjustment. 24 In the present study, adjustment was made for several comorbid diseases, ruptured versus unruptured AAA, nature of admission, and patient demographics. Several of these variables were associated with increased in-hospital death rates and were entered into the multivariate analysis. After adjusting for case mix in this fashion, there was no change in the magnitude of the volume–outcome interaction. This was consistent with several previous studies, all with varying methods of case mix adjustment, that showed that differences in outcomes between HVHs and LVHs are not attributable to variations in patient characteristics. There is no doubt that using a physiologic risk-adjustment tool, such as the acute physiology and chronic health evaluation (APACHE) III score, would provide more accurate comparisons, especially for patients with ruptured AAAs. 22 However, the large-scale nature of the present study precludes such data acquisition. Another limitation is that the NIS does not include all U.S. hospitals. The participating hospitals, representing 20% of all hospitals, were stratified according to geographic region, urban or rural location, teaching status, ownership, and bed size to accurately represent care across the entire nation. Finally, this study did not take into account the impact of rapidly developing endovascular AAA repair. In 1996 and 1997, however, few endovascular AAA repairs were being performed outside investigational centers, and this technology would therefore not have an impact. In the future, a change in both patient characteristics and hospital performance profiles will likely occur as endovascular techniques become more widespread.
This study shows that HVHs have lower in-hospital death rates compared with LVHs using a nationally representative database. Further, there is a nearly ninefold variation in the surgical death rate for intact AAA repair that can be explained by hospital volume, gender, and age. The magnitude of the effect of volume on outcomes is significant but does not appear as large as the estimates from state databases. These findings have important implications for health policy makers and healthcare providers. Patients in need of AAA repair may be best referred to HVHs. Patients who are older than 65 years, who are female, or who have multiple comorbid diseases are at a higher risk of in-hospital death and would likely benefit the most from referral to HVHs.
Footnotes
Correspondence: Gilbert R. Upchurch, Jr., MD, 1500 East Medical Center Drive, Taubman Center 2210, Ann Arbor, MI 48109-0329.
E-mail: riversu@umich.edu
Accepted for publication September 28, 2001.
References
- 1.Ernst CB. Abdominal aortic aneurysm. N Engl J Med 1993; 328: 1167–1172. [DOI] [PubMed] [Google Scholar]
- 2.Huber TS, Wang JG, Derrow AE, et al. Experience in the United States with intact abdominal aortic aneurysm repair. J Vasc Surg 2001; 33: 304–311. [DOI] [PubMed] [Google Scholar]
- 3.Luft HS, Bunker JP, Enthoven AC. Should operations be regionalized? The empirical relationship between surgical volume and mortality. N Engl J Med 1979; 301: 1364–1369. [DOI] [PubMed] [Google Scholar]
- 4.Dudley RA, Johansen KL, Brand R, et al. Selective referral to high-volume hospitals: estimating potentially avoidable deaths. JAMA 2000; 283: 1159–1166. [DOI] [PubMed] [Google Scholar]
- 5.Wennberg DE, Lucas FL, Birkmeyer JD, et al. Variation in carotid endarterectomy mortality in the Medicare population. JAMA 1998; 279: 1278–1281. [DOI] [PubMed] [Google Scholar]
- 6.Hannan EL, O’Donnell JF, Kilburn H Jr, et al. Investigation of the relationship between volume and mortality for surgical procedures performed in New York State hospitals. JAMA 1989; 262: 503–510. [PubMed] [Google Scholar]
- 7.Lawrence PF, Gazak C, Bhirangi L, et al. The epidemiology of surgically repaired aneurysms in the United States. J Vasc Surg 1999; 30: 632–640. [DOI] [PubMed] [Google Scholar]
- 8.Dardik A, Burleyson GP, Bowman H, et al. Surgical repair of ruptured abdominal aortic aneurysms in the state of Maryland. J Vasc Surg 1998; 28: 413–421. [DOI] [PubMed] [Google Scholar]
- 9.Dardik A, Lin JW, Gordon TA, et al. Results of elective abdominal aortic aneurysm repair in the 1990s: A population-based analysis of 2335 cases. J Vasc Surg 1999; 30: 985–995. [DOI] [PubMed] [Google Scholar]
- 10.Pearce WH, Parker MA, Feinglass J, et al. The importance of surgeon volume and training in outcomes for vascular surgical procedures. J Vasc Surg 1999; 29: 768–776. [DOI] [PubMed] [Google Scholar]
- 11.Katz DJ, Stanley JC, Zelenock GB. Operative mortality rates for intact and ruptured abdominal aortic aneurysms in Michigan: an eleven-year statewide experience. J Vasc Surg 1994; 19: 804–815. [DOI] [PubMed] [Google Scholar]
- 12.Manheim LM, Sohn MW, Feinglass J, et al. Hospital vascular surgery volume and procedure mortality rates in California, 1982–1994. J Vasc Surg 1998; 28: 45–56. [DOI] [PubMed] [Google Scholar]
- 13.Birkmeyer JD. High-risk surgery—follow the crowd. JAMA 2000; 283: 1191–1193. [DOI] [PubMed] [Google Scholar]
- 14.Healthcare Cost, Utilization Project (HCUP-6). Nationwide Inpatient Sample, Release 6. Rockville, MD. Agency for Health Care Research and Quality, 1997.
- 15.Public Health Service, Health Care Financing Administration. International Classification of Diseases, 9th revision, Clinical Modification. Washington, DC: U.S. Department of Health and Human Services, 1991.
- 16.Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J Clin Epidemiol 1993; 46: 1075–1079. [DOI] [PubMed] [Google Scholar]
- 17.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method for classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373–383. [DOI] [PubMed] [Google Scholar]
- 18.Birkmeyer JD. Should we regionalize major surgery? Potential benefits and policy considerations. J Am Coll Surg 2000; 190: 341–349. [DOI] [PubMed] [Google Scholar]
- 19.Harmon JW, Tang DG, Gordon TA, et al. Hospital volume can serve as a surrogate for surgeon volume for achieving excellent outcomes in colorectal resection. Ann Surg 1999; 230: 404–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cebul RD, Snow RJ, Pine R, et al. Indications, outcomes, and provider volumes for carotid endarterectomy. JAMA 1998; 279: 1282–1287. [DOI] [PubMed] [Google Scholar]
- 21.Royston P. An extension of Shapiro and Wilk’s W test for normality to large samples. Applied Statistics 1982; 31: 115–124. [Google Scholar]
- 22.Kazmers A, Jacobs L, Perkins A, et al. Abdominal aortic aneurysm repair in Veterans Affairs Medical Centers. J Vasc Surg 1996; 23: 191–200. [DOI] [PubMed] [Google Scholar]
- 23.Birkmeyer JD, Lucas FL, Wennberg DE. Potential benefits of regionalizing major surgery in Medicare patients. Eff Clin Pract 1999; 2: 277–283. [PubMed] [Google Scholar]
- 24.Romano PS. Can administrative data be used to compare the quality of health care? Med Care Rev 1993; 50: 451–477. [DOI] [PubMed] [Google Scholar]


