Abstract
Objective
To determine the incidence, significance, and anatomy of spermatic cord and round ligament lipomas.
Methods
This was a retrospective review of 280 hernia repairs on 217 patients performed by a single surgeon (M.E.A.) from January 1996 to January 2000. The incidence of cord lipoma and relationship to inguinal hernia were evaluated. Further, when identified at the time of laparoscopic preperitoneal hernia repair, the anatomy of the lipomas was studied both at the time of surgery and again on review of videotapes.
Results
One hundred ninety-nine laparoscopic and 81 open inguinal hernia repairs were performed on 192 male patients and 25 female patients. Sixty-three lipomas of the cord were identified for an incidence of 22.5%. Overall, 18 cord lipomas were found in groins without hernias, and these were identified before surgery in 10 (2 by physical examination, 7 by groin ultrasound, and 1 by magnetic resonance imaging). The remaining nine were misidentified as a hernia before surgery. Fourteen of these patients presented with groin pain and four were asymptomatic. Forty-five lipomas were associated with hernias and were characterized as a hernia by examination in 43 instances. There were 32 (51%) cord lipomas associated with indirect hernias, 11 (17%) with direct hernias, and 1 each with pantaloon and femoral hernias. Nine lipomas were found in women, seven presenting with groin pain and six found without an associated peritoneal defect. Two patients presented with symptomatic cord lipomas after laparoscopic hernia repair. A lipoma of the cord is herniated fat that appears to originate from the retroperitoneal fat outside and posterior to the internal spermatic fascia and protrudes through the internal ring lateral to the cord. They are generally not visible by transperitoneal inspection unless manually reduced.
Conclusions
Lipomas of the cord and round ligament occur with a significant incidence. They can cause hernia-type symptoms in the absence of a true hernia (associated with a peritoneal defect). They should be considered in the patient with groin pain and normal examination results. They can be easily overlooked at the time of laparoscopic hernia repair, and this can lead to an unsatisfactory result.
Lipomas of the spermatic cord or round ligament are encountered often during anterior repairs of inguinal hernias but have garnered little press. Cord lipomas are identified usually as an incidental finding in association with an actual hernia and are easily dealt with by separation from the cord structures and resection. In some cases they are the only finding and no associated hernia sac is identified. With laparoscopic herniorrhaphy, the identification of cord lipomas is more difficult, and thus the incidence and their significance take on a new light. The senior author (M.E.A.) has been keenly aware and interested in this subject and has prospectively looked specifically for lipomas and carefully recorded these cases in the operative dictation for subsequent retrospective analysis.
The purposes of this review are to describe the anatomy of cord lipomas, to identify any relationship to the type of inguinal hernia, to determine the incidence of lipomas in those explored for hernia repair, and to determine their significance, especially in patients with no associated hernia defect.
METHODS
This is a retrospective review of 280 consecutive hernia repairs performed by or under the direction of the senior author (M.E.A.) between January 1996 and January 2000. Hernia repairs were performed either from an anterior open approach in a Lichtenstein fashion, or by a preperitoneal laparoscopic method. The laparoscopic method consisted of an initial pelvic laparoscopy to look for a peritoneal defect and to facilitate placement of preperitoneal trocars. After this, the preperitoneal space was dissected, the hernia sac (if present) reduced, and a large piece of mesh placed without fixation. We currently do this through only 5-mm trocars. This technique has been recently described by Spitz and Arregui. 1
The decision to perform the repair open or laparoscopically was based on the patient’s wishes, comorbidities, and surgical history and the presence of bilateral or recurrent hernias. Either way, the type of hernia and presence of a cord lipoma was recorded. A lipoma of the spermatic cord or round ligament was identified as an isolated discrete collection of fatty tissue arising from the retroperitoneal tissue and protruding through the internal ring that could be easily separated from the cord structures.
Preoperative evaluation consisted initially of only a history and physical examination; in more recent patients, ultrasound of the groin was performed. Preoperative suspicion of a cord lipoma was annotated. Intraoperative evaluation during laparoscopic repair consisted of both transperitoneal examination and preperitoneal exploration, with external manipulation of the inguinal canal performed to reduce and identify a lipoma. Most of the laparoscopic repairs were videotaped, which facilitated the review of those that had a recurrence. During open hernia repair, the spermatic cord was evaluated for lipoma directly. Follow-up consisted of the initial postoperative visit and 1-year follow-up.
