Abstract
Objective
To evaluate whether gastrin-releasing peptide (GRP) and GRP receptor (GRP-R) expression correlate with tumor behavior and to examine the mitogenic actions of GRP on neuroblastomas.
Summary Background Data
Neuroblastoma is the most common solid tumor of infants and children. Despite recent advances in multimodality treatment regimens, the survival for advanced-stage tumors remains dismal. Neuroblastomas are known to produce GRP; however, the proliferative effects of GRP on neuroblastomas have not been elucidated.
Methods
Sections of paraffin-embedded neuroblastomas from 33 patients were analyzed for GRP and GRP-R protein expression by immunohistochemistry. Functional binding of GRP-R to the Ca2+ signaling pathway was examined. In addition, the proliferative effect of GRP on neuroblastoma cells (SK-N-SH, IMR-32, SH-SY5Y, LAN-1) was determined.
Results
Immunohistochemical analysis showed GRP and GRP-R protein expression in neuroblastomas; an increased expression of GRP-R was noted in a higher percentage of undifferentiated tumors compared with tumors that were benign. GRP-R mRNA was confirmed in neuroblastoma cell lines. GRP treatment resulted in intracellular calcium [Ca2+]i mobilization in two cell lines (SK-N-SH, LAN-1). GRP treatment stimulated growth of all four neuroblastoma cell lines; this effect was inhibited in SK-N-SH cells by pretreatment with GRP antibody.
Conclusions
These findings show increased GRP-R expression in the more aggressive and undifferentiated neuroblastomas. The synchronous expression of GRP and its receptor, GRP-R, suggests a role for these proteins in tumor growth. Moreover, these findings show enhanced proliferation of neuroblastoma cells in vitro after GRP treatment, suggesting that GRP may act as an autocrine and/or paracrine growth factor for neuroblastomas. Treatment with specific GRP-R antagonists may provide novel adjuvant therapy for neuroblastomas in children.
Neuroblastoma is the most common extracranial solid malignancy of childhood and has a death rate exceeding 50%, particularly in older children. 1 Neuroblastoma tumors are derived from neural crest precursors and are classified as ganglioneuromas (benign type), ganglioneuroblastomas (mixed type), or neuroblastomas (malignant type). 2,3 They occur at sites in the sympathetic nervous system distributed from the head and neck region to the pelvis. 4 Variations in tumor location and degree of histopathologic differentiation can result in a wide range of clinical and biologic characteristics; however, the two most important clinical prognostic variables are disease stage and the age of the patient at diagnosis. 5,6 Interestingly, the clinical behavior of neuroblastoma is varied and unpredictable and can range from spontaneous tumor regression, maturation from a malignant to a benign lesion, or progressive malignant change. 2,3 This unpredictable feature of neuroblastoma suggests that malignant transformation of cells may result, in part, from a failure to respond to normal signals regulating morphologic differentiation.
Neuroblastomas are classified as amine precursor uptake decarboxylase tumors because they secrete peptides and other substances including vasoactive intestinal polypeptide and vasoactive metabolites such as catecholamines, serotonin, and acetylcholine. 7 These peptides may be involved in the regulation of tumor growth and differentiation. Further, the concentration of certain peptides (e.g., vanillylmandelic acid) has been correlated with the clinical behavior of neuroblastomas. Despite an ever-increasing ability to determine prognosis based on biologic features of neuroblastoma, such as N-myc amplification, 8 ploidy, 9 and deletion or loss of heterozygosity of chromosome 1p, 10 the cellular mechanisms involved in the growth regulation of this tumor remain unknown. Devising new therapeutic strategies for treating patients with high-risk neuroblastoma based on a better molecular understanding of neuroblastoma growth regulation remains a challenge.
Gastrin-releasing peptide (GRP), the mammalian equivalent of bombesin, is a neuroendocrine peptide shown to have a growth stimulatory effect on various tissues. 11,12 Investigators in our laboratory have shown that GRP is a potent mitogen for normal intestinal mucosa 13 and pancreas. 14 In addition, we have demonstrated that GRP, binding to its G-protein coupled receptor (GRP-R), exerts a trophic effect on cancers of the lung, 14 pancreas, 15 stomach, 16 and colon. 17 We have also shown that bombesin exerts its mitogenic action in gastric cancer by functionally binding to GRP-R and activating the intracellular calcium [Ca2+] pathway. 18 Others have shown the trophic effects of GRP on breast 19 and prostate cancer cell lines. 20,21 Moreover, Cuttitta et al. 12 have shown that GRP is an autocrine growth factor for human small cell lung cancer. GRP mRNA expression has been identified in certain neuroblastomas;22 however, the expression of the GRP and the role of this peptide in neuroblastoma growth have not been elucidated.
The purpose of our study was to define the potential mitogenic actions of GRP in human neuroblastomas. We examined paraffin-embedded archival samples of neuroblastomas of varying histopathology for expression of GRP and GRP-R by immunohistochemistry and correlated the expression of these proteins with clinical tumor behavior. In addition, we assessed human neuroblastoma cell lines for the presence of GRP-R mRNA, the effect of GRP on [Ca2+]i mobilization, the secretion of GRP, and the proliferative effects of GRP on these cell lines.
METHODS
Materials
Human GRP was obtained from Bachem (Torrance, CA) and dissolved in sterile water containing 0.1% bovine serum albumin. BIM26226 (GRP-R antagonist: [D-F5 Phe,6D-Ala11]BBS(6–13)OMe was a gift from Biomeasure Inc. (Milford, MA). Carbachol was purchased from Calbiochem (San Diego, CA). Fura-2/acetoxymethyl ethyl ester was purchased from Molecular Probes (Eugene, OR). Cell culture medium RPMI 1640 and fetal bovine serum (FBS) were from Cellgro (Herndon, VA) and Sigma Chemical Co. (St. Louis, MO), respectively. GRP-R antibody was a gift from Dr. James F. Battey (NIH, Bethesda, MD). GRP antibody was purchased from Peninsula Laboratories Inc. (San Carlos, CA). The ABC Staining System Kit used for immunohistochemistry was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). First Strand Buffer (5×) and Superscript II enzyme were purchased from Gibco (Carlsbad, CA). Polymerase chain reaction (PCR) buffer (10×) and Taq polymerase were from Promega (Madison, WI). GRP-R PCR primers were synthesized by Oligos Etc. Inc. (Wilsonville, OR). All other reagents were molecular biology-grade and obtained from either Sigma or Amresco (Solon, OH).