RESULTS
A total of 280 hernia repairs were performed, 251 on male patients and 29 on female patients, with an average age of 57 years (range 6–86). This involved a total of 217 patients, as 63 patients (54 male patients, 9 female patients) had bilateral repairs. A total of 63 lipomas of the spermatic cord or round ligament were identified.
Hernias were classified as indirect, direct, pantaloon (combined direct and indirect), femoral, or none. There were 137 indirect with 32 lipomas, 99 direct with 11 lipomas, 18 pantaloon with 1 lipoma, 11 femoral with 1 lipoma, and 20 groins explored with no hernia sacs, with 18 having cord lipomas. Hence, the incidence of lipoma associated with an indirect hernia was 23% and with a direct hernia 11%. The overall incidence of cord lipoma in those explored for hernia repair was 22.5%.
There were 199 laparoscopic hernia repairs, finding 39 lipomas, and 81 open repairs, finding 24 lipomas. Two patients who underwent laparoscopic repairs returned with recurrent symptoms and were found to have a cord lipoma. Both of these had development of a lipoma in the interim: one had a lipoma removed from that side already and the other had a thorough search (documented by video) performed at the initial repair.
Eighteen cord lipomas were identified in patients who did not have a hernia defect. Of these lipomas, 12 were in male patients and 6 in female patients. Fourteen of these were symptomatic and four had no symptoms of pain but did have a palpable bulge. On examination, seven were felt to have a hernia and two to have lipomas. Nine had no findings on physical examination. All six of the female hernias in this group had no findings on physical examination but had significant groin pain. The women of this group were in general obese, with an average weight of 169 lbs and an average height of 62 inches. Five of the six women with a round ligament lipoma and no hernia had improvement or complete resolution of their chronic groin pain after laparoscopic repair and lipoma removal. Overall, of the 14 patients with preoperative symptoms, 11 had resolution of symptoms, 2 had improvement, and 1 continued to have groin pain.
Twelve of these patients with a lipoma and no hernia were evaluated before surgery by ultrasound, with 11 having a positive finding. A lipoma was seen by ultrasound in seven, and a hernia was mistakenly identified in six. However, we have not usually made an attempt to distinguish a lipoma from a true hernia. One patient who did not have findings on examination or ultrasound had a cord lipoma identified by magnetic resonance imaging. We suspect that because this was a small lipoma that was intermittently protruding, it was not bulging out at the time we performed the examination and was therefore missed.
Lipomas were noted to protrude through the internal inguinal ring to lie on the anterolateral aspect of the spermatic cord, much like an indirect hernia. When viewed from a preperitoneal dissection, a filmy connection containing the blood supply could be visualized to extend to the retroperitoneum, inferior to the cord structures. The lipoma and blood supply was found outside the internal spermatic fascia (Fig. 1).
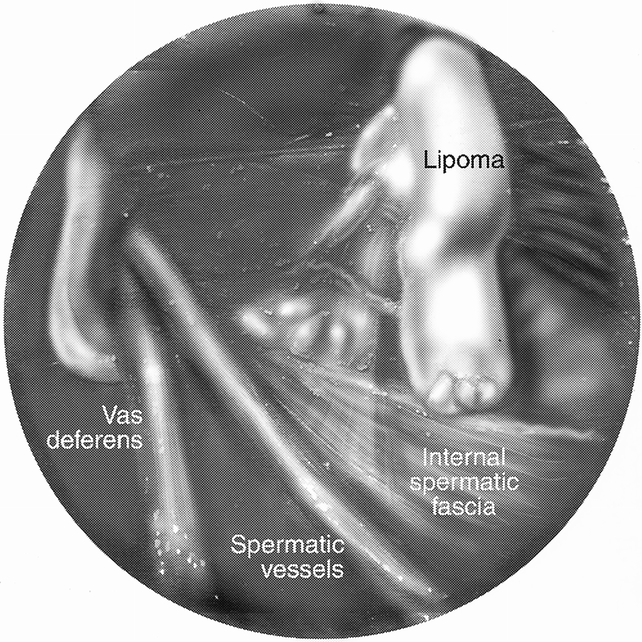
Figure 1. Preperitoneal view of lipoma, showing anatomy.
DISCUSSION
Lipomas of the cord have been relatively ubiquitous in the practice of hernia repair. Their presence has been widely accepted, usually as an incidental finding at the time of hernia repair. Their significance was inconsequential because they were routinely resected from the spermatic cord during an exploration for a hernia sac. With laparoscopic herniorrhaphy, cord lipomas are more apt to be missed; thus, it may be time to rethink the significance of cord lipomas.