Cell Culture
The neuroblastoma cell line, LAN-1, was a gift from Dr. Robert C. Seeger (University of Southern California, Los Angeles, CA). SH-SY5Y, SK-N-SH, and IMR-32 human neuroblastoma cell lines were purchased from the American Type Culture Collection (ATCC, Manassas, VA). Cells were maintained in RPMI 1640 with 10% FBS at 37°C in a humidified atmosphere consisting of 5% CO2 and 95% air.
Cell Growth Study
SK-N-SH, IMR-32, SH-SY5Y, and LAN-1 cells were plated in triplicate onto 24-well plates (15,000 cells/well) using RPMI 1640 media containing 5% heat-treated FBS. After 24 hours, medium was replaced with fresh RPMI 1640 without FBS, and the cells were serum-starved for 24 hours. After serum starvation, new medium containing 0.1% FBS (for SK-N-SH, IMR-32, and LAN-1) or medium without FBS (for SH-SY5Y cells) was added along with GRP (10−8 mol/L). The cells were treated daily with GRP.
For the conditioned medium cell growth study, SK-N-SH cells were plated using medium containing 5% heat-treated FBS. The next day, serum-free medium was placed. After serum starvation for 24 hours, exogenous GRP (10− mol/L) was added and incubated for an additional 24 hours. The cells were then washed three times to remove the exogenous GRP, and fresh serum-free medium was placed. The cells were incubated for an additional 48 hours, and this medium was designated as the conditioned medium. The control medium for this experiment was obtained in the same manner as the conditioned medium without the initial addition of exogenous GRP. At the end of 48 hours, this conditioned medium was collected and used to treat another set of plated cells that had been serum-starved for 24 hours as described above. The treated cells were collected at the end of 48 hours of incubation and counted. To determine whether endogenously secreted GRP was responsible for the mitogenic effect, GRP antibody (1 μg/mL) was incubated with the conditioned medium for 3 hours before the addition of this medium to another set of serum-starved cells.
For both sets of growth studies, the medium was removed at the end of each treatment period and cells were harvested with 0.25% trypsin-EDTA, resuspended in Isotone III and counted using a Coulter Cell Counter (Coulter Electronics Inc., Hialeah, FL). The experiments were repeated twice on separate occasions.
Intracellular Calcium ([Ca2+]i) Ratio Imaging
Real-time recording of [Ca2+]i was performed in cultured cells using methods described previously. 23,24 Cells grown on glass coverslips for 24 hours were washed with Krebs-Ringer’s-Henseleit (KRH) buffer and loaded with 2 μmol/L fura-2 for 50 minutes at 25°C to minimize dye compartmentalization. Loaded cells were washed three times with KRH and incubated for 60 minutes at 25°C in the dark in KRH with 0.1% bovine serum albumin, mounted on a Leiden Cover Slip Dish, and placed in an Open Perfusion Micro-Incubator (Medical Systems Corp., Greenvale, NY) covered in 3 mL KRH with 0.1% bovine serum albumin. Cells were imaged using a Nikon Diaphot inverted microscope (Garden City, NY), coupled to a dual monochromator system, and GRP or carbachol-mediated [Ca2+]i mobilization was calculated by the method of Grynkiewicz et al. 24 Fluorescence was detected using an intensified CCD camera (Dage-MTI, Inc., Michigan City, IN), and images were processed with ImageMaster software (Photon Technologies International, Inc., South Brunswick, NJ).
Immunohistochemical Analysis
Paraffin-embedded sections (4 μm) were deparaffinized in three xylene washes followed by a graded alcohol series. Slides were then washed with phosphate-buffered saline and sections were incubated with anti-GRP-R (1:500 dilution) or anti-GRP antibody (1:100 dilution) for 1 hour at room temperature. After three washes with phosphate-buffered saline, the sections were incubated for 30 minutes with biotinylated secondary antibody. AB enzyme reagent was added for 30 minutes to the sections, followed by a phosphate-buffered saline wash. Finally, one to three drops peroxidase substrate was added for stain development. All sections were counterstained with hematoxylin. The sections were dehydrated with a series of ethanol and xylene washes and permanently mounted and observed by light microscopy. For negative controls, primary antibody was omitted from the above protocol.
Reverse Transcriptase–Polymerase Chain Reaction
RNA was extracted from cells by the method previously described. 23 For the reverse transcriptase (RT) reaction, random primers were mixed with 5 μg total RNA. After 70°C incubation, appropriate amounts of 5× First Strand Buffer, 0.1 mol/L DTT, and 10 mmol/L dNTP were added and incubated at 42°C for 2 minutes, followed by Superscript II enzyme as recommended by the manufacturer. After the addition of Superscript II enzyme, the mixture was heated at 42°C for 50 minutes and then 70°C for 15 minutes. The PCR reaction was performed by adding the cDNA generated from the RT reaction with appropriate amounts of 10× PCR Buffer, 25 mmol/L MgCl2, 10 mmol/L dNTP, 40 pmol/L sense GRP-R (ATCTATGTCATCCCTGCAG) and antisense GRP-R (GATCTGCTTCTTGACATGTA) primers, and Taq polymerase and cycled in a Stratagene PCR Robocycler according to the following profile: an initial denaturation for 3 minutes at 95°C followed by 35 cycles at 94°C for 1 minute, 50°C for 1 minute, and 72°C for 1.5 minutes. An aliquot of the PCR reaction was resolved by gel electrophoresis with 1% agarose gel containing ethidium bromide. GRP-R bands of approximately 710 base pairs were visualized by ultraviolet illumination.
Statistical Analysis
In vitro experiments were repeated on at least two separate occasions. Cell counts were expressed as mean ± SEM; statistical analyses were performed using one-way analysis of variance for comparisons between the treatment groups. P < .05 was considered significant.
RESULTS
Expression of GRP-R and GRP in Human Neuroblastomas
The purpose of the first part of our study was to assess a large number of archival human tumor samples for GRP-R and GRP protein expression. Thirty-three paraffin-embedded tumor samples, which consisted of undifferentiated neuroblastomas (n = 20), mixed-type ganglioneuroblastomas (n = 7), and ganglioneuromas (n = 6) from patients of varying ages, were analyzed. The histopathologic classification of tumor samples was based on nuclear features, cell shape, degree of neuropil formation, and organization. 2,3 The mean age at diagnosis of neuroblastoma for all histopathologic types was 3.3 years.
Tumor sections were stained for GRP-R expression. A variable degree of GRP-R expression was noted in 24 of the neuroblastoma tumors (Fig. 1; representative sections from 6 of the 24 samples). An increased expression of GRP-R was noted in undifferentiated tumors compared with benign ganglioneuromas, as scored by a blinded pathologist (D.R.K.). Based on amounts of GRP-R protein staining. Significant GRP-R expression was noted in undifferentiated neuroblastoma cells. Ganglioneuromas, represented by large matured ganglion cells, also expressed GRP-R, but significantly less when compared with ganglioneuroblastomas or neuroblastomas.
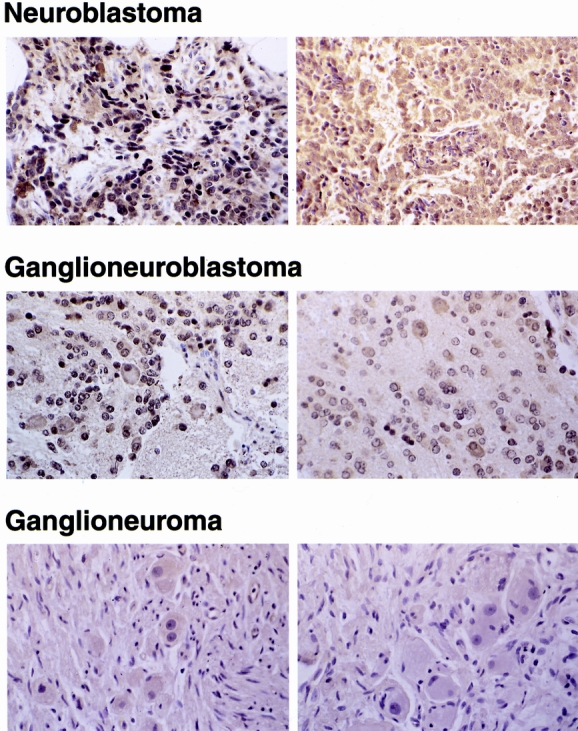
Figure 1. Expression of gastrin-releasing peptide receptor (GRP-R) in neuroblastomas. Two representative histologic sections from each of three subtypes of neuroblastomas showing differential GRP-R expression by immunohistochemical staining (×100). Increased expression of GRP-R protein (shown by the brown staining) is noted in undifferentiated neuroblastomas compared with glioneuroblastomas or ganglioneuromas.
In addition to GRP-R, tumor sections were examined for expression of GRP. Consistent with the findings of Sebesta et al., 25 GRP protein expression was also identified in the majority of tumor samples (27/33); however, unlike GRP-R expression, GRP expression did not appear to correlate with tumor histology. In other words, GRP protein was expressed at similar levels in the neuroblastomas and ganglioneuromas (Fig. 2; representative sections from 4 of the 27 samples). Collectively, these findings suggest that GRP may be an important endogenous peptide that is involved in the regulation of tumor growth. In addition, correlation of an increased GRP-R expression with more undifferentiated tumors may be an important predictor of clinical tumor behavior.
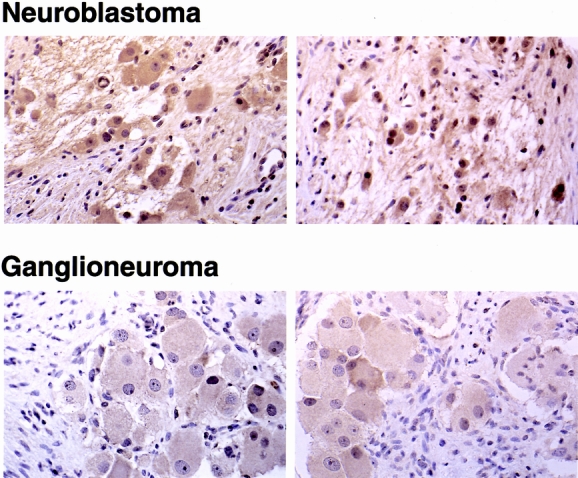
Figure 2. Expression of gastrin-releasing peptide (GRP) in neuroblastomas. Representative histologic sections of immunohistochemical staining with GRP antibody in neuroblastomas compared with ganglioneuromas (×100). Large mature ganglion cells show GRP protein (brown staining) in the cytoplasm.
Expression of GRP-R mRNA in SK-N-SH and IMR-32 Neuroblastoma Cell Lines
To further elucidate the role of GRP-R and GRP in neuroblastomas, we analyzed well-characterized human neuroblastoma cell lines. Two neuroblastoma cell lines, SK-N-SH and IMR-32, were analyzed for the presence of GRP-R mRNA by RT-PCR. A transcript size of 710 base pairs, representing a fragment of human GRP-R, was identified in both cell lines (Fig. 3). The identification of GRP-R mRNA in these cell lines corroborated our findings of GRP-R protein expression in resected human specimens.
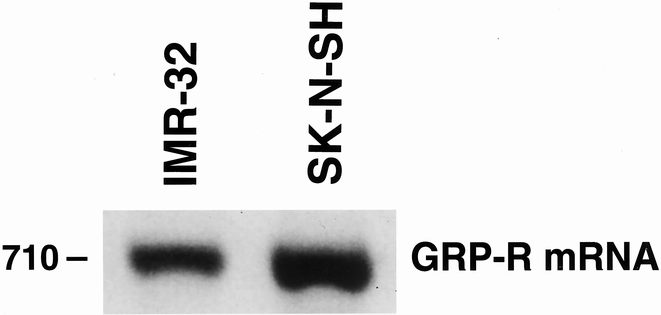
Figure 3. Reverse transcriptase–polymerase chain reaction analysis of neuroblastoma cell lines. RNA was extracted from human neuroblastoma cells (SK-N-SH, IMR-32) and reverse transcriptase–polymerase chain analysis was performed. A transcript size of 710 base pairs, representing a fragment of human gastrin-releasing peptide receptor (GRP-R), was identified in both cell lines.
GRP-R Is Functionally Coupled to the Ca2+ Pathway in SK-N-SH and LAN-1 Cells
To determine whether the GRP-R is functionally coupled to the Ca2+ signaling pathway in neuroblastoma cells, we next measured the effects of GRP (100 nmol/L) on the concentration of free [Ca2+]i in SK-N-SH cells (Fig. 4). GRP treatment resulted in a rapid increase in [Ca2+]i in SK-N-SH cells. This increase was noted in a majority of the cells after GRP treatment.