The term “lipoma” itself may be a misnomer because in most instances it is a protrusion of the retroperitoneal fat rather than a benign neoplasm, as the term “lipoma” implies. Tobin et al 2 in 1946 described the connective tissue between the peritoneum and the abdominal wall as consisting of three strata. They suggested that the intermediate stratum was the origin of lipomas of the cord. This intermediate stratum is contiguous through the retroperitoneum as it encases the kidneys, ureters, and the spermatic cord structures with a fatty areolar tissue. Fawcett and Rooney 3 performed a prospective examination of 140 hernia repairs, finding 46 fatty protrusions of the extraperitoneal fat and only one “true” lipoma. They made a distinction that a “true” lipoma was identified only if there was no connection to the extraperitoneal fat and it was confined to the inguinal canal. Nyhus et al 4 described lipomas of the cord as preperitoneal fat that projects through the internal inguinal ring, usually at the lateral aspect of the cord. In our experience with hernia repairs, both laparoscopic and open, it appears that lipomas of the cord, whether seen as a protrusion of extraperitoneal fat or as separate, all have some connection to the retroperitoneal tissues. As described above and as shown in Figure 1, we have found this to be from the lateral aspect of the cord structures. The blood supply usually comes from beneath the internal spermatic fascia.
By the nature of our definition of cord lipomas, they are all “indirect.” The incidence of cord lipoma was greater among patients with indirect hernia defects as opposed to those with direct defects. Although it is difficult to say that this represents a cause-and-effect relationship, it is possible that lobular retroperitoneal fat (i.e., lipoma) insinuates itself through the internal ring and over time dilates it. This could then predispose to indirect hernia formation.
In our series we showed that in many instances the lipoma is the only pathology identified during an exploration for a hernia repair. It is quite clear in these situations that the lipoma needs to be resected. The question is what can be extrapolated from those situations to the incidental lipomas that are found at the time of hernia repair when a true hernia is also identified. In our series we had two patients return with a “recurrence” after laparoscopic repair, only to find that there was no hernia recurrence at all, only a cord lipoma. These were believed to have been formed in the interim, because one patient had already had a lipoma removed from that side and in the other a very thorough search for a lipoma had been done at the initial repair. Although these may have been formed in the interim, others have described recurrences resulting from missed lipomas of the cord. Felix et al 5 described a large multicenter study of more than 10,000 laparoscopic hernia repairs, and of the 35 recurrences, 4 were due to missed lipomas of the cord. This is echoed by Gersin et al, 6 who described six cases of lipomas of the cord. In Gersin et al’s description, there were six patients with a hernia by examination with no peritoneal defect at laparoscopy, each with a cord lipoma. Two of these patients had previously abandoned transabdominal preperitoneal (TAPP) repairs because no peritoneal defect had been found on laparoscopy of the pelvis. Contrary to their findings, we have found that cord lipomas can be identified at the time of pelvic laparoscopy (Figs. 2 and 3). This is done by manual external manipulation at the inguinal canal, thus pushing the lipoma back through the internal ring (Fig. 4). Also contrary to Gersin et al’s findings is that at preperitoneal exploration, the lipoma is not readily identified and again often requires external compression as well as gentle internal traction on the cord structures. Despite our maneuvers for identifying these lipomas at laparoscopic repair, it is of concern that we may be missing some lipomas, because our incidence of lipomas found at laparoscopic repair was 19.6%, whereas it was 29% on open repair.
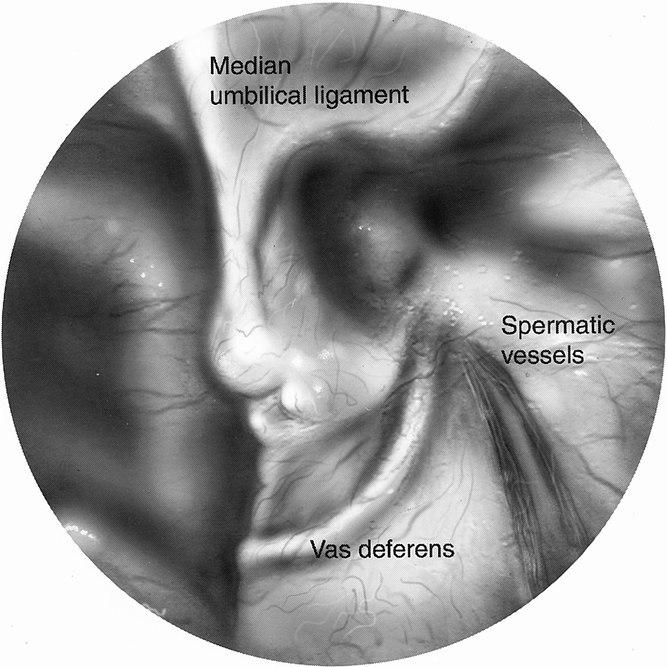
Figure 2. Peritoneal view of internal ring before external compression.