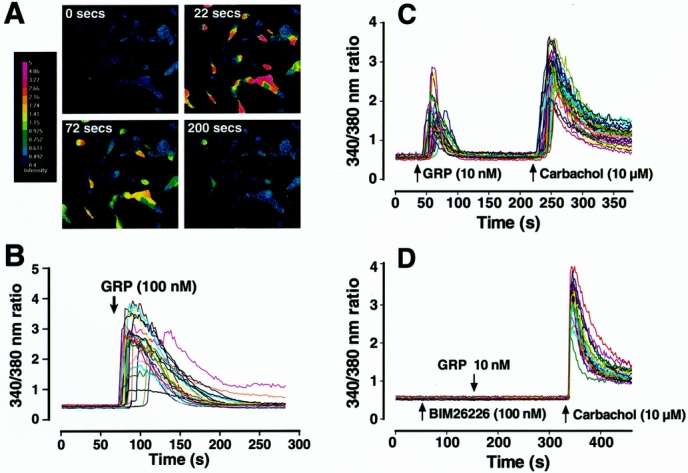
Figure 4. Intracellular calcium [Ca2+]i mobilization in SK-N-SH (A, B) and LAN-1 cells (C, D). (A) Pseudocolor images represent the relative [Ca2+]i as shown by the color bar (left). Red-shifted colors indicate higher Ca2+ activation. (B) Graph depicts [Ca2+]i over a time course after GRP treatment (100 nmol/L). Each line represents a single cell measurement. (C) Graph depicts [Ca2+]i over a time course after GRP (10 nmol/L) treatment in LAN-1 cells. To ensure the ability of cells to reproduce a calcium response, GRP treatment was followed with carbachol (10 μmol/L) treatment, which also produced a brisk [Ca2+]i response. (D) Graph depicting [Ca2+]i concentration pretreated with BIM26226 (100 nmol/L), a specific GRP-receptor antagonist, before GRP (10 nmol/L) treatment in LAN-1 cells.
We next assessed the effect of GRP on [Ca2+]i in another neuroblastoma cell line, LAN-1 (see Fig. 4). GRP (10 nmol/L) treatment also resulted in a rapid [Ca2+]i mobilization in these cells. A carbachol-induced increase in [Ca2+]i after GRP stimulation confirmed that GRP-treated cells can elicit a calcium response on stimulation. To confirm the specificity of GRP-R-coupled increases in [Ca2+]i, BIM26226 (a specific GRP-R antagonist) was applied before GRP stimulation. BIM26226 blocked increases in [Ca2+]i, further showing GRP specificity.
Unlike SK-N-SH and LAN-1 cells, GRP treatment did not induce [Ca2+]i mobilization in IMR-32 and produced only a minimal increase (<1% population) in SH-SY5Y cells (data not shown). Therefore, not all neuroblastoma cell lines respond by [Ca2+]i mobilization on GRP stimulation, suggesting that other signaling pathways may be involved in the response to GRP that do not involve [Ca2+]i.
GRP Treatment Stimulates Neuroblastoma Cell Growth
To determine whether GRP is a mitogenic factor for neuroblastoma cells, we next assessed GRP-induced proliferation of neuroblastoma cells. Four neuroblastoma cell lines (SK-N-SH, IMR-32, LAN-1, SH-SY5Y) were plated at equal numbers (15,000 cells/well) and serum-starved for 24 hours. The medium was then changed to a low serum concentration (0.1% for SK-N-SH, IMR-32, and LAN-1 and serum-free for SH-SY5Y), and cells were treated with GRP (10−8 mol/L) daily beginning on day 0. Cell number was counted using a Coulter Cell Counter on days 1, 2, and 3. Significant increases in cell growth were noted in all four cell lines after treatment with GRP (10−8 mol/L) (Fig. 5). Despite lack of significant [Ca2+]i mobilization found in IMR-32 and SH-SY5Y cells, GRP treatment resulted in an increase in cell growth. This finding suggests that the mitogenic action of GRP on neuroblastoma cells may involve Ca2+-dependent, as well as Ca2+-independent cellular signaling pathways.
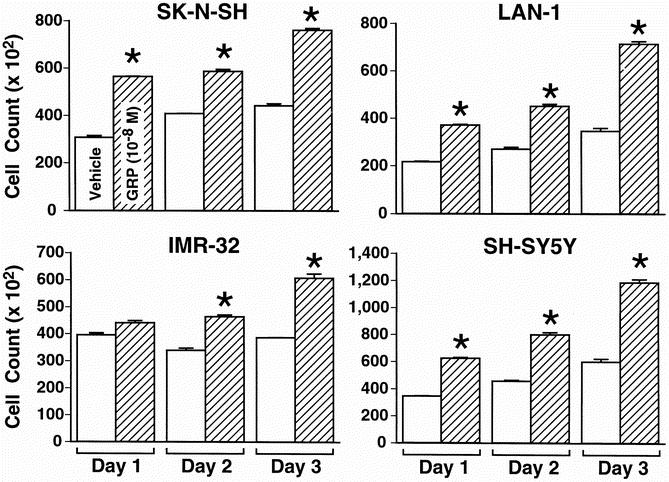
Figure 5. Gastrin-releasing peptide (GRP) treatment increases the growth of neuroblastoma cells. GRP treatment increases cell growth in all four (SK-N-SH, LAN-1, IMR-32, SH-SY5Y) neuroblastoma cell lines, when compared to control. Mean ± SEM for triplicate determination; *P < .05 vs. control.
Finally, to further ascertain whether GRP may act in either an autocrine or paracrine fashion to stimulate cell growth, we next performed studies using conditioned medium obtained from stimulated neuroblastoma cell cultures. To obtain the conditioned medium, serum-starved SK-N-SH cells were stimulated with exogenous GRP (10−8 mol/L) for 24 hours. At the end of 24 hours of incubation, the stimulated cells were washed thoroughly with fresh medium to remove exogenous GRP. After these washes, fresh medium was added and cells incubated for an additional 48 hours; this final medium constituted the conditioned medium. A stimulation of SK-N-SH cell growth was noted with the addition of the conditioned medium (Fig. 6). This proliferative effect was inhibited by the addition of anti-GRP antibody to the conditioned medium. These data further suggest a potential role for GRP as either an autocrine or paracrine growth factor in neuroblastoma cells.
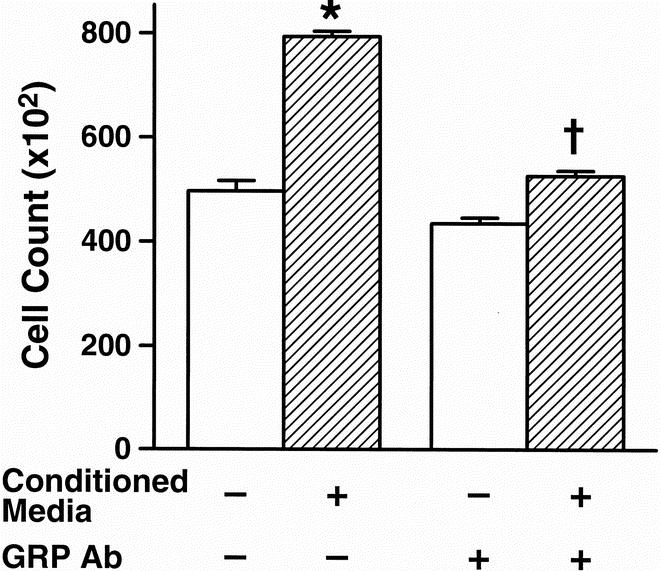
Figure 6. Conditioned medium-stimulated growth of SK-N-SH cells is inhibited with anti-gastrin-releasing peptide (GRP) antibody. The cells maintained with conditioned medium significantly increased growth at 48 hours. Further, anti-GRP antibody significantly blocked the growth effect of cells plated in the conditioned medium. Mean ± SEM for triplicate determination; *P < .05 vs. control, †P < .05 vs. conditioned medium alone.
DISCUSSION
GRP, the mammalian equivalent of bombesin, binds to GRP-R to stimulate growth of a number of normal and neoplastic tissues. 11,26 In this study, we found an increased expression of GRP-R in the undifferentiated neuroblastomas compared with the differentiated ganglioneuromas. GRP functionally binds to GRP-R to stimulate the release of calcium from intracellular stores, which was shown in two of the four neuroblastoma cell lines studied. Exogenous GRP treatment stimulated neuroblastoma cell growth, and proliferative effects of conditioned medium on neuroblastoma cells were inhibited with anti-GRP antibody. Our findings suggest a role for GRP as either an autocrine or paracrine growth factor for human neuroblastomas.
Several studies have attempted to correlate the level of GRP-R and the biologic behavior of tumor types. 25,27 Markwalder and Reubi 27 reported GRP-R overexpression in neoplastic and preneoplastic prostate tissues. Sebesta et al. 25 analyzed human neuroblastoma tumors and found that GRP-R mRNA was qualitatively present in all tumors examined. However, no correlation was found for GRP-R mRNA expression to the biologic behavior of neuroblastomas. In our study, we found GRP-R protein expression in a spectrum of undifferentiated neuroblastomas to benign tumors (ganglioneuromas). Further, a higher percentage of GRP-R protein expression was found in more undifferentiated tumors, suggesting that the mitogenic effects of GRP may be more profound in undifferentiated tumors as a result of increased GRP-R expression. This finding corroborates the results from a study by Sebesta et al. 25 in which GRP mRNA was found in all of the neuroblastoma tumor samples.
In addition to GRP-R expression, we found that GRP protein was expressed throughout the majority of neuroblastoma tumor samples. In contrast, GRP expression did not correlate with tumor histologic types. Both well-differentiated ganglion cells from benign ganglioneuromas as well as undifferentiated neuroblastoma cells expressed GRP. Certain neuroblastoma cell markers, such as nerve growth factor and its associated high-affinity receptor (gp 140Trk-A), have been associated with the prognosis of neuroblastoma patients. 28,29 An association between high levels of Trk gene expression and favorable outcome in patients with neuroblastomas has also been observed. 30 Although we did not find differential GRP expression in all stages of tumors in our study, an apparent differential expression of GRP-R may contribute to the more aggressive behavior of neuroblastomas. Therefore, antagonists to GRP-R may inhibit the growth stimulatory effects of GRP, providing a potential novel adjuvant therapy for patients with neuroblastoma.
Transmembrane signaling by bombesin or GRP involves a guanine-nucleotide-binding regulatory protein (G-protein) and activation of adenyl cyclase in other cells. 31–33 The binding of bombesin to its receptor activates protein kinase C via diacylglycerol and causes mobilization of Ca2+ from internal stores mediated by inositol 1,4,5-triphophate. 31,34 Bombesin can also induce mitogenesis by arachidonic acid release. 35 Bombesin/GRP stimulation activates adenyl cyclase, leading to an increase in intracellular cyclic adenosine monophosphate (cAMP), which stimulated c-fos and c-myc expression and promoted mitogenesis in Swiss 3T3 cells. 36 We found that the human neuroblastoma cell lines SK-N-SH and LAN-1 exhibit a robust [Ca2+]i mobilization on GRP stimulation. In contrast, minimal or no response was noted in SH-SY5Y and IMR-32 cells. However, the lack of [Ca2+]i mobilization in IMR-32 or SH-SY5Y cells does not preclude cAMP induction in IMR-32 and SH-SY5Y cells, which was not specifically evaluated in this study. Our results suggest that the signal transduction pathways linked to GRP-R may be dependent on cell line. Similarly, Casanueva et al. 37 reported that bombesin stimulates the growth of HT-29 colon carcinoma cells without an increase in [Ca2+]i mobilization. GRP may also act via the tyrosine kinase pathway to produce its mitogenic actions in certain cells. 38 Future studies will assess the role of other signaling pathways associated with GRP-mediated actions in neuroblastoma cells.
We found that GRP stimulates growth of all four neuroblastoma cell lines assessed and that endogenous GRP can further enhance cell growth in SK-N-SH cells. Sawin et al. 22 previously reported that SK-N-SH cells secrete GRP into the medium as assayed by radioimmunoassay. To demonstrate whether endogenous GRP can elicit a trophic effect, we first stimulated quiescent SK-N-SH neuroblastoma cells with exogenous GRP to produce conditioned medium. In SK-N-SH cells incubated with conditioned medium, significant growth stimulation was observed. Further, this proliferative effect of the conditioned medium was abrogated with anti-GRP antibody, suggesting that GRP was responsible for the growth effect of the conditioned medium.
GRP has been reported to be an autocrine growth factor for certain cancers, such as small cell lung carcinoma. 12,14 Various in vitro and in vivo studies using cell lines expressing GRP-R such as small cell lung, breast, colon, and pancreatic cancer cells have clearly shown a mitogenic effect of bombesin/GRP on these tumors. 12,14,17,20 These studies were followed by reports of a growth inhibitory effect of specific GRP-R antagonists, such as RC-3095, in multiple cell lines. 39–41 More sophisticated cytotoxic analogs of bombesin/GRP were also synthesized, such as bombesin-like carrier peptide conjugated to a potent derivative of doxorubicin (AN-201, AN-215), to target small cell lung and prostate cancer cells. 42,43 These were also found to be effective in inhibiting tumor cell growth. Another approach that has been used to treat GRP-R-positive tumors is to target GRP by producing monoclonal anti-GRP antibodies, such as 2A11, which culminated in a phase 1 trial to treat patients with small cell lung cancer. 44 Although this study did not show a significant antitumor response, the safety of using such an agent in clinical trials was shown. To detect and stratify the response of GRP/GRP-R-targeted treatment of GRP-R-positive tumors, technetium-99m-labeled GRP analogs have also been developed for the purpose of GRP-R scintigraphy. 45 Our finding of GRP/GRP-R expression in neuroblastomas further supports the possibility of existing technology to treat and diagnose patients with neuroblastoma.