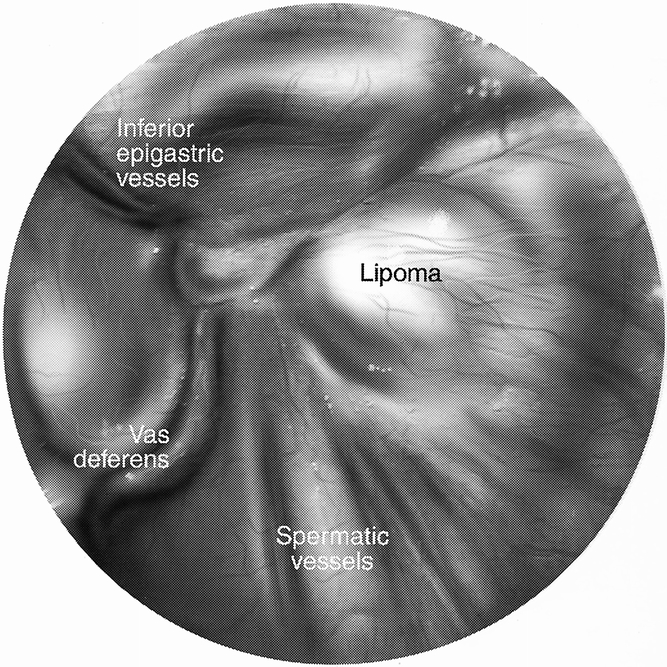
Figure 3. Peritoneal view of the internal ring with external compression.
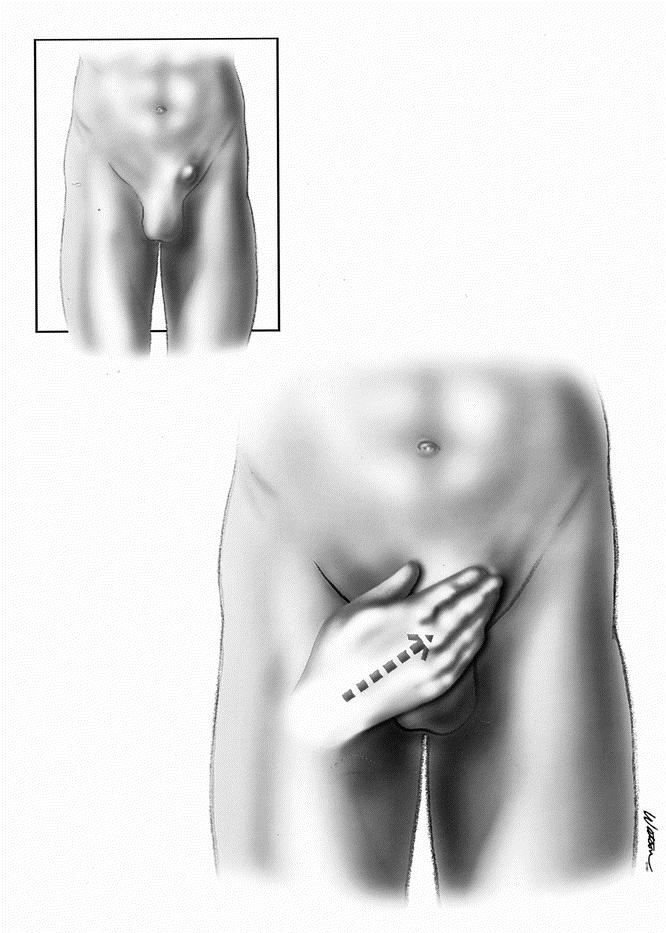
Figure 4. Method of external compression.