In conclusion, we showed expression of GRP and GRP-R in a majority of human neuroblastomas, with an increased expression of GRP-R noted in more undifferentiated tumors. We also demonstrated that GRP-R is functionally coupled to the Ca2+ signaling pathway in two of the four neuroblastoma cell lines examined. GRP treatment stimulated growth of all four neuroblastoma cell lines, suggesting that mitogenic effects of GRP in neuroblastomas may involve both Ca2+-dependent as well as Ca2+-independent intracellular pathways. The effect of conditioned medium to promote growth was inhibited by anti-GRP antibody. Collectively, these results suggest a role for GRP as either an autocrine or paracrine growth factor in neuroblastoma cells. Therapeutic agents that act to block GRP-R may provide a novel adjuvant treatment modality for neuroblastomas in children.
Acknowledgments
The authors thank Dr. James F. Battey (NIH, Bethesda, MD) for the GRP-R antibody, Kirk Ives and Jell Hsieh for their technical support, and Eileen Figueroa and Karen Martin for manuscript preparation.
Discussion
DR. MARTY HESLIN (Birmingham, AL): Thank you for the opportunity to discuss this paper. I would like to thank the group from Galveston for again bringing excellent work to this membership and congratulate them on looking at a clinical problem and bringing it to the laboratory to try to elucidate the mechanisms for potential tumor growth. After reading the manuscript, I have three questions.
First, is there any data, or have you investigated utilizing gastrin or GRP as a potential tumor marker and its relationship with tumor response and/or tumor recurrence?
The second question relates to the potential role of GRP or GRP-R as a phenomenon that would induce tumor production. So that is there a mutation in the gene of the GRP-R, the receptor itself, that may act to provide a selection for these tumors to grow? That is, is this an alteration in the gene or its promotor, or is this simply a phenomenon that is associated with alterations in signaling pathways in the neuroblastoma cells?
The last question relates to something that was in the manuscript and wasn’t presented but deals with the GRP-R antagonists. There are certain compounds which you mentioned, such as in RC 3095, which function to inactivate the GRP-R pathway. As you know, there is other work in small cell lung cancer where RC 3095 is associated with a reduction in the proliferation of small cell cancer, and it is also associated with the reduction in the expression of the epidermal growth factor receptor. C 225, an epidermal growth factor receptor antagonist, has been used in other malignancies. Have you explored this in any potential synergistic treatment or novel treatment in these types of tumors?
I would like to thank the Association for the opportunity to discuss this paper and again congratulate you.
DR. JAMES A. O’NEILL, JR. (Nashville, TN): I rise to point out this paper’s potential importance and to ask a few questions that relate to the thesis that has been proposed.
First of all, Dr. Chung is to be congratulated for choosing a challenging tumor, because the undifferentiated neuroblastoma has a survival in the range of 10% to 15%. So it is the worst of the tumor models, if you will, in childhood to attack. Second, he has chosen to pursue the avenue of identifying growth factors in order to determine if growth can be impeded.
The first question comes about really related to his first slide. You will note that he pointed out that there were a number of substances, markers, et cetera, produced by neuroblastoma. Inherent in that tumor model is the fact that it is polyclonal. So it raises the question about multiplicity of growth factors involved. And obviously the ultimate success of this approach will be based on whether this is the dominant growth factor or not. But it is encouraging in that it is expressed broadly.
However, there is another avenue that perhaps he didn’t mention that may even be more important: that is that if it does pan out that this particular growth factor correlates with the particularly bad prognosis tumors—that is, the very undifferentiated ones—it may be one way of selecting out patients who have a particularly poor prognosis and to immediately shift them from the usual conventional treatment approaches to alternate approaches, most of which are bone marrow transplant-based. I would ask him to comment on that.
In that respect, there are a number of other indicators of bad prognosis for which there might be comparison and correlation, notably the increases in ferritin in serum in patients with undifferentiated neuroblastomas, the increased expression of the oncogene N-myc, and things of that nature. Therefore, in your archival material do you have any of those correlates of particularly bad prognosis in addition to histology—which, after all, is not always a sufficient benchmark?
Nonetheless, this is a very stimulating paper and potentially important.
DR. RICHARD J. ANDRASSY (Houston, TX): Dr. Chung and his coworkers in Galveston have continued the work of Dr. Evers and Dr. Townsend looking at tumor markers and tumor aggressiveness, and I certainly commend them.
I think you have heard from Dr. O’Neill that neuroblastoma is a very aggressive tumor. It is one of our worst prognostic tumors to deal with. We continue to look for markers. As mentioned, one good marker has been the N-myc oncogene.
It was also stated that GRP and the GRP receptor are seen in a number of tumors, as the Galveston group has already shown, so obviously it is not exactly specific. Now they are showing that the neural tumors such as neuroblastoma may express this.
I have several questions to ask them, and they were alluded to briefly.
Does this receptor expression correlate with the size, the invasiveness, or outcome? How are we going to use this clinically?
Are other peptides expressed by these tumors that may confuse this growth factor potential? Do other tumors, such as Wilms tumor that may be confused with neuroblastoma, express GRP or its receptor?
Could you also help me understand a little bit about the measurement of the intracellular calcium signaling pathway? My understanding is that this is pretty much a snapshot. Do you have some evidence clinically or in other studies to suggest that this would correlate with clinical growth? I would also like to know if you have looked at any GRP receptor antagonists.
Finally, some other investigators have suggested that when there is coexpression of GRP and GRP receptor, that this may actually lead to a more differentiated phenotype. I was wondering if you have any thoughts of why your work suggests that undifferentiated tumors correlate with GRP expression.
DR. JOHN M. KELLUM (Richmond, VA): This is a very interesting paper. We know that gastrin itself is a putative trophic hormone. I am wondering if the authors looked at gastrin receptors in this tumor material and whether neuroblastomas actually make gastrin.
Second, since we know in gastric mucosa that GRP or bombesin cells are intimately related to somatostatin cells, I ask whether the authors have looked at the effect of somatostatin or somatostatin analog on tumor growth.
DR. DAI H. CHUNG (Galveston, TX): I would like to thank all the discussants for their insightful comments and questions.
Dr. Heslin asked whether we can use gastrin or GRP as tumor markers in determining neuroblastoma recurrences. We have not specifically correlated the expression of GRP or gastrin to neuroblastoma tumor recurrences in our patient samples. However, we are currently analyzing whether there is differential expressions of GRP/GRP-R in correlation to other clinical information, such as age of patient, overall outcome, and other tumor markers.