As per the above discussion, any lipoma of the cord found at exploration, either with or without an associated hernia, should be removed, although it is not always easy to pull a lipoma through a 5-mm trocar. The options are to remove it piecemeal, which is tedious but probably the right answer; to leave it attached at the base and place it between the mesh and the peritoneum, which we have done with success; or to separate it completely and leave in situ, which we do not recommend. We have only anecdotal results to report on this decision because we commonly perform the former two, and have had one patient who at initial surgery had a direct hernia and a lipoma of the cord. The lipoma was reduced draped over the cord structures and placed in the space of Retzius. He returned with the lipoma having migrated into the direct space, presenting as an asymptomatic hernia recurrence. At exploration he did not have a peritoneal defect, and the previously reduced lipoma was found in the direct hernia sac formed by the protruding transversalis fascia. The lipoma was removed at this setting.
With the knowledge that a lipoma of the cord can be the sole etiology of hernia symptoms, we have taken steps to identify these before surgery. We have been using ultrasound of the groin with some success, and in one patient mentioned previously magnetic resonance imaging identified a symptomatic cord lipoma. A lipoma of the cord or round ligament is sometimes the source of chronic groin pain in a patient with no physical findings. The number of women with groin pain secondary to a lipoma is disproportionately high, and although a relatively small number, we showed a propensity for lipomas to present in women as groin pain in the absence of physical findings. This may be due to the usually small size of the internal ring in women, which could allow a smaller lipoma to be symptomatic, although it is difficult to be certain because we have seen women with relatively large and asymptomatic lipomas. What is true is that lipomas of the round ligament seem to be more difficult to diagnose. This may be due to a propensity to associate these complaints with a gynecologic source, or in the case of some of our patients obesity may make the diagnosis more difficult.
Lipomas of the spermatic cord or round ligament have been shown to be a true pathology of the groin, because they can be the etiology of significant symptoms. A lipoma of the cord is herniated preperitoneal fat, which causes symptoms as a true hernia, which is herniated peritoneal contents. Whether found with or without a hernia at exploration for hernia repair, they must be dealt with. This does not imply that lipomas that are asymptomatic and found incidentally by ultrasound or intraoperative examination during contralateral repair should be resected, but they should certainly be annotated in case of future symptoms. Laparoscopic hernia repair makes it easy to overlook a cord lipoma, but the surgeon must think about a cord lipoma in every case and pursue it actively, lest he or she have the patient return with persistent complaints of a groin bulge or pain. Further, one should consider the possibility of a cord lipoma when dealing with those patients with groin pain in the absence of a hernia. Much like occult hernias, imaging studies such as ultrasound, computed tomography, and possibly magnetic resonance imaging can be applied to identify these lipomas before surgery.
Footnotes
Correspondence: Michael C. Lilly, MD, 81 MSGS/SGCQ, 301 Fisher Street, Suite 1A132, Keesler AFB, MS 39534.
E-mail: michael.lilly@keesler.af.mil
Accepted for publication October 5, 2001.
References
- 1.Spitz JD, Arregui ME. Sutureless laparoscopic extraperitoneal inguinal herniorrhaphy using reusable instruments: Two hundred three repairs without recurrence. Surg Laparosc Endosc 2000; 10: 24–29. [PubMed] [Google Scholar]
- 2.Tobin CE, Benjamin JA, Wells JC. Continuity of the fasciae lining the abdomen, pelvis and spermatic cord. Surg Gynecol Obst 1946; 83: 575–595. [PubMed] [Google Scholar]
- 3.Fawcett AN, Rooney PS. Inguinal cord lipoma. Br J Surg 1997; 84: 1169–1168. [PubMed] [Google Scholar]
- 4.Nyhus LM, Bombeck CT, Klein MS. Hernias. In Sabiston DC, ed. Textbook of Surgery: The Biological Basis of Modern Surgical Practice, 14th ed. Philadelphia: WB Saunders; 1991: 1134–1148.
- 5.Felix E, Scott S, Crafton B, et al. Causes of recurrence after laparoscopic hernioplasty. Surg Endosc 1998; 12: 226–231. [DOI] [PubMed] [Google Scholar]
- 6.Gersin KS, Heniford BT, Garcia-Ruiz A, Ponsky JL. Missed lipoma of the spermatic cord: A pitfall of transabdominal preperitoneal laparoscopic hernia repair. Surg Endosc 1999; 13: 585–587. [DOI] [PubMed] [Google Scholar]