We do not know whether there is a mutation in the GRP-R gene that results in an increased neuroblastoma tumor growth; however, we have recently found that inhibition of the PI3-K pathway can downregulate the GRP-R promotor activity and subsequently decrease cell survival in neuroblastoma cells. We plan to further investigate the mechanism of GRP-R regulation by the PI3-K pathway.
We do not know whether EGF-receptor antagonists can inhibit neuroblastoma cell growth. Since GRP can activate EGF receptors in a heterologous fashion in other cancer cells, it is possible that a similar action may be occurring in neuroblastoma cells. We plan to further investigate this possibility.
Dr. O’Neill and Dr. Andrassy asked whether we looked at other peptide markers in neuroblastomas. We have not specifically examined the expression of various gut peptides for this particular study; however, we are in the process of analyzing tumor samples for expression of RNA and proteins for various gut peptides, such as neurotensin, gastrin, and somatostatin.
Dr. O’Neill also asked whether the expression of GRP/GRP-R correlated with other tumor markers of neuroblastoma, such as ferritin and N-myc. Since many of these neuroblastoma tumor markers are analyzed routinely, we plan to investigate the potential relationship of GRP/GRP-R to the expressions of ferritin and N-myc in neuroblastomas.
Dr. Andrassy asked whether GRP-R expression correlates with neuroblastoma tumor size and/or invasiveness. We did not specifically assess this, but we plan to perform in vivo nude mice experiments using GRP and GRP antagonists to determine the effects of GRP on neuroblastoma tumor growth or inhibition. Our preliminary data from in vitro experiments show that GRP stimulates IMR-32 neuroblastoma cell growth, and this proliferative effect is reversed using GRP (BIM26226) antagonist. On binding of ligands to receptors that are coupled to intracellular calcium pathway, there is generally a rapid peak increase in calcium level; this returns to baseline as the intracellular calcium stores are reestablished. We have not examined the expression of GRP/GRP-R in Wilms tumor.
Dr. Kellum asked whether we examined the presence of somatostatin and gastrin in neuroblastomas. We have found that neuroblastomas also express gastrin but have not specifically analyzed for somatostatin and its effects on cell growth.
Footnotes
Presented at the 113th Annual Session of the Southern Surgical Association, December 3–5, 2001, Hot Springs, Virginia.
Supported by grants from the National Institutes of Health (PO1 DK35608, RO1 DK48498) and American Cancer Society Institutional Research Grant (# 2002-01). S.K. is a recipient of a pilot grant from the Texas Gulf Coast Digestive Diseases Center (P30 DK56338).
Correspondence: Dai H. Chung, MD, Department of Surgery, The University of Texas Medical Branch, 301 University Blvd., Galveston, TX 77555-0353.
E-mail: dhchung@utmb.edu
Accepted for publication December 2001.
References
- 1.Davis S, Rogers MA, Pendergrass TW. The incidence and epidemiologic characteristics of neuroblastoma in the United States. Am J Epidemiol 1987; 126: 1063–1074. [DOI] [PubMed] [Google Scholar]
- 2.Joshi VV, Silverman JF. Pathology of neuroblastic tumors. Semin Diagn Pathol 1994; 11: 107–117. [PubMed] [Google Scholar]
- 3.Kelly DR, Joshi VV. Neuroblastoma and related tumors. In: Parham DM, ed. Pediatric neoplasia: morphology and biology. Philadelphia: Lippincott-Raven; 1996: 105–152.
- 4.Ciccarone V, Spengler BA, Meyers MB, et al. Phenotypic diversification in human neuroblastoma cells: expression of distinct neural crest lineages. Cancer Res 1989; 49: 219–225. [PubMed] [Google Scholar]
- 5.Joshi VV, Cantor AB, Brodeur GM, et al. Correlation between morphologic and other prognostic markers of neuroblastoma. A study of histologic grade, DNA index, N-myc gene copy number, and lactic dehydrogenase in patients in the Pediatric Oncology Group. Cancer 1993; 71: 3173–3181. [DOI] [PubMed] [Google Scholar]
- 6.Brodeur GM. Neuroblastoma. In: Pizzo PA, Poplack, DG, ed. Principles and practice of pediatric oncology. Philadephia: Lippincott; 1997: 761–797.
- 7.Maris JM, Matthay KK. Molecular biology of neuroblastoma. J Clin Oncol 1999; 17: 2264–2279. [DOI] [PubMed] [Google Scholar]
- 8.Brodeur GM, Seeger RC, Schwab M, et al. Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage. Science 1984; 224: 1121–1124. [DOI] [PubMed] [Google Scholar]
- 9.Look AT, Hayes FA, Nitschke R, et al. Cellular DNA content as a predictor of response to chemotherapy in infants with unresectable neuroblastoma. N Engl J Med 1984; 311: 231–235. [DOI] [PubMed] [Google Scholar]
- 10.Fong CT, Dracopoli NC, White PS, et al. Loss of heterozygosity for the short arm of chromosome 1 in human neuroblastomas: correlation with N-myc amplification. Proc Natl Acad Sci USA 1989; 86: 3753–3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greeley GH Jr, Partin M, Spannagel A, et al. Distribution of bombesin-like peptides in the alimentary canal of several vertebrate species. Regul Pept 1986; 16: 169–181. [DOI] [PubMed] [Google Scholar]
- 12.Cuttitta F, Carney DN, Mulshine J, et al. Bombesin-like peptides can function as autocrine growth factors in human small-cell lung cancer. Nature 1985; 316: 823–826. [DOI] [PubMed] [Google Scholar]
- 13.Chu KU, Evers BM, Ishizuka J, et al. Role of bombesin on gut mucosal growth. Ann Surg 1995; 222: 94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alexander RW, Upp JR Jr, Poston GJ, et al. Effects of bombesin on growth of human small cell lung carcinoma in vivo. Cancer Res 1988; 48: 1439–1441. [PubMed] [Google Scholar]
- 15.Parekh D, Ishizuka J, Townsend CM Jr, et al. Characterization of a human pancreatic carcinoid in vitro: morphology, amine and peptide storage, and secretion. Pancreas 1994; 9: 83–90. [DOI] [PubMed] [Google Scholar]
- 16.Bold RJ, Kim HJ, Ishizuka J, et al. A human gastric cancer cell line possesses a functional receptor for gastrin-releasing peptide. Cancer Invest 1998; 16: 12–17. [DOI] [PubMed] [Google Scholar]
- 17.Narayan S, Guo YS, Townsend CM Jr, et al. Specific binding and growth effects of bombesin-related peptides on mouse colon cancer cells in vitro. Cancer Res 1990; 50: 6772–6778. [PubMed] [Google Scholar]
- 18.Kim HJ, Evers BM, Guo Y, et al. Bombesin-mediated AP-1 activation in a human gastric cancer (SIIA). Surgery 1996; 120: 130–136. [DOI] [PubMed] [Google Scholar]
- 19.Burns DM, Walker B, Gray J, Nelson J. Breast cancer cell-associated endopeptidase EC 24.11 modulates proliferative response to bombesin. Br J Cancer 1999; 79: 214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bologna M, Festuccia C, Muzi P, et al. Bombesin stimulates growth of human prostatic cancer cells in vitro. Cancer 1989; 63: 1714–1720. [DOI] [PubMed] [Google Scholar]
- 21.Logothetis C, Hoosein N. The inhibition of the paracrine progression of prostate cancer as an approach to early therapy of prostatic carcinoma. J Cell Biochem Suppl 1992; 16H: 128–134. [DOI] [PubMed] [Google Scholar]
- 22.Sawin RS, Brockenbrough J, Ness JC. Gastrin releasing peptide is an autocrine growth factor for human neuroblastoma. Surg Forum 1992; 43: 606–608. [Google Scholar]
- 23.Ehlers RA, Kim S, Zhang Y, et al. Gut peptide receptor expression in human pancreatic cancers. Ann Surg 2000; 231: 838–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 1985; 260: 3440–3450. [PubMed] [Google Scholar]
- 25.Sebesta JA, Young A, Bullock J, et al. Gastrin-releasing peptide: a potential growth factor expressed in human neuroblastoma tumors. Curr Surg 2001; 58: 86–89. [DOI] [PubMed] [Google Scholar]
- 26.Rozengurt E, Sinnett-Smith J. Bombesin stimulation of DNA synthesis and cell division in cultures of Swiss 3T3 cells. Proc Natl Acad Sci USA 1983; 80: 2936–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Markwalder R, Reubi JC. Gastrin-releasing peptide receptors in the human prostate: relation to neoplastic transformation. Cancer Res 1999; 59: 1152–1159. [PubMed] [Google Scholar]
- 28.Matsushima H, Bogenmann E. Expression of trkA cDNA in neuroblastomas mediates differentiation in vitro and in vivo. Mol Cell Biol 1993; 13: 7447–7456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaplan DR, Hempstead BL, Martin-Zanca D, et al. The trk proto-oncogene product: a signal transducing receptor for nerve growth factor. Science 1991; 252: 554–558. [DOI] [PubMed] [Google Scholar]
- 30.Nakagawara A, Arima-Nakagawara M, Scavarda NJ, et al. Association between high levels of expression of the TRK gene and favorable outcome in human neuroblastoma. N Engl J Med 1993; 328: 847–854. [DOI] [PubMed] [Google Scholar]
- 31.Spindel ER, Giladi E, Segerson TP, Nagalla S. Bombesin-like peptides of ligands and receptors. Recent Prog Horm Res 1993; 48: 365–391. [DOI] [PubMed] [Google Scholar]
- 32.Rozengurt E. Bombesin stimulation of mitogenesis. Specific receptors, signal transduction, and early events. Am Rev Respir Dis 1990; 142: S11–15. [DOI] [PubMed] [Google Scholar]
- 33.Qin Y, Halmos G, Cai RZ, et al. Bombesin antagonists inhibit in vitro and in vivo growth of human gastric cancer and binding of bombesin to its receptors. J Cancer Res Clin Oncol 1994; 120: 519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sethi T, Langdon S, Smyth J, et al. Growth of small cell lung cancer cells: stimulation by multiple neuropeptides and inhibition by broad spectrum antagonists in vitro and in vivo. Cancer Res 1992; 52: 2737S–2742S. [PubMed] [Google Scholar]
- 35.Millar JB, Rozengurt E. Arachidonic acid release by bombesin. A novel postreceptor target for heterologous mitogenic desensitization. J Biol Chem 1990; 265: 19973–19979. [PubMed] [Google Scholar]
- 36.Benya RV, Fathi Z, Kusui T, et al. Gastrin-releasing peptide receptor-induced internalization, down-regulation, desensitization, and growth: possible role for cyclic AMP. Mol Pharmacol 1994; 46: 235–245. [PubMed] [Google Scholar]
- 37.Casanueva FF, Perez FR, Casabiell X, et al. Correlation between the effects of bombesin antagonists on cell proliferation and intracellular calcium concentration in Swiss 3T3 and HT-29 cell lines. Proc Natl Acad Sci USA 1996; 93: 1406–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liebow C, Crean DH, Lee MT, et al. Synergistic effects of bombesin and epidermal growth factor on cancers. Proc Natl Acad Sci USA 1994; 91: 3804–3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yano T, Pinski J, Groot K, et al. Stimulation by bombesin and inhibition by bombesin/gastrin-releasing peptide antagonist RC-3095 of growth of human breast cancer cell lines. Cancer Res 1992; 52: 4545–4547. [PubMed] [Google Scholar]
- 40.Miyazaki M, Lamharzi N, Schally AV, et al. Inhibition of growth of MDA-MB-231 human breast cancer xenografts in nude mice by bombesin/gastrin-releasing peptide (GRP) antagonists RC-3940-II and RC-3095. Eur J Cancer 1998; 34: 710–717. [DOI] [PubMed] [Google Scholar]
- 41.Radulovic S, Miller G, Schally AV. Inhibition of growth of HT-29 human colon cancer xenografts in nude mice by treatment with bombesin/gastrin releasing peptide antagonist (RC-3095). Cancer Res 1991; 51: 6006–6009. [PubMed] [Google Scholar]
- 42.Kiaris H, Schally AV, Nagy A, et al. Targeted cytotoxic analogue of bombesin/gastrin-releasing peptide inhibits the growth of H-69 human small-cell lung carcinoma in nude mice. Br J Cancer 1999; 81: 966–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Plonowski A, Nagy A, Schally AV, et al. In vivo inhibition of PC-3 human androgen-independent prostate cancer by a targeted cytotoxic bombesin analogue, AN-215. Int J Cancer 2000; 88: 652–657. [DOI] [PubMed] [Google Scholar]
- 44.Chaudhry A, Carrasquillo JA, Avis IL, et al. Phase I and imaging trial of a monoclonal antibody directed against gastrin-releasing peptide in patients with lung cancer. Clin Cancer Res 1999; 5: 3385–3393. [PubMed] [Google Scholar]
- 45.Van de Wiele C, Dumont F, Vanden Broecke R, et al. Technetium-99m RP527, a GRP analogue for visualisation of GRP receptor- expressing malignancies: a feasibility study. Eur J Nucl Med 2000; 27: 1694–1699. [DOI] [PubMed] [Google Scholar]


